2019 年 59 巻 1 号 p. 31-38
2019 年 59 巻 1 号 p. 31-38
The viscous behaviors of CaO-SiO2-11.00wt%MgO-11.00wt%Al2O3-43.00wt%TiO2 slag systems were investigated by the rotating cylinder method in this paper, and the effects of CaO/SiO2 on the viscosity, break point temperature and activation energy for viscous flow of slag were analyzed. Meanwhile, the slag structural characterizations were studied by Fourier transformation infrared (FTIR) spectroscopy and Raman spectroscopy, and the phase compositions of pre-melted slags were detected by X-ray diffraction (XRD) analyses. The results show that, when the CaO/SiO2 increases from 0.20 to 0.80, the viscosity, break point temperature and activation energy for viscous flow of slag decrease. FTIR and Raman spectroscopy results indicate that the complex viscous units in slag are gradually depolymerized with the increase of CaO/SiO2, which improve the fluidity of slag. The depolymerization mechanism of silicate networks can be expressed by 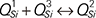 . The complex O–Ti–O deformation units are depolymerized into the simple structural units (
. The complex O–Ti–O deformation units are depolymerized into the simple structural units (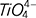 monomers,
monomers, 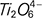 chains and Ti–O stretching vibrations in 6-coordinated Ti4+) and the [AlO4]- tetrahedrons are simplified to the [AlO6]- octahedrons. In addition, the relative amounts of low melting point complicated pyroxene in the pre-melted slag increase, improving the meltability of slag and decreasing the break point temperature.
chains and Ti–O stretching vibrations in 6-coordinated Ti4+) and the [AlO4]- tetrahedrons are simplified to the [AlO6]- octahedrons. In addition, the relative amounts of low melting point complicated pyroxene in the pre-melted slag increase, improving the meltability of slag and decreasing the break point temperature.
Vanadium-bearing titanomagnetite (VT) is an important mineral carrier of Fe, V and Ti elements in nature and has extremely high utilization value.1) There reserves 10 billion tons of VT in Panzhihua District in China and the blast furnace (BF) process has been adopted to process VT since the 1970s.2,3) The pig iron containing vanadium and titanium-bearing BF slag are produced. Because of the excessive additives and introduction of impurities in BF process, the TiO2 content in the obtained titanium-bearing BF slag is only about 20.00–25.00wt%. Currently, it is difficult to extract TiO2 from these titanium-bearing BF slags, which results in a waste of titanium resources.4,5) In recent years, the direct reduction-electric furnace process has been significantly developed to utilize VT.6,7) The TiO2 grade in the obtained titanium-bearing slag has been improved and higher than 40.00 wt%. By the appropriate hydrometallurgical process, the titanium-bearing components in this type slag can be efficiently extracted.8,9)
Importantly, the practical experience indicates that the slag system plays an important role in the smelting process. The viscous behaviors (i.e., viscosity, break point temperature, activation energy for viscous flow) of slag can not only affect the productivity and operation stability, but also influence the slag/metal separation and recovery of valuable elements.10) Therefore, in order to effectively achieve the comprehensive use of VT by the direct reduction-electric furnace process, a fundamental research on the viscous behaviors of titanium-bearing slag formed in this production process is necessary.
Over recent years, many experts have reported the viscous behaviors of titanium-bearing slag.11,12,13,14,15) Most of these researches were aimed at the properties of titanium-bearing BF slags, mold fluxes and refining slags. Usually, the basicity CaO/SiO2 in these slag systems is higher than 0.80, and the TiO2 content is lower than 30.00 wt%. In comparisons with the titanium-bearing BF slag, the titanium-bearing slag obtained in the direct reduction-electric furnace process is of low basicity (less than 0.80) and high TiO2 content (exceed 40.00 wt%).16) At present, few studies on the viscous behaviors of this type titanium-bearing slag have been reported in the literatures. Meanwhile, the structure characteristics of high TiO2 content slag with low CaO/SiO2 are also not investigated in detail.
