2018 年 58 巻 5 号 p. 876-885
2018 年 58 巻 5 号 p. 876-885
Experimental and theoretical studies have been carried out to study the effects of slag and metal electrode compositions on alloying elements in ingot during Drawing-Ingot-Type electroslag remelting (ESR) with a focus of developing a numerical model to control titanium and aluminum. The mass transfer model based on the penetration and film theories is established to analyze the Fe+FeO, Ti+TiO2, Al+Al2O3 and Si+SiO2 concentration changes along the height of ESR ingot. The results show that the combination of slag containing high CaO content, extra TiO2 incessantly added into slag in the first slag-temperature-rising period, and extra SiO2 incessantly added into slag during the whole ESR process is suitable for Drawing-Ingot-Type ESR of AISI321 steel. The mass transfer model shows that the remelting rate has little effect on the change of silicon, aluminum and titanium content in ingot, while the titanium content in electrode has significant effect on silicon, aluminum and titanium content in ingot. Since the titanium in AISI321 stainless steel ingot ranges from 0.4% to 0.8%, the titanium in electrode needs to be larger than 0.9% because of the titanium’s reaction with silica. In addition, the alloying element content in ingot under different remelting rate, electrode and slag composition are estimated based on the mass transfer model.
Electroslag remelting (ESR) is used to produce special steel and superalloy due to its exclusively outstanding superiorities, such as removal of non-metallic inclusion, homogeneous composition and outstanding solidification structure of ingots.1,2) As to homogeneous composition, slag composition and remelting process play essential roles in controlling titanium and aluminum content during Drawing-Ingot-Type ESR process. As the Drawing-Ingot-Type ESR needs the slag having the excellent high temperature plasticity, more than 4% silica needs to be added into molten slag.3) Unfortunately, the excess silica in slag can decrease the aluminum and titanium content in ingot by reactions of 4[Al]+3(SiO2)=(Al2O3)+[Si] and [Ti]+(SiO2)=(TiO2)+[Si], which makes the active alloying element control difficultly. Therefore, it is important to have a good understanding of titanium and aluminum control during Drawing-Ingot-Type ESR with stainless steel under different remelting process, slag and metal electrode composition.
Many measures have been taken to improve the homogeneous composition of ESR ingot, and few studies have reported the titanium and aluminum control during Drawing-Ingot-Type ESR with stainless steel. Many investigators4,5,6,7,8,9,10,11) have studied the homogeneous composition control and they found that slag composition and remelting process has significant effects on control of alloying elements. Hou4,5) studied the relationship between titanium, aluminum and silicon based on the thermodynamic equilibrium, and illustrated that CaO has significant effect on the activities of Al2O3, TiO2 and SiO2. Schwerdtfeger10) investigated the change of alloying elements during ESR with stainless steel and illustrated the influence of titania and slag temperature on titanium content in ingot.
To the best of authors’ knowledge, few investigations on controlling of Ti and Al during Drawing-Ingot-Type ESR process have been reported so far. The present study focuses on Ti and Al control during Drawing-Ingot-Type ESR of stainless steel. In the present work, a 50 kg ESR furnace experiment has been carried out for providing Ti and Al control technique based on kinetic analysis.
Three ESR experiments were carried out by using a 50-kg ESR furnace under different kinds of slag for 30 minutes. The inner diameter of water cooled copper mold is 134 mm, and the diameter of rustless consumable electrode is 60 mm. The chemical composition of electrode is listed in Table 1. The ESR equipment is shown in Fig. 1(a), which can be used to investigate the change of Si, Al and Ti during Drawing-Ingot-Type ESR process shown in Fig. 1(b): the essential issue of Drawing-Ingot-Type ESR with stainless steel is to control Al and Ti when there is more than 4% SiO2 in slag.
| C | Mn | Cr | Ni | Si | Al | Ti |
|---|---|---|---|---|---|---|
| 0.10 | 0.52 | 18.77 | 8.35 | 0.59 | 0.04 | 0.68 |

