2018 Volume 58 Issue 8 Pages 1474-1479
2018 Volume 58 Issue 8 Pages 1474-1479
The optimum composition of mixtures of iodine, ethanol, and ethylene glycol as selective extraction media for free magnesia in steelmaking slags was explored. Hydration of free magnesia in steelmaking slags increases its volume to expand the slags, resulting in deteriorate pavement of roads when the steelmaking slags were used as a road-constructing material. Therefore, accurate analytical methods to determine free magnesia in the steelmaking slags have been sought in steel industries. Ethanolic solutions of iodine without ethylene glycol afforded higher selectivity for the extraction of magnesia than ternary mixtures of iodine, ethanol, and ethylene glycol. Although the addition of up to 5 wt% of ethylene glycol to the ethanolic iodine solution increased the amount of magnesia extracted, it also increased the extraction of other magnesium species present in the steelmaking slags, resulting in decreased selectivity toward magnesia. The dissolution of magnesia in ethanolic iodine solution was promoted by the alkalinity of the magnesia. Meanwhile, the dissolution of magnesia promoted the redox reaction between ethanol and iodine in the extraction medium.
Most of the steelmaking slags produced in the steel manufacturing process are recycled as materials for road construction and engineering. Steelmaking slags often contain magnesia (MgO), which is derived from the added dolomite and remains as a non-smelted form or a crystallized form produced during the solidification process.1) The magnesia of this type in steelmaking slags is referred to as free magnesia. When free magnesia is converted to Mg(OH)2 by hydration, its volume expands by 2.2 times.2) Calcium oxide (CaO) shows a similar expansion behavior to magnesia upon hydration. These increases in the volumes of magnesia and calcium oxide in steelmaking slags result in the expansion of the slags. When steelmaking slags are used as road construction materials, this expansion may cause serious problems, such as deterioration of the road. Thus, the relationship between the expansion of the steelmaking slags and the contents of free magnesia and/or free calcium oxide therein has been of great concern with respect to the recycling of the slags. As the accurate evaluation of this relationship requires knowledge of the accurate contents of free magnesia and free calcium oxide in the slags, the development of accurate methods to determine these is also necessary. Thus, accurate analytical methods to determine free magnesia in the steelmaking slags have been sought in steel industries.
For the determination of free CaO in steelmaking slags, an extraction method using ethylene glycol has been established and was proposed as a recommended method by The Iron and Steel Institute of Japan in 2013.3) In contrast, accurate standard methods for the determination of free magnesia in steelmaking slags have not been established or recommended to date, although several methods have been reported, such as extraction followed by atomic spectrometric determination,4,5,6,7) solid-state 25Mg-NMR spectroscopy,8) and infrared spectroscopy after hydration with heavy water.9) Extraction methods using suitable extraction media have been considered as potential options for practical analyses, because free CaO in steelmaking slags can successfully be extracted into ethylene glycol.3) Hanada and co-workers previously reported a procedure for the extraction and determination of free magnesia in steelmaking slags using a ternary mixture composed of iodine, ethanol and ethylene glycol.6) This method not only permits the effective extraction of the free magnesia but can also be combined with the extraction of free CaO in the same slag samples for sequential analyses. However, the ternary medium simultaneously extracted other magnesium species from the slags, indicating that it would be desirable to explore the optimum composition for the selective extraction of magnesia. In this study, the optimum composition of the ternary medium is investigated and the mechanism of the extraction of magnesia using the ternary medium is also discussed.
Two kinds of steelmaking slags and a blast furnace slag were used as sample materials, which were kindly donated by The Iron and Steel Institute of Japan. The obtained slags were pulverized and sieved to less than 75 μm before use. The elemental compositions of each slag are summarized in Table 1. Highly pure magnesia (99.99%, 4 N) was obtained from Kanto Chemicals. All of the other reagents used were of analytical reagent grade. Ultrapure water (<18 MΩ), purified using a PURELAB Ultra system (Organo, Japan), was used throughout the experiments. The concentration of magnesium extracted into each medium was determined using a contrAA-300 flame atomic absorption photometer (Analytik Jena, Germany).