In the present work, the effects of CaO/SiO2 on the viscous behaviors of CaO-SiO2-11.00wt%MgO-11.00wt%Al2O3-43.00wt%TiO2 system were investigated. The viscosity, break point temperature and activation energy for viscous flow of slag were considered in detail. The range of CaO/SiO2 was 0.20–0.80. Meanwhile, the effect mechanisms were expounded using the X-Ray Diffraction (XRD) analyses, Fourier transformation infrared (FTIR) spectroscopy and Raman spectroscopy.
The experimental slag samples were synthesized using the analytical reagent oxides of CaO, SiO2, MgO, Al2O3 and TiO2, based on the approximate compositions of titanium-bearing slag obtained in the smelting separation process of vanadium-bearing titanomagnetite metallized pellets. The main chemical compositions of basic titanium-bearing slag are listed in Table 1. In order to reduce the error and improve the accuracy of experiments, the CaO, SiO2, MgO, Al2O3 and TiO2 reagents were firstly calcined in a muffle furnace to decompose any carbonates and hydroxides. When these treated oxides were mixed adequately in a desired proportion, the mixtures were subsequently pre-melted with a molybdenum crucible under Ar atmosphere at 1823 K for 30 min to obtain the homogeneous samples. After being cooled naturally in the air, the slag samples were crushed into powder with the particles of size less than 0.074 mm and used for the XRD analyses and viscosity measurements. The experimental scheme and chemical compositions of designed titanium-bearing slags are shown in Table 2.
| CaO | SiO2 | MgO | Al2O3 | TiO2 | CaO/SiO2 |
|---|---|---|---|---|---|
| 10.65 | 20.72 | 11.32 | 11.22 | 43.58 | 0.514 |
| NO. | Initial designed compositions/wt% | Final compositions by XRF/wt% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO/SiO2 | CaO | SiO2 | Al2O3 | MgO | TiO2 | CaO/SiO2 | CaO | SiO2 | Al2O3 | MgO | TiO2 | |
| 1 | 0.20 | 5.83 | 29.17 | 11.00 | 11.00 | 43.00 | 0.18 | 5.34 | 29.66 | 10.51 | 10.58 | 43.49 |
| 2 | 0.35 | 9.07 | 25.93 | 11.00 | 11.00 | 43.00 | 0.33 | 8.85 | 26.81 | 10.67 | 10.63 | 42.66 |
| 3 | 0.50 | 11.67 | 23.33 | 11.00 | 11.00 | 43.00 | 0.49 | 11.79 | 24.05 | 10.53 | 10.44 | 42.78 |
| 4 | 0.60 | 13.13 | 21.88 | 11.00 | 11.00 | 43.00 | 0.61 | 13.41 | 21.99 | 10.47 | 11.27 | 42.45 |
| 5 | 0.70 | 14.41 | 20.59 | 11.00 | 11.00 | 43.00 | 0.71 | 14.20 | 20.00 | 10.59 | 11.42 | 43.43 |
| 6 | 0.80 | 15.56 | 19.44 | 11.00 | 11.00 | 43.00 | 0.78 | 15.06 | 19.31 | 11.36 | 10.51 | 43.32 |
In this study, the viscosity measurements of experimental pre-melted slags were carried out by the rotating cylinder method with a digital viscometer. The schematic diagram of apparatus is given in Fig. 1. Six U-shaped MoSi2 heating elements were used in the electric resistance furnace to heat and melt the slag samples. The temperature of slag could reach 1843 K. The experimental temperatures were controlled by two B-Type thermocouples inserted into the furnace with an error less than ±3 K. The crucible and rotating spindle employed for the viscosity measurements were both made of molybdenum. Their dimensions are also listed in Fig. 1. Before the viscosity measurements, the viscometer was calibrated using the standard oil with known viscosity at room temperature.