Schematic diagram of equipment: (a) ESR, and (b) Drawing-Ingot-Type ESR.
In each heat, the current and voltage were kept at 3000 A and 38 V, and mass remelting rate is about 66 kg·h−1. The 3200 g slag mixtures were roasted at 873 K (600°C) for four hours in a resistant furnace before experiment. The ratio of oxides in each slag and ESR process are listed in Table 2. In all experiments, the deoxidizer Al was added into molten slag at the rate of 2 kg t−1 (kg/per ton steel) under air atmosphere. In experimental A under the condition of slag S1, 80 g silica was added into molten slag at the 5th and 13th minute of ESR process, respectively. Both in experimental B (with slag S2) and C (with slag S3), 150 g TiO2 was incessantly added into slag in the first 10 minutes, and SiO2 was added into molten slag during the whole ESR process. The SiO2 addition rate is about 3.2 kg t−1 in experimental B, as well as about 1.8 kg t−1 in experimental C.
| Exp. | Slag | CaF2 | CaO | Al2O3 | TiO2 | SiO2 | MgO | Atmosphere | Deoxidizer | TiO2 | SiO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | S1 | 56 | 5 | 20 | 8 | 1 | 10 | Air | 2 kg t−1 | 0 | 80×2 g |
| B | S2 | 59 | 5 | 20 | 2 | 4 | 10 | Air | 2 kg t−1 | 150 g | 3.2 kg t−1 |
| C | S3 | 51 | 20 | 20 | 2 | 4 | 3 | Air | 2 kg t−1 | 150 g | 1.8 kg t−1 |
During each ESR process, slag samples were taken from the high temperature slag in water cooled copper mold at 5, 9, 13, 17, 29 cm height of ingot by using the quartz tube,12) and the contents of titania, alumina and silica in slag samples were analyzed. The steel samples taken along the height of ingot were used to analyze the silicon, titanium and aluminum content. The silicon and titanium were determined by direct reading spectrometry, and soluble aluminum was analyzed by inductively coupled plasma-mass spectroscopy.
Kinetic model based on penetration and film theories is established to analyze the change of Ti, Al and Si in experiments.12) As shown in Fig. 2, the process controlling solute redistribution in molten metal is the reactions among Fe+FeO, Ti+TiO2, Al+Al2O3 and Si+SiO2. The concentration of FeO at slag-metal interface

Concentration gradients of multiphase reactions at slag-metal interface.
If it is assumed that the reactions among Fe+FeO, Ti+TiO2, Al+Al2O3 and Si+SiO2 systems have reached equilibrium at the slag-metal interface, the thermodynamic parameters can be expressed by Eqs. (1), (2), (3), (4), (5), (6).8,9,10) Since the concentrations of MnO and Cr2O3 in molten slag are extremely low in the present experiment, the Mn+MnO and Cr+Cr2O3 systems are not considered. And because liquid iron is alloying element solvent, the transport of iron in metallic phase is discounted. Further, AlO1.5 is employed to simplify the calculation.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
Where γi and Xi are the activity coefficient and molar fraction of oxide component in molten slag; fi is the activity coefficient of alloying element in molten metal, and can be calculated by Eq. (7).
The first-order interaction parameters17) used in current work are listed in Table 3. The available second-order interaction parameters18,19,20) are expressed as
| (7) |
| C | Mn | Cr | Ni | Si | Al | Ti | |
|---|---|---|---|---|---|---|---|
| Al | 0.091 | 0.035 | 0.03 | −0.017 | 0.056 | 0.043 | 0.004 |
| Ti | −0.19 | −0.043 | 0.055 | – | −0.025 | 0.0037 | 0.042 |
| Si | 0.18 | −0.015 | −0.004 | 0.005 | 0.10 | 0.058 | 1.23 |
On the basis of the previous study5) and ion and molecule coexistence theory,13,14,15,16) the activity coefficient of each oxide component in slag γi can be measured after determining the slag composition (CaO–CaF2–Al2O3–SiO2–MgO–TiO2–FeO).
3.1.2. Slag-metal Reaction Mass Transfer ModelAccording to the description of penetration and film theories, the Ti mass transfer Eq. (8) can be obtained, and the derivational process has been studied in the previous study.12) For the same reason, the mass transfer model Eqs. (8), (9), (10), (11) are acquired.21)
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
| (15) |
Where C[i] and C(iO) are the concentration of i in metallic phase and iO in slag phase, mol/m3;
As the metal volume Vm is far less than the slag volume Vs during each metal droplet reacting with slag, the oxide component in slag would change very little within a time interval. Thus, the Eq. (16) can be obtained, and the mass transfer differential Eqs. (8), (9), (10), (11) can be integrated to Eq. (17). After solving the Eq. (17), the differential Eqs. (8), (9), (10), (11) are replaced with integral Eqs. (18), (19), (20), (21), which are also the mass transfer equations:
| (16) |
| (17) |
| (18) |
| (19) |
| (20) |
| (21) |
Where
As shown in Fig. 2 and Eqs. (1), (2), (3), according to the conservation law of oxygen atoms, the Eq. (22) is acquired. And then FeO at slag-metal interface
| (22) |
If this