| Slag | CaO | MgO | SiO2 | Total Fe | Al2O3 |
|---|---|---|---|---|---|
| Blast furnace slag | 41 | 7.4 | 33.8 | 0.4 | 13.4 |
| Steel-making slag 1 | 37 | 10 | 26 | 6.4 | 14 |
| Steel-making slag 2 | 34 | 8.6 | 18 | 13 | 12 |
Magnesium species were extracted from the slags using ternary media according to the following procedure. After transferring prescribed weights of ethanol and ethylene glycol to a 100 mL Erlenmeyer flask with a glass cap, a prescribed weight of iodine was added to prepare the extraction medium. The final weight of the resulting medium was adjusted to 25 g. After mixing the medium for 30 min using a magnetic stirrer to completely dissolve the iodine, 0.1 g of a slag sample was added and the mixture was heated and stirred under defined conditions. Subsequently, the mixture was filtered through a membrane filter with a pore size of 0.45 μm. A portion of the filtrate (0.6 g) was then placed in a 50 mL volumetric flask and L-ascorbic acid was added until the iodine was completely reduced. The final volume of the mixture was diluted to 50 mL with water. The concentrations of magnesium and other elements, such as calcium, silicon, aluminum, and iron, in the solution were determined using a flame atomic absorption photometer. The amounts of each element extracted into the medium were calculated as the oxide forms.
2.3. Determination of Mass of Iodine Reduced and Alkali Content Consumed during Magnesia ExtractionFirst, the amount of magnesia dissolved in the extraction medium was determined according to the above procedure. Briefly, 0.1 g of magnesia was added to an ethanolic iodine solution at 80°C, and the resulting mixture was stirred for 4 h with heating in an oil bath and then filtered through a membrane filter with a pore size of 0.45 μm. To a 50 mL volumetric flask were added a 0.1 g aliquot of the filtrate, 5 mL of 1 mol/L HCl, and 2 mL 10 g/L La(NO3)3 solution. Then, L-ascorbic acid was added to the flask until the iodine was completely reduced. After adjusting the volume of the solution to 50 mL, the magnesium concentration was determined using a flame atomic absorption photometer.
To determine the amount of unreacted iodine in the filtrate, a 2.0 or 4.0 g aliquot of the filtrate was transferred to a 200 mL conical beaker followed by 20 mL of 1 mol/L KI solution and 10 mL of 2.00 mol/L H2SO4. The resulting solution was titrated with 5 × 10−2 mol/L Na2S2O3 solution as the titrant. Furthermore, to determine the consumption of the alkali content during the redox reaction in the medium, the mixture resulting from the redox titration was subjected to a neutralization titration against 0.1 mol/L sodium hydroxide solution.
In general, certified reference materials should be used to evaluate the accuracy of newly developed determination methods. However, there are no certified reference materials for steelmaking slags in which the free magnesia content has been defined, which renders it difficult to develop accurate determination methods for free magnesia in steelmaking slags. Instead, we used a blast furnace slag as a reference material that does not contain free magnesia, since magnesia is not added in the blast furnace process and therefore free magnesia does not exist in resulting blast furnace slags. Note that the other common mineral constituents in blast furnace slag are similar to those in steelmaking slags.
First, we explored the composition of an extraction medium not to extract magnesium species from the blast furnace slags. For this study, we regarded the amount of magnesium species extracted from steelmaking slags into the extraction medium that did not extract any other magnesium species from the blast furnace slag as the content of free magnesia because types of magnesium species except free magnesia in steelmaking slags are practically identical to those of magnesium species in blast furnace slags, as mentioned above. The mass of magnesium species extracted into the medium was converted into the mass of magnesia. At the start of the evaluation, we examined the extraction medium reported by Hanada et al.,6) with which free magnesia was effectively extracted from steelmaking slags. Briefly, the extraction procedure was as follows. First, 18 g of iodine was dissolved in 200 mL of ethanol, and 10 mL of this ethanolic iodine solution was mixed with 15 mL of ethylene glycol. To the resulting medium was added 0.1 g of a slag sample and the mixture was then stirred with heating at 80°C for 60 min. Although this procedure is convenient for practical analyses, it is not suitable for the systematic exploration of the composition of the ternary medium because the volume of a mixed solvent composed of two different solvents is usually less than the total volume of the two solvents. Since the volume change that arises upon mixing two solvents makes it difficult to optimize the conditions, we prepared the extraction media based on the weights of the constituents. Prescribed weights of the three constituents were mixed and stirred for 30 min to dissolve the iodine. Sampling of the media to determine the amount of magnesia extracted was also performed on the basis of weight.