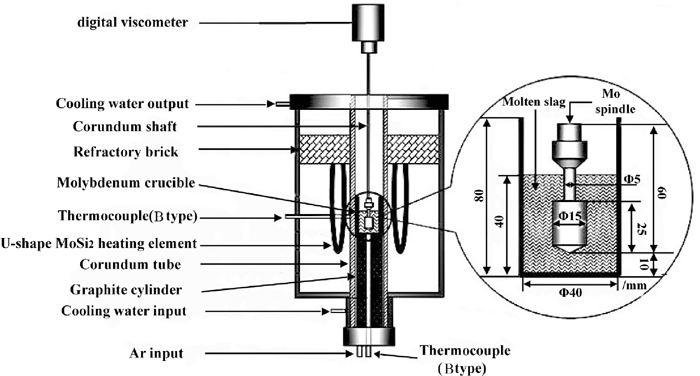
Schematic diagram of viscosity measurement apparatus.
140 g pre-melted slag powder was filled in a molybdenum crucible. The crucible was then placed at the even temperature zone of electric resistance furnace and heated to 1843 K with a heating rate of 5 K/min. After the experimental pre-melted titanium-bearing slag completely melted, its amount was about 40 mm in depth. Meanwhile, the molten slag was held for 30 min to stabilize the temperature and homogenize the compositions. Upon the stabilizations of signal, the molybdenum rotating spindle was slowly immersed in the molten slag and kept at a distance of 10 mm above the crucible bottom. The crucible and spindle were properly aligned along the axis of viscometer. Subsequently, the viscosity measurements started. In the whole experimental process, Argon gas (purity 99.999%) was flowed with 0.3 NL/min to protect the molybdenum crucible, molybdenum spindle and apparatus structures.
According to the previous research,17) the viscosity measurements were performed with the continuous cooling method in the present work, to obtain the relationship between viscosity and temperature in a wide temperature range for the different CaO/SiO2 experimental titanium-bearing slags. The furnace was programmed to cool at a rate of 3 K/min. When the viscosity of slag started to rise rapidly and reached about 3.500 Pa·s, the measurements were ended. Three times viscosity measurements were carried out for the experimental slags, and the average value was adopted as the viscosity of slag at various temperatures. After the viscosity measurements, the experimental slags were reheated up to 1843 K for 1 h and then rapidly quenched in the cold water. Subsequently, FTIR and Raman Spectroscopy were used to confirm the slag structure, and X-ray fluorescence (XRF) was adopted to analyze the chemical compositions of quenched slags.
The average value of three times viscosity measurements was determined as the viscosity of slag at various temperatures. The measurement results for No. 4 experimental titanium-bearing slag (CaO/SiO2 0.60) are listed in Table 3. It can be seen that the accuracy of viscosity measurements is 0.001 Pa·s and the experimental results are matched well each other. Considering the experimental uncertainties which are usually associated with the viscosity measurements, the measurement methods in this study are credible. The chemical compositions of quenched slags obtained after the viscosity measurements are analyzed by XRF in Table 2. The final compositions of quenched slags are close to the initial designed compositions.
| Temperature/K | first viscosity measurement/Pa·s | second viscosity measurement/Pa·s | third viscosity measurement/Pa·s | Average viscosity/Pa·s |
|---|---|---|---|---|
| 1843 | 0.216 | 0.216 | 0.214 | 0.215 |
| 1823 | 0.224 | 0.227 | 0.225 | 0.225 |
| 1793 | 0.242 | 0.234 | 0.239 | 0.238 |
| 1773 | 0.253 | 0.256 | 0.255 | 0.255 |
| 1753 | 0.303 | 0.299 | 0.301 | 0.301 |
| 1733 | 0.364 | 0.363 | 0.357 | 0.361 |
| 1723 | 0.410 | 0.405 | 0.406 | 0.407 |
| 1713 | 0.495 | 0.493 | 0.490 | 0.493 |
After the viscosity measurements, the viscosity-temperature (η-T) curves of different CaO/SiO2 experimental titanium-bearing slags were acquired. The results are shown in Fig. 2. It is clear that the viscosity of slag increases when the temperature decreases. There exists a break point of temperature in the every η-T curve. When the temperature is higher than the break point temperature, the variations of viscosity are slow, and the fluidity and thermostability of slag are well. While the temperature is decreased to be lower than the break point temperature, the viscosity increases rapidly and can reach 3.500 Pa·s in a narrow temperature range. The slag melt changes from a Newtonian fluid to a non-Newtonian fluid.18) The fluidity and stability of slag become worse.