Flow chart of established mass transfer model.
It was assumed that the diffusion coefficient
| (23) |
| (24) |
| (25) |
The slag-metal reaction model parameters necessary for the solution of Eqs. (18), (19), (20), (21), (22) are: (i) Geometric parameters: geometrical dimensions of the metal film at the tip of consumable electrode, metal droplet, and metal pool; (ii) Kinetic data: flow field of slag during ESR process, and the reaction time at the tip of consumable electrode, metal droplet and metal pool process; (iii) Distribution of temperature at the tip of consumable electrode, metal droplet and metal pool. The relevant parameters above can be calculated according to the previous study,12) and the results used for mass transfer model are listed in Table 4.
| Parameter | Location (reaction site) | ||
|---|---|---|---|
| Film/slag | Droplet/slag | Pool/slag | |
| kSi, kAl, kTi, cm/s | 0.0070 | 0.22 | 0.042 |
| kAl2O3, kTiO2, kFeO, cm/s | 0.011 | 0.11 | 0.022 |
| kSiO2, cm/s | 0.0048 | 0.051 | 0.0097 |
| Reaction surface area, cm2 | 36.91 | 8.38 | 141.03 |
| Liquid metal volume, cm3 | – | 0.35 | 399.57 |
| Area/Volume, cm−1 | – | 24 | 0.35 |
| Residence time, s | – | 0.19 | – |
| Volume/Reaction time, cm3/s | 2.55 | 1.84 | – |
The liquidus temperature of 1Cr21Ni5Ti is considered as 1730 K (1457°C), which is calculated by Thermo-calc. To simplify the mass transfer model, the temperature at the tip of consumable electrode is set as constant temperature, 1750 K (1477°C), with a certain superheat.11) According to the measured results under the similar ESR condition in the reference,11) the temperature at the metal pool/slag interface is considered as 1950 K (1677°C) in the present study. The average temperature of falling metal droplets in slag layer are assumed to be 1950 K (1677°C), which is equivalent to metal pool/slag temperature.
The ESR process consists with two processes: the one is slag temperature rising period at the beginning of ESR process, and another is slag temperature being stable, which can be distinguished by the change of remelting rate during the whole ESR process. Since the slag temperature increasing rate at the beginning of ESR process is continually decreasing, the increment of slag temperature (TSlag) at the interface of droplet metal/slag and pool metal/slag in the first 780 seconds (13 cm height of the ingot) can be expressed by ellipse Eq. (26). And the change of molten metal pool volume (VPool) with time can also be determined by ellipse Eq. (27), as shown in Fig. 4.
| (26) |
| (27) |

Variations of temperature and metal pool volume with time in ESR experiment.
As the consumable electrode is oxidized by air, the iron oxide at the surface of electrode is added into slag.23) The increment of iron oxide (IFeO) during each time interval can be determined on the total losses of all alloying elements (Al, Ti and Si) in remelted ingot during this time interval. Thus, the Eq. (28) is equivalent to the FeO increment per second, and the change of FeO increment per second with time is shown in Fig. 5. It is clear that the increment of iron oxide per second can be expressed by the line of IFeO=0.115 g/s.
| (28) |

Change of FeO increment per second with time in ESR experiment.
Where: Wm is the mass remelting rate, g/s; Mi is the molar mass of i element.
3.3. Concentration Change of Al, Ti and Si in Experimental ABased on the analysis above, the mass-transfer model of oxidation of alloying elements during ESR of stainless steel has been established. Figures 6, 7, 8 show the change curves of Ti, Al and Si along the height of ingot in experimental A, in comparison with the calculated results of numerical model established above. In Figs. 6, 7, 8 the experimentally determined concentration curves are shown together with the calculated results.

