2018 Volume 58 Issue 8 Pages 1437-1442
2018 Volume 58 Issue 8 Pages 1437-1442
The effect of silicon on TiN formation in liquid iron was studied by measuring the nitrogen solubility and TiN solubility product in liquid Fe–Ti alloys with silicon additions under various nitrogen partial pressures in the temperature range of 1823–1923 K. The first- and second order interaction parameters of silicon on titanium,  and
and  were determined as −0.038 and 0, respectively, and the second-order cross-product parameter of titanium and silicon on nitrogen in liquid iron,
were determined as −0.038 and 0, respectively, and the second-order cross-product parameter of titanium and silicon on nitrogen in liquid iron, 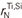 was determined as 0. Temperature dependence of these parameters were negligible.
was determined as 0. Temperature dependence of these parameters were negligible.
Titanium is often added in liquid steel to improve mechanical properties via the grain refinement during hot rolling. Also, TiN inclusions formed as secondary inclusions during solidification of ferritic stainless steel are known to act as nucleation sites of δ-ferrite helping the formation of equi-axed cast structure. In order to control the TiN formation based on the supersaturation of Ti and N in various steel grades, it is necessary to have an accurate thermodynamic information on the effect of various alloying elements on titanium and nitrogen in liquid iron.
Among alloying elements in steels, the effect of silicon on TiN formation is not clearly known since the thermodynamic data on the interaction parameter between Si and Ti scatter to a large extent from −0.0256 to 2.1 at 1873 K1,2,3,4) as shown in Fig. 1. According to those high