Figure 1 shows the effects of the choice of alcohol and iodine content on the extraction of magnesium species from the slags. Regardless of the alcohol used, the amounts of magnesium species extracted from the steelmaking slags increased with increasing iodine content, indicating that iodine plays a crucial role in the extraction of magnesium species. Among the alcohols studied, ethanol extracted the greatest amount of magnesium species from the steelmaking slags. In contrast, no magnesium species were extracted from the blast furnace slag using any of the alcohols at any of the iodine contents tested. Thus, ethanolic iodine solutions were considered to selectively extract free magnesia from steelmaking slags and were used in the following experiments.

Effects of the choice of alcohol and amount of iodine on the extraction of Mg species as MgO. Suspensions of 0.1 g samples of the slags in 25 g of alcoholic media containing iodine were stirred for 3 h at 80°C.
Next, we investigated the influence of the iodine content in ethanol on the extraction of free magnesia. The maximum iodine content tested in the experiments shown in Fig. 1 was 2.5 g, because of the poor solubility of iodine in 2-chloroethanol. However, when ethanol was used, it was possible to investigate the effect of iodine contents up to 4.5 g owing to the high solubility of iodine in ethanol. Figure 2 shows the effect of iodine contents of up to 4.5 g on the extraction of magnesium. The extraction of calcium and silicon species was also investigated, considering that dicalcium silicate (Ca2SiO4) is one of the major constituents in steelmaking slags. The amount of each element extracted was estimated as the weight of the oxide forms, as in the case of magnesium. The results for steelmaking slag 2 revealed that the extraction of magnesium species generally increased with increasing iodine content up to 3.0 g and fluctuated thereafter. Note that no magnesium species were extracted from the blast furnace slag at any of the iodine contents tested, indicating the selectivity for free magnesia.

Effect of amount of iodine in ethanol on the extraction of Mg, Ca, and Si species as the oxide forms. Suspensions of 0.1 g samples of the slags in 25 g of ethanolic media containing iodine were stirred for 3 h at 80°C.
The extraction of calcium and silicon species also increased with increasing iodine content in ethanol. The two profiles were quite similar and the mass of calcium species extracted was almost twice that of the mass of silicon species extracted across the range of iodine contents tested, suggesting that the calcium and silicon species were extracted as Ca2SiO4. Note that these elements are also extracted from the blast furnace slag because blast furnace slag also contains Ca2SiO4. This result indicates that ethanolic iodine solution is not a selective medium for the extraction of magnesia. However, atomic spectrometry methods such as atomic absorption spectrometry, atomic emission spectrometry, and atomic mass spectrometry can overcome this disadvantage owing to their high elemental selectivity.
3.2. Influence of Ethylene Glycol ContentEthylene glycol has been used as a solvent for the selective extraction of free calcium oxide from steelmaking slag,3) and it has also been used as a major component in ternary media for the extraction of free magnesia.6) We previously studied the dissolution mechanism of CaO in ethylene glycol.10) It was found that ethylene glycol is dehydrated by the CaO to produce acetaldehyde, which subsequently undergoes oligomerization under the basic conditions caused by the CaO. We consider that the acetaldehyde is easily oxidized by the iodine in the extraction medium. Thus, the addition of ethylene glycol to ethanolic solutions of iodine is expected to be effective for the extraction of magnesia from steelmaking slags. Figure 3 shows the relationship between the ratio of ethanol to ethylene glycol and the amount of magnesium species extracted. The ratio of the two constituents was varied under the condition that the total weight of the extraction medium was fixed at 22.5 g. The addition of 5% ethylene glycol significantly improved the extraction of magnesium species as well as that of calcium and silicon species, indicating that the extraction performance of the medium was improved by the addition of ethylene glycol. However, this improvement of the extraction performance also facilitates the extraction of other magnesium species present in the blast furnace slag, resulting in decreased selectivity for free magnesia. Therefore, ethylene glycol was not added to the extraction medium for the sake of maintaining the selectivity for free magnesia.

Effect of weight ratio of ethylene glycol to ethanol in the extraction media on the extraction of Mg, Ca, and Si species as the oxide forms. Suspensions of 0.1 g samples of the slags in 25 g of ethylene glycol and ethanol media containing 2.5 g of iodine were stirred for 3 h at 80°C.
Figure 2 suggests that the extraction of magnesia involves chemical reactions between iodine, ethanol, and magnesia. The effects of heating temperature and duration were investigated because chemical reactions are generally accelerated by heating. Based on the results shown in Fig. 3, an ethanol medium containing 2.5 g of iodine and 5 wt% of ethylene glycol was used to enlarge the profiles of the extraction of Mg species from the steel making slags. The amount of extracted magnesium species increased with increasing temperature up to a temperature of 80°C, as shown in Fig. 4(a). As the ethanolic iodine solution did not boil even at 80°C, it was considered that the dissolved iodine and other species extracted from the slags raised the practical boiling point of the extraction medium. It was found from Fig. 4(b) that a heating time of 4 h was required to reach a plateau in the extraction of magnesia from the slag sample with a high magnesia content.