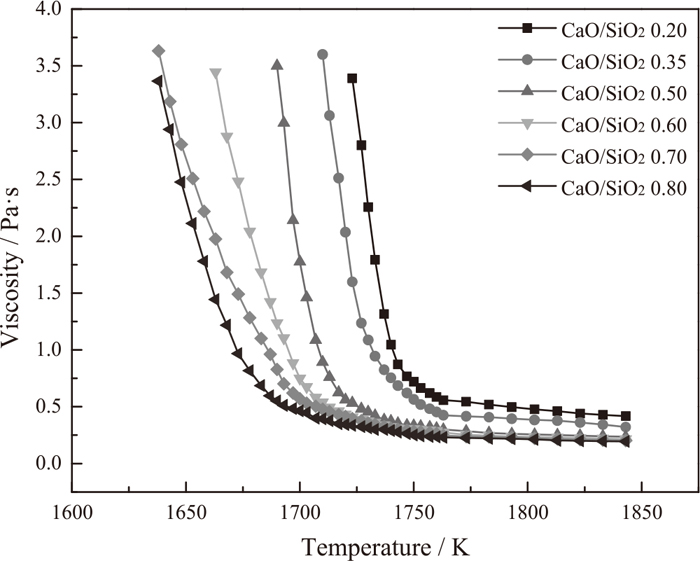
η-T curves of different CaO/SiO2 experimental titanium-bearing slags.
According to Fig. 2, the effects of CaO/SiO2 on the viscosity of CaO-SiO2-11.00wt%MgO-11.00wt%Al2O3-43.00wt%TiO2 slag systems above the break point temperature are obtained and given in Fig. 3. Obviously, the viscosity of experimental titanium-bearing slag continuously decreases with the increase of CaO/SiO2 from 0.20 to 0.80 at a given temperature. In addition, the decreasing rate of viscosity is weakened when the CaO/SiO2 increases. For example, at the temperature of 1823 K, an increase of CaO/SiO2 from 0.20 to 0.50 results in a relatively appreciable decrease of 0.194 Pa·s in viscosity, whereas a subsequent increase of CaO/SiO2 from 0.50 to 0.80 is less effective in lowering the viscosity (the viscosity is decreased by approximately 0.045 Pa·s).
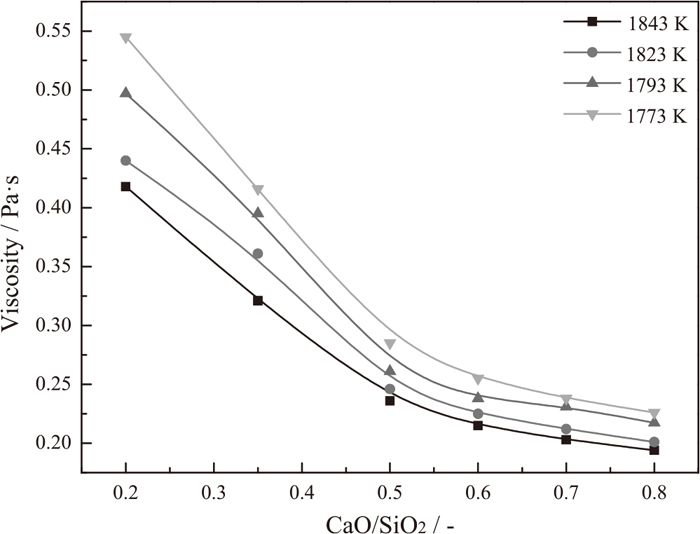
Effects of CaO/SiO2 on the viscosity of titanium-bearing slags at various temperatures.
According to the related researches, the SiO2 and Al2O3 components in the molten slag can form the complex network units, which are combined through the bridging oxygen (O0), such as [SiO4]- tetrahedral and [AlO4]- tetrahedral.18,19) It is well known that the depolymerizations of these complex viscous units can simplify the slag structure and decrease the viscosity of slag.20) While the CaO/SiO2 increases, the availability of free oxygen ions (O2−) in the molten slag is increased with the dissociations of CaO. These provided O2– can react with the O0 in the network structure to form the non-bridging oxygen (O−), and the complex networks are depolymerized to the small units, such as monomers.21) Thus, the viscosity of experimental slags decreases with the increase of CaO/SiO2, and the fluidity is improved.