Change curve of titanium in ingot, and of titania in slag with height of ingot for experimental A.

Change curve of aluminum in ingot, and of alumina in slag with height of ingot for experimental A.

Change curve of silicon in ingot, and of silica in slag with height of ingot for experimental A.
As shown in Figs. 6, 7, 8, after 80 g silica was added into molten slag at 5 th and 13 th minute during the ESR process (5 cm and 13 cm height of ingot), plenty of aluminum and titanium are oxidized into slag according to [Ti]+(SiO2)=(TiO2)+[Si] and 4[Al]+3(SiO2)=2(Al2O3)+3[Si].
As can be seen in Fig. 7(a), with the increase of slag temperature in the first 13 minutes (from 1750 K to 1950 K, 1477°C to 1677°C), the aluminum increases during 1–5 cm and 5–13 cm height of ingot, respectively. As the temperature increases in the first 13 cm height of ingot, the increase of lgK(Ti,Al) in Eq. (30) makes the lg
| (29) |
| (30) |
Based on the mass transfer model, Exp.B and Exp.C were carried out, and the results are shown in Figs. 9, 10, 11. As shown in Fig. 9(a), it is clear that: (i) the titanium ranges from 0.25% to 0.39% in Exp.B, while the titanium ranges from 0.33% to 0.42% in Exp.C; (ii) the aluminum is close to the line of 0.025% in Exp.B, while the aluminum ranges from 0.045% to 0.055% in Exp.C. Since the titanium in AISI 321 ranges from 5(C-0.02)% to 0.8% and the carbon content is 0.10%, the titanium in AISI 321 should range from 0.4% to 0.8%. Thus, the slag containing high CaO is appropriate to Drawing-Ingot-Type ESR of high-carbon AISI 321 stainless steel. Moreover, the remelting rate and titanium in electrode should be increased to guarantee the requirement of titanium in ingot, which will be investigated below.

Change curve of titanium in ingot, and of titania in slag with height of ingot for experimental B and C.

Change curve of aluminum in ingot, and of alumina in slag with height of ingot for experimental B and C.

Change curve of silicon in ingot, and of silica in slag with height of ingot for experimental B and C.
Based on the Eqs. (18), (19), (20), when diameters of water cooled copper mold and electrode are fixed, the t/Vm decreases with the increase of Wm. In order to investigate the influence of remelting rate on alloying element during Drawing-Ingot-Type ESR process, the numerical values of Ti, Al and Si along the height of ingot are calculated based on the model established above by using slag S3 under 66, 78 and 90 kg/h remelting rates. In each simulation experiment, 150 g TiO2 was added into molten slag in the first 7 minutes. The deoxidizer Al and SiO2 are added into molten slag during the whole ESR process: the Al and SiO2 addition rates are 2 kg t−1 and 1.8 kg t−1, respectively.
As can be seen in Fig. 12, the faster the remelting rate is, the more the titanium content in ingot there is. But the effect of remelting rate on variations of each alloying element is little.

Change curve of titanium/titania with height of ingot calculated by developed model with different remelting rate.

Change curve of aluminum/alumina with height of ingot calculated by developed model with different remelting rate.