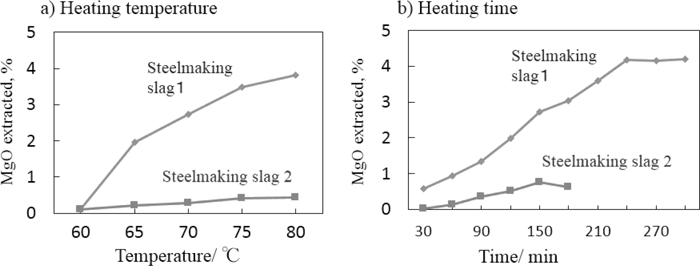
Effects of (a) heating temperature (for 30 min) and (b) heating time (at 80°C) on the extraction of Mg species as MgO. Suspensions of 0.1 g samples of the slags in 25 g of ethanol media containing 2.5 g of iodine and 5 wt% of ethylene glycol were stirred.
Based on the results described above, the extraction of magnesium species from 0.1 g slag samples was investigated using a medium composed of 3.5 g of iodine and 21.5 g of ethanol with heating at 80°C for 4 h as the optimized conditions. The content of magnesium species extracted into the medium was calculated as the oxidized form magnesia. The contents of free magnesia in the steelmaking slag 1 and steelmaking slag 2 samples were found to be 3.02 ± 0.16% and 0.54 ± 0.09%, respectively, based on three replicate analyses; these values were lower than those determined by a previous method (unpublished results).6) We consider that these lower values can be attributed to the omission of ethylene glycol from the extraction medium in order to increase the selectivity toward magnesia. However, owing to the lack of ethylene glycol in the extraction medium, the amounts of magnesium species extracted from the blast furnace slag and merwinite (Ca2Mg(SiO4)2)) were 0.042% and 0.12%, respectively, which are negligible in terms of practical analysis. In addition, no magnesium species were detected in the extraction medium when an FeO–MgO (50:50) solid solution was examined. Thus, the extraction conditions used in this study can be used to selectively extract free magnesia from steelmaking slag. However, improving the extraction of magnesia still remains a challenge from the viewpoint of quantitative extraction.
3.5. Extraction MechanismIn an effort to investigate the chemical reactions that occur in the ethanolic iodine solution during the extraction of magnesia, we examined the relationship between the amount of magnesia dissolved in the medium and the amount of iodine reduced for various iodine contents. Figure 5 shows the amounts of iodine reduced and magnesia dissolved in 25 g of ethanolic iodine solution as a function of the mass of iodine in the solution. As the iodine content was increased, the amounts of both dissolved magnesia and reduced iodine increased, indicating that the addition of iodine promotes the dissolution of magnesia, which in turn facilitates the reduction of iodine. Figure 5 also depicts the ratio of the amount of reduced iodine to the amount of dissolved magnesia as a function of the mass of iodine in the medium. This ratio was found to fluctuate between 60% and 80%, suggesting a certain relationship between the amount of dissolved magnesia and the amount of reduced iodine. However, the fluctuation renders it difficult to discuss the mechanism quantitatively. We considered that the fluctuation was due to the direct reduction of iodine by ethanol in the medium without the involvement of magnesia. To test this hypothesis, we examined the relationship between the reduction of iodine and the heating time in the absence of magnesia. As shown in Fig. 6, the amount of reduced iodine increased with the reaction time, confirming that iodine directly oxidized ethanol. The fact that the complete dissolution of iodine in ethanol took longer for greater amounts of iodine also supports the occurrence of a direct redox reaction between iodine and ethanol. Iodide ions resulting from the reduction of iodine react with iodine molecules to produce triiodide ions (I3−), which further promote the dissolution of iodine in ethanol. Considering the direct reduction of iodine in the absence of magnesia, the total amount of reduced iodine is the sum of that due to direct reduction and that due to the main part of the reduction promoted by dissolved magnesia. Since the direct redox reaction was not precisely controlled, the ratio of the amount of reduced iodine to the amount of dissolved magnesia fluctuated.

Effects of mass of iodine in ethanol on the dissolution of MgO and reduction of I2. Suspensions of 0.1 g samples of MgO in 25 g of ethanol media containing iodine were stirred for 4 h at 80°C.