In addition, Zheng et al.14) and Kim et al.19) have reported that the Ti4+ cations formed by TiO2 in the molten slag are larger in size and smaller in electronegativity, compared with the Si4+ cations. The bonds between Ti4+ and oxygen (Ti–O bonds) are expected to be weaker than that between Si4+ and oxygen (Si–O bonds). Hence, the polymerization strength of network structures in slag declines when the Ti4+ cations are incorporated into the network structures. Besides, some simple and small structural units can be also brought into slag with the TiO2 additions, such as TiO44− monomers. Therefore, in the present slag systems, 43.00 wt% TiO2 may create an environment for decreasing the viscosity of slag. Thus, only a slight change of viscosity can be observed at high temperatures and high CaO/SiO2. The structural characterizations of various experimental titanium-bearing slags are given in the following sections in detail.
3.3. Effects of CaO/SiO2 on the Break Point TemperatureBy referring to the study performed by Qi et al.,17) the break point temperature (TBr) of slag in the present work is determined as the temperature that corresponds to the tangency point of a 45° line and η-T curve. Because the viscosity of slag significantly increases when the temperature is lower than TBr, the TBr can represent the boundary between better fluidity and worse fluidity of slag in one sense and is an important viscous property of molten slag.
Table 4 illustrates the TBr of various experimental slags. Similarly, the TBr decreases with the increase of CaO/SiO2 from 0.20 to 0.80. When the CaO/SiO2 is 0.20, the TBr exhibits a highest value of 1747 K. When the CaO/SiO2 is 0.80, the TBr exhibits a lowest value of 1687 K. As mentioned in the relevant investigations, the meltability of slag (i.e., melting temperature) may affect the variations of TBr.14,17,18,22) Nevertheless, the TBr is distinguished from the melting temperature. The melting temperature is the initial point where the solid crystals are precipitated from the molten slag, and the TBr associated with the supercooling degree lies within the solid–liquid coexisting region. It is inferred that the difference between the TBr and the melting temperature is related to the thermodynamic driving force by which the solid crystals can be precipitated from the molten slags. Generally, when the melting temperature of slag increases, the crystallization capacity of molten slag at high temperature becomes relatively stronger. In the continuous cooling process, the crystal phases are more easier to precipitate from the molten slag and the melt easily shifts from a Newtonian fluid to a non-Newtonian fluid, which is contributed to the increase of TBr of slag.
| CaO/SiO2/- | 0.20 | 0.35 | 0.50 | 0.60 | 0.70 | 0.80 |
| TBr/K | 1747 | 1743 | 1718 | 1707 | 1697 | 1687 |
In order to explore the effects of CaO/SiO2 on the meltability of experimental titanium-bearing slags, the pre-melted slag samples were analyzed by XRD in Fig. 4. The MgTi2O5, complicated pyroxene (such as CaMgSi2O6, CaMg0.7Al0.6Si1.7O6, CaMg0.5AlSi1.5O6, CaSiTiO5, CaMg0.85Al0.45Si1.7O6, Ca0.8Mg1.2Si2O6), Mg2SiO4 and MgAl2O4 are detected in these slags. The basic phase is MgTi2O5. When the CaO/SiO2 increases, the diffraction peak intensity of MgTi2O5 varies slightly and decreases on the whole. However, the diffraction peak intensity of complicated pyroxene obviously increases. It indicates that the relative amounts of complicated pyroxene in the pre-melted slag samples increase. According to Ren,23) the complicated pyroxene has a low melting point. As a typical, the melting point of CaMgSi2O6 is 1664 K. Consequently, with the increase of CaO/SiO2, the melting temperature of experimental pre-melted titanium-bearing slags decreases, and the meltability of slag is improved. Thus, the TBr of slag decreases.