Change curve of silicon/silica with height of ingot calculated by developed model with different remelting rate.
As shown in Fig. 9(a), the titanium in ingot ranges from 0.33% to 0.42%. Unfortunately, the AISI 321 stainless steel requires titanium ranging from 0.4% to 0.8%. For example, the GH3044 requires titanium ranging from 0.30% to 0.70%, the 1Cr21Ni5Ti requires titanium ranging from 0.35% to 0.8%.
In order to discuss the effects of titanium in electrode on alloying element content in ingot during Drawing-Ingot-Type ESR process, the titanium content in electrode are assumed as 0.7%, 0.9% and 1.1%, respectively. In each simulation experiment, 150 g TiO2 was added into molten slag in the first 7 minutes, the deoxidizer Al addition rates is 2 kg t−1, and the remelting rate is about 90 kg/h during ESR process. It should be noted that the SiO2 addition rates are 1.8 kg t−1 under electrode with 0.7% Ti, 3.5 kg t−1 under electrode with 0.9% Ti, and 5.2 kg t−1 under electrode with 1.1% Ti, respectively. And then the corresponding results under the condition of different titanium content in electrode combined with slag S3 are calculated based on the mass transfer model, as shown in Figs. 15, 16, 17.
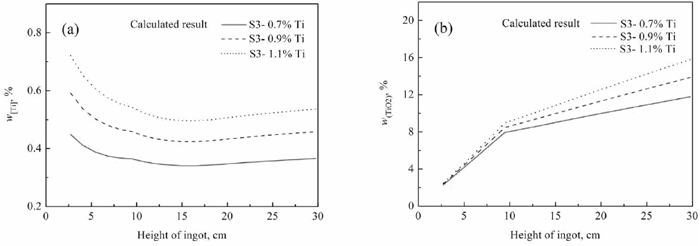
Change curve of titanium/titania with height of ingot calculated by developed model with 0.7, 0.9 and 1.1% titanium in electrode.
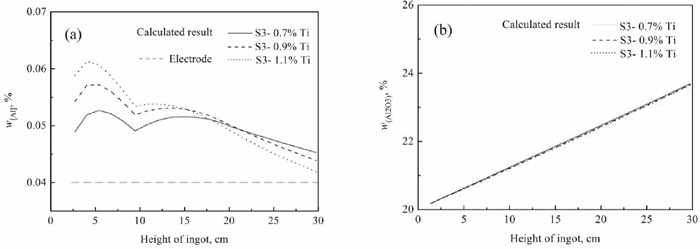
Change curve of aluminum/alumina with height of ingot calculated by developed model with 0.7, 0.9 and 1.1% titanium in electrode.

Change curve of silicon/silica with height of ingot calculated by developed model with 0.7, 0.9 and 1.1% titanium in electrode.
As shown in Fig. 15(a), when there is 0.7% titanium content in electrode, the titanium content in ingot ranges from 0.34% to 0.45%; in the case of 0.9% titanium content in electrode, the ingot containing 0.42%–0.59% titanium would be acquired; under the condition of 1.1% titanium in electrode, the titanium in ingot ranges from 0.50% to 0.72%.
The current work investigates the influence of CaO in molten slag on the change of Al, Ti and Si in ingot based on kinetics during Drawing-Ingot-Type ESR process. After selecting CaO content, the current work also studies the effects of remelting rate and titanium in electrode on the concentrations of titanium, aluminum and silicon in ingot further. This work focuses on providing slag mixtures, electrode and ESR process which can be used to control titanium and aluminum during Drawing-Ingot-Type ESR of steel containing Ti, Si and Al. In addition, it should be pointed out that the TiO2 in each experiment is larger than 10% in slag (shown in Fig. 15(b)), and the effect of slag composition on the surface quality of ingot would be investigated in a industrial Drawing-Ingot-Type ESR furnace based on this study further.
In order to investigate the effect of remelting process, slag and electrode composition on titanium and aluminum content in Drawing-Ingot-Type ESR ingot, experiments were performed with a 50 kg ESR furnace, and the slag-metal reaction was investigated from the viewpoint of kinetics. The results were presented as follows.
(1) The oxidation kinetics of alloying elements based on penetration and film theories reveals the transfer behavior of Ti, Al and Si during Drawing-Ingot-Type ESR process, and the numerical results calculated by mass transfer model are in line with the experimental results.
(2) The more CaO in slag is, the less titanium is oxidized by its reaction with silica. With the increase of slag temperature at the beginning of ESR process, the TiO2 should be added into slag to improve the homogenous of titanium and aluminum. The SiO2 should be added into molten slag during the whole process to certificate more than 4% silica in slag.
(3) The numerical results calculated by mass transfer model show that the remelting rate has little effect on the variations of aluminum, titanium and silicon content in ingot.
(4) Since the titanium in AISI321 steel ranges from 0.4% to 0.8%, the titanium in electrode should be larger than 0.9% because of the reaction [Ti]+(SiO2)=(TiO2)+[Si]. The alloying element content in ingot under different remelting rate, electrode and slag composition can be calculated according to the mass transfer model.
This project is supported by National Nature Science Foundation of China with grant No. 51674070 and U1560203. This project is also supported by Nature Science Foundation of China with grant No. 51674172, and Jiangsu Province with grant No. BK20150334 and 20150336. In addition, this project supported by Open Foundation of The State Key Laboratory of Refractories and Metallurgy with the grant No. G201607.