Effect of heating time on the reduction of iodine in 25 g of ethanol media containing 2 g of iodine at 80°C.
We studied the effect of the iodine content on the reduction of iodine and the dissolution of magnesia in the presence of a constant amount of magnesia in Fig. 5. We then investigated the effect of the mass of added magnesia on the reduction of iodine and the dissolution of magnesia using a constant iodine content in ethanol. As shown in Fig. 7, increasing the amount of magnesia led to an increase in the amount of reduced iodine. Note that a portion of the added magnesia remained undissolved in the medium during the extraction under every set of experimental conditions. This result indicates that the addition of a greater amount of magnesia promotes greater dissolution of magnesia, even though part of the magnesia remained as an undissolved solid. We consider that magnesia could promote its own dissolution owing to its alkalinity, based on our previous report that showed that increasing the amount of calcium oxide promoted its dissolution in ethylene glycol.10) To investigate this hypothesis, KOH was used instead of magnesia because this compound has a high solubility in ethanol, allowing the effect of alkalinity to be studied under homogeneous conditions without the precipitation of KOH. As the amount of KOH was increased, the amount of reduced iodine increased monotonically, as shown in Fig. 8, indicating that the alkali component (OH−) promoted the reduction of iodine. Note again that Fig. 8 shows that a direct redox reaction between iodine and ethanol progresses even without KOH. To investigate the role of KOH in the redox reaction between iodine and ethanol, the amount of KOH remaining after completion of the redox reaction was determined. After the redox titration of the remaining iodine, an acid–base titration was performed using sodium hydroxide solution. The amount of unreacted KOH was determined from the difference between the amount of added sulfuric acid and the amount of sodium hydroxide titrated. The results are summarized in Table 2. Regardless of the amount of KOH added, KOH did not remain in the solution after completion of the redox reaction, indicating that the added KOH was completely consumed during the redox reaction.

Effects of mass of MgO on extraction of MgO and reduction of I2. Suspensions of 0.1 g samples of MgO in 25 g of ethanol media containing 2 g of iodine were stirred for 4 h at 80°C.

Effect of mass of added KOH in ethanolic media containing 2 g of iodine on the reduction of iodine after 4 h at 80°C.
| KOH added before extraction/ ×10−3 mol | H2SO4 added after extraction/ ×10−3 mol | NaOH titrated/ ×10−3 mol | Remaining KOH after extraction/ ×10−3 mol |
|---|---|---|---|
| 0 | 3.95 | 3.94 | 0.01 |
| 1.26 | 3.89 | 3.93 | −0.04 |
| 2.74 | 3.93 | 3.94 | −0.01 |
| 4.89 | 3.93 | 3.91 | 0.02 |
| 7.48 | 3.91 | 3.94 | −0.03 |
KOH was dissolved into ethanolic iodine solution composed of 22.5 g of ethanol and 2.5 g of iodine.
The redox reaction of ethanol and iodine is well known. This reaction is promoted by OH−, as shown in the following reactions.
| (1) |
| (2) |
| (3) |
In an effort to investigate whether reaction (3) proceeded, the amount of acetic acid remaining in the medium after the reaction was measured by another acid–base titration against sodium hydroxide solution. No acetic acid was detected in the medium, confirming that reaction (3) proceeded and consumed the acetic acid formed in reaction (2). The generation of bubbles, which seemed to be carbon dioxide, was observed from the medium as the reaction proceeded.
Considering the results discussed above, the following extraction mechanism is presented, as illustrated in Fig. 9. In ethanolic iodine solution, the redox reactions (1), (2), and (3) are promoted by the OH− ions formed from magnesia to produce iodide ions, which form triiodide ions upon further reaction with iodine. The triiodide ions form ionic associates with the Mg2+ ions formed from magnesia. The resulting ionic associates dissolve in the ethanolic iodine solution, thereby explaining the dissolution of magnesia in ethanolic iodine solution.

Reaction scheme showing the proposed mechanism for the dissolution of MgO in ethanolic iodine solution.
Ethanolic iodine solution was used as a selective medium for the extraction of free magnesia from steelmaking slags. Although the addition of up to 5% ethylene glycol to the medium increased the amount of magnesia extracted from the slags, it decreased the selectivity of the extraction toward magnesia. The redox reaction of iodine with ethanol was promoted by the extracted magnesia, while iodide ions produced by the redox reaction subsequently formed triiodide ions to further promote the dissolution of magnesia.