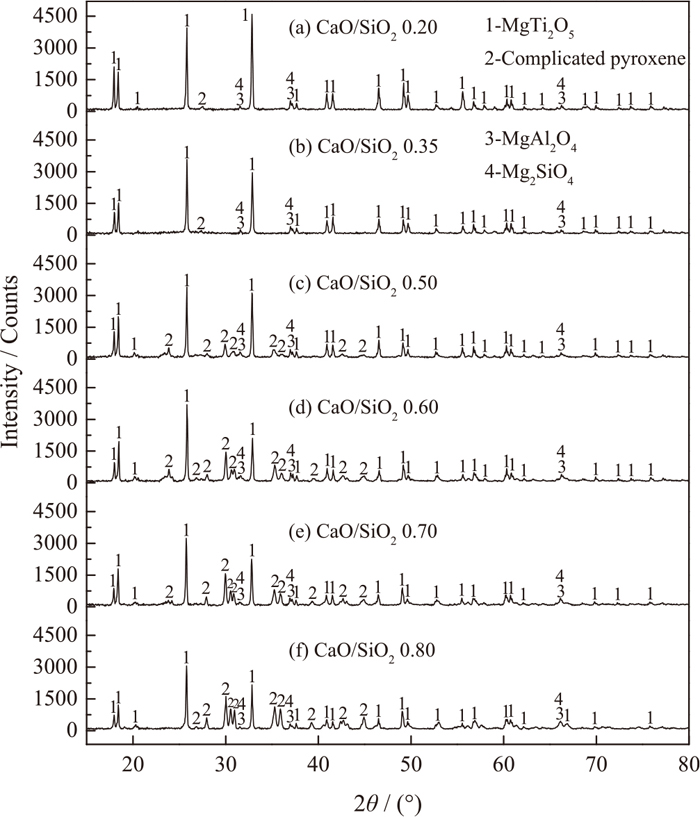
XRD analyses of pre-melted experimental slag samples.
After the viscosity measurements, the reheated molten experimental slags were rapidly quenched in the cold water, and the FTIR spectrometer was subsequently employed to analyze the slag structure for revealing the variation mechanisms of viscosity in this study. The detailed XRD analyses of quenched titanium-bearing slags with different CaO/SiO2 are presented in Fig. 5(a). As expected, the XRD patterns show no obvious characteristic peaks, suggesting that the quenched samples are totally amorphous and representative of molten slag structures. Figure 5(b) provides the FTIR spectra for the different CaO/SiO2 titanium-bearing slags. The detected results can be separated into the vibration band in the 800–1200 cm–1 range, 600–800 cm–1 range and 400–600 cm–1 range. In the FTIR analyses of glassy slag structure, the vibration region between approximately 800 cm−1 to 1200 cm–1 has been assigned to the [SiO4]- tetrahedral symmetric stretching vibration.11) The vibration band from 600 cm−1 to 800 cm−1 is identified as the [AlO4]- tetrahedral asymmetric stretching vibration.19,24) The vibration band in the 400–600 cm–1 range corresponds to the Al–O–Si bending vibration.24,25) As shown in Fig. 5(b), with the increase of CaO/SiO2 from 0.20 to 0.80, the low limit of [SiO4]- tetrahedral vibration shifts to a lower wavenumber from about 802 cm−1 to 795 cm−1, and the width of [SiO4]- tetrahedral vibration band becomes broad. The depth of [SiO4]- tetrahedral vibration trough also decreases. It indicates that the [SiO4]- tetrahedral symmetric stretching vibration becomes less pronounced and the silicate networks in the molten slag are depolymerized with increasing the CaO/SiO2. In addition, the troughs of [AlO4]- tetrahedral asymmetric stretching vibration and Al–O–Si bending vibration are both dampened with the increase of CaO/SiO2. When the CaO/SiO2 exceeds 0.35, the [AlO4]- tetrahedral asymmetric stretching vibration band and Al–O–Si bending vibration band are almost disappeared in the FTIR spectra curves. The aluminate networks in slag melt are also depolymerized. These observed variations suggest that the slag structure of experimental titanium-bearing slags is gradually simplified with the increase of CaO/SiO2. As a result, the viscosity of slag decreases.