Relationship between
Therefore, in the present study, the effect of silicon on TiN solubility product was directly determined by addition of silicon in TiN saturated Fe–Ti–N melt under various nitrogen partial pressures in the temperature range from 1823 to 1923 K. Also, the simultaneous effect of titanium and silicon on nitrogen in liquid iron was determined by measuring the nitrogen solubility in Fe–Ti–Si melts. The experimental results were thermodynamically analyzed to determine the accurate values of interaction parameters among silicon, titanium and nitrogen in Fe–Ti–Si–N system.
The gas-liquid metal-nitride and gas-liquid metal equilibration experiments were carried out to determine TiN solubility product and N solubility in liquid Fe–Ti–Si alloys, respectively, using a 15 kW/30 kHz high frequency induction furnace. Five hundred grams of high purity electrolytic iron was melted in an Al2O3 crucible (outer diameter (OD): 56 mm, inner diameter (ID): 50 mm, height (H): 96 mm), and the melt temperature was directly measured by a Pt/Pt-13 mass%Rh thermocouple sheathed with an 8 mm OD alumina tube immersed in the melt. After the melt temperature was reached to a desired value, the Ar-10% H2 gas was blown onto the melt surface at a high flow rate of ~2 L/min to deoxidize the melt. After 2 hours of gas blowing, the oxygen content in the melt decreased to a value less than 20 mass ppm, and then the gas was switched to a mixture of N2 and Ar-10% H2 gases to keep the aimed nitrogen partial pressures. The flow rate of the gas mixture was 1 L/min. Detailed descriptions of the experimental apparatus and procedure are available in the authors’ recent studies.11,12,13)
In order to determine the effect of silicon on the TiN solubility product in liquid iron, titanium (99.999%purity) was first added in pure liquid iron until a TiN layer was formed on the surface of the melts under a given nitrogen partial pressure, and then silicon (99.999%purity) was repeatedly added up to 2.1 mass%. The formation of TiN in the melt could be also confirmed by a sharp decrease in nitrogen content in the melt checked by the in situ analysis of metal samples during the experiment. It was also confirmed visually through the glass window at the top frame of the furnace. After each silicon addition, a new TiN solubility equilibrium was attained within 1 h.
In case of the nitrogen solubility measurement in Fe–Ti–Si melt to determine the simultaneous effect of Ti and Si on N in liquid iron, titanium was first added up to 0.083 mass% under different nitrogen partial pressures at 1823 and 1873 K, and then silicon was repeatedly added up to 2.3 mass%. After silicon additions, the new equilibrium nitrogen solubility in liquid iron was attained within 1 hour. TiN did not form during the nitrogen solubility measurement at reduced nitrogen partial pressures of 0.05 and 0.1 atm.
The metal sample of about 10 g was extracted by a 4 mm ID quartz tube connected to a syringe (10 ml), and it was quenched rapidly in water within 2 s. The metal samples were carefully cut for the chemical analysis. Titanium and silicon in the metal sample were analyzed by the inductively coupled plasma atomic emission spectroscopy (ICP-AES, SPECTRO ARCOS apparatus, manufactured by Spectro Analytical Instruments, Kleve, Germany) using appropriate standard solutions containing the same amount of Fe as the sample solutions. The analytical limit of Ti and Si in the metal sample was 5±1 mass ppm. The nitrogen and oxygen contents in the metal sample were analyzed by the nitrogen/oxygen analyzer (LECO TC-600 apparatus; LECO Corporation, St. Joseph, MI) with an accuracy of ±2 mass ppm.
The reaction equilibrium for the dissolution of pure solid TiN in liquid iron can be written as:
| (1) 11) |
| (2) |
At a given temperature, the TiN solubility product, log[%Ti][%N] in Eq. (2) depends on fTi and fN values in liquid steel. For a Fe–Ti–Si–N melts, those activity coefficients can be expressed as the following relations using the Wagner’s interaction parameter formalism (WIPF).14)
| (3) |
| (4) |
| System | Interaction parameter | Value (1873 K) | Temp.(K) [%i] range | pN2 (atm) | Ref. |
|---|---|---|---|---|---|
| Fe–Ti–N | −0.21 | 1823–1923 [% Ti]<0.2 | 0.05–0.3 | 11 | |
| 0 | |||||
| 0.048 | 1823–1923 [% Ti]<0.52 | ||||
| 0 | |||||
| Fe–Si–N | 0.047 | 1823–1923 [% Si]<12.53 | 0.3–1 | 9 | |
| 0.0013 | |||||
| Fe–Ti–Si–N | 0 | 1823–1873 [% Ti]<0.083, [% Si]<2.3 | 0.05, 0.1 | Present study | |
| 0.038 | 1773–1873 [% Ti]<0.36, [% Si]<2.2 | 0.1–0.7 | |||
| 0 |
In the present study, the effect of silicon addition on TiN solubility product in liquid iron was determined under various nitrogen partial pressures in the temperature range from 1823 to 1923 K. The experimental results of TiN solubility measurement in Fe–Ti–Si–N melts are summarized in Table 2, and they are plotted in Fig. 2 together with TiN solubility lines for Fe–Ti–N system.11) Figure 3 also shows the effect of silicon additions on the TiN solubility product in the melt at different temperatures. As the silicon content increases, the TiN solubility product is nearly constant at various nitrogen contents in the melt controlled by the nitrogen partial pressure in the system.
| Temp. (K) | pN2 (atm) | [%Ti] | [%Si] | [%N] | [%O] | TiN sat. |
|---|---|---|---|---|---|---|
| 1823 | 0.1 | 0.000 | 0.000 | 0.0141 | 0.0012 | |
| 0.039 | 0.000 | 0.0147 | 0.0011 | |||
| 0.080 | 0.000 | 0.0154 | 0.0018 | |||
| 0.209 | 0.000 | 0.0058 | 0.0008 | sat. | ||
| 0.355 | 0.000 | 0.0037 | 0.0016 | sat. | ||
| 0.343 | 0.338 | 0.0038 | 0.0014 | sat. | ||
| 0.335 | 0.611 | 0.0036 | 0.0012 | sat. | ||
| 0.324 | 1.055 | 0.0040 | 0.0008 | sat. | ||
| 0.320 | 1.587 | 0.0036 | 0.0006 | sat. | ||
| 0.319 | 2.110 | 0.0040 | 0.0011 | sat. | ||
| 0.2 | 0.000 | 0.000 | 0.0366 | 0.0016 | ||
| 0.023 | 0.000 | 0.0202 | 0.0013 | |||
| 0.053 | 0.000 | 0.0206 | 0.0007 | |||
| 0.059 | 0.000 | 0.0206 | 0.0005 | sat. | ||
| 0.129 | 0.000 | 0.0098 | 0.0001 | sat. | ||
| 0.178 | 0.000 | 0.0068 | 0.0014 | sat. | ||
| 0.140 | 1.030 | 0.0083 | 0.0008 | sat. | ||
| 0.114 | 1.490 | 0.0100 | 0.0016 | sat. | ||
| 0.090 | 1.898 | 0.0130 | 0.0017 | sat. | ||
| 1873 | 0.1 | 0.000 | 0.000 | 0.015 | 0.0019 | |
| 0.079 | 0.000 | 0.0154 | 0.0008 | |||
| 0.148 | 0.000 | 0.0125 | 0.0004 | sat. | ||
| 0.218 | 0.000 | 0.0087 | 0.0004 | sat. | ||
| 0.207 | 0.311 | 0.0089 | 0.0016 | sat. | ||
| 0.180 | 0.615 | 0.0096 | 0.0015 | sat. | ||
| 0.142 | 0.761 | 0.0131 | 0.0011 | sat. | ||
| 0.7 | 0.000 | 0.000 | 0.0384 | 0.0005 | ||
| 0.047 | 0.000 | 0.0372 | 0.0012 | |||
| 0.087 | 0.000 | 0.0229 | 0.0016 | sat. | ||
| 0.123 | 0.000 | 0.0154 | 0.0008 | sat. | ||
| 0.115 | 0.628 | 0.0165 | 0.0018 | sat. | ||
| 0.102 | 0.935 | 0.0184 | 0.0004 | sat. | ||
| 0.087 | 1.221 | 0.0214 | 0.0004 | sat. | ||
| 0.068 | 1.547 | 0.0265 | 0.0014 | sat. | ||
| 1923 | 0.1 | 0.000 | 0.000 | 0.013 | 0.0011 | |
| 0.139 | 0.000 | 0.014 | 0.0019 | |||
| 0.241 | 0.000 | 0.012 | 0.0016 | sat. | ||
| 0.340 | 0.000 | 0.009 | 0.0011 | sat. | ||
| 0.332 | 0.254 | 0.009 | 0.0005 | sat. | ||
| 0.311 | 0.584 | 0.009 | 0.0004 | sat. | ||
| 0.296 | 0.867 | 0.010 | 0.0004 | sat. | ||
| 0.276 | 1.116 | 0.011 | 0.0017 | sat. | ||
| 0.260 | 1.382 | 0.012 | 0.0018 | sat. | ||
| 0.3 | 0.000 | 0.000 | 0.025 | 0.0018 | ||
| 0.075 | 0.000 | 0.028 | 0.0011 | |||
| 0.116 | 0.000 | 0.025 | 0.0019 | sat. | ||
| 0.152 | 0.000 | 0.019 | 0.0007 | sat. | ||
| 0.207 | 0.000 | 0.014 | 0.0005 | sat. | ||
| 0.165 | 0.342 | 0.017 | 0.0006 | sat. | ||
| 0.131 | 0.597 | 0.021 | 0.0007 | sat. |

Equilibrium [%Ti]–[%N] relations in Fe–Ti–Si–N melts saturated with TiN at (a) 1823, (b) 1873 and (c) 1923 K.

Effect of Si addition on TiN solubility product in Fe–Ti–Si–N melts.
Figure 4 shows an example of TiN solubility data with silicon additions at 1873 K together with the solubility lines calculated for Fe–Ti–N11) and Fe–Ti–Si–N.1,2,3,4) In the present experiment, titanium was added up to 0.123 mass% in pure liquid iron under a nitrogen partial pressure of 0.7 atm. After confirming the formation of TiN layer on the surface of melt, silicon was added repeatedly up to 1.55 mass%. As the silicon content increased in the melt, the titanium content decreased significantly but the nitrogen content increased accordingly to keep the TiN solubility product under a given nitrogen partial pressure. The present experimental result indicates that silicon does not have a large effect on the equilibrium TiN solubility product in liquid iron. The dotted lines in Fig. 4 are the calculated TiN solubility lines for a Fe-1.55%Si–Ti–N melt at 1873 K using different values of

Comparison of experimental data with TiN solubility diagrams for Fe–Si–Ti–N melt at 1873 K.
In Fe–Ti–Si–N melt, both titanium and silicon are present with opposing effects on nitrogen solubility. In order to determine the simultaneous effects of silicon and titanium on nitrogen in liquid iron,
| Temp. (K) | pN2 (atm) | [%Ti] | [%Si] | [%N] | [%O] |
|---|---|---|---|---|---|
| 1823 | 0.05 | 0.076 | 0 | 0.0104 | 0.0021 |
| 0.077 | 0.46 | 0.0097 | 0.0008 | ||
| 0.076 | 0.94 | 0.0092 | 0.0019 | ||
| 0.075 | 1.40 | 0.0088 | 0.0015 | ||
| 0.075 | 1.86 | 0.0085 | 0.0016 | ||
| 0.073 | 2.26 | 0.0077 | 0.0005 | ||
| 1873 | 0.1 | 0.083 | 0 | 0.0145 | 0.0002 |
| 0.082 | 0.47 | 0.0141 | 0.0007 | ||
| 0.082 | 0.94 | 0.0134 | 0.0002 | ||
| 0.081 | 1.34 | 0.0131 | 0.0007 | ||
| 0.080 | 1.80 | 0.0122 | 0.0009 |

Effect of Si on N solubility in Fe–Ti–Si melts at 1823 and 1873 K.
The dissolution of nitrogen in liquid iron alloys can be written as
| (5) 5) |
| (6) |
| (7) |
The

Relation of Eq. (7) to determine the value of
Therefore, the specific effect of silicon on titanium can be determined from the TiN solubility product data in Table 2 as a function of silicon content in Fe–Ti–Si–N melts. Eqs. (2), (3), (4) can be rearrange as:
| (8) |
Figure 7 shows the values of

Relationship between
Figure 8 is a test for the systematic variation in the interaction parameter values,
| (9) |

Variation of the interaction parameters,
As shown in the figure, interaction parameter values vary with the atomic number within some ranges from positive to negative values or vice versa depending on their nature of interaction. However, it is noted that some reported values are apart from other values.
In the present study, the effect of silicon on TiN solubility product was directly determined under various nitrogen partial pressures in the temperature range from 1823 to 1923 K. The simultaneous effect of titanium and silicon on nitrogen in liquid iron was also determined. The first and second-order interaction parameters among silicon, titanium and nitrogen in liquid Fe–Ti–Si–N alloy can be expressed as:
([%Ti]<0.36, [%Si]<2.2, 1773–1873 K)
([%Ti]<0.083, [%Si]<2.3, 1823–1873 K)
This study was supported by the R&D Center for Valuable Recycling (Global-Top Environmental Technology Development Program) funded by the Ministry of Environment (Project No.: 11-C22-ID).