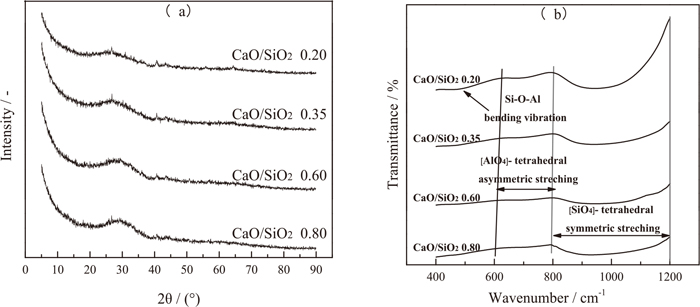
XRD analyses (a) and FTIR spectra (b) of quenched titanium-bearing slags with different CaO/SiO2.
Raman Spectroscopy was also used to analyze the structure characteristics of quenched slags. Figure 6(a) presents the room temperature Raman spectra curves of different CaO/SiO2 titanium-bearing slags. The strong bands located in the region between 550 cm−1 and 950 cm−1 and the shoulder bands lied between 950 cm−1 and 1100 cm−1 are observed in the every spectra curve. With the increase of CaO/SiO2, the bands in the 600–950 cm−1 range become more pronounced, and the peak shifts from 829 cm−1 to 819 cm−1. However, the shoulder bands in the 950–1100 cm−1 range are weakened, and the corresponding shoulder in the spectra curve almost disappears with the CaO/SiO2 of 0.60 and 0.80.
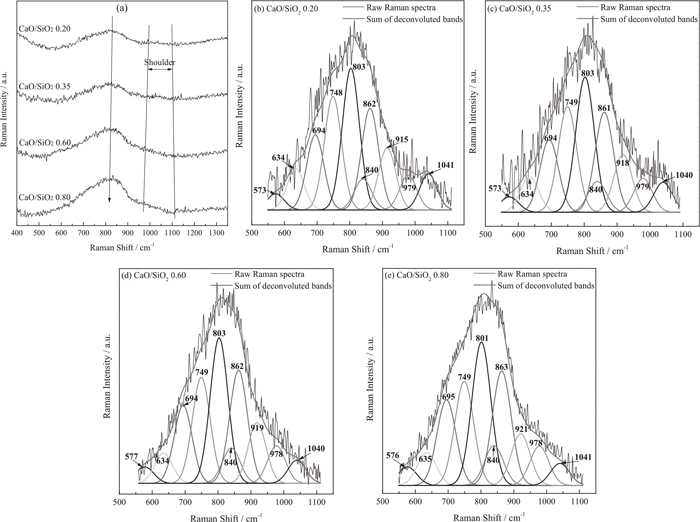
Raman spectra (a) and deconvoluted results of different CaO/SiO2 experimental titanium-bearing slags (b–e).
The deconvolutions of Raman spectra curves are essential to carry out the detailed investigations on the slag structure. In the present work, the Raman spectra curves of experimental titanium-bearing slags were mainly deconvoluted in the 550–1100 cm−1 range. The deconvolution fitting process was conducted by the works performed by Jin et al.26) The results have been illustrated in Figs. 6(b)–6(e). There exists ten bands in the analyzed frequency range as ~570 cm−1, ~630 cm−1, ~690 cm−1, ~750 cm−1, ~800 cm−1, ~840 cm−1, ~860 cm−1, ~920 cm−1, ~980 cm−1 and ~1040 cm−1, respectively. For the Raman signals of glassy slags, Mysen et al.27) have assigned the band at 840–860 cm−1, 900–920 cm−1, 960–980 cm−1 and ~1040 cm−1 to the monomer units (
| Raman shifts/cm−1 | Raman assignments | References |
|---|---|---|
| ~570 | [AlO6]- octahedron stretching vibrations | [28] |
| ~630 | [AlO4]- tetrahedral stretching vibrations | [28] |
| 690–700 | Ti–O stretching vibrations in 6-coordinated Ti4+ | [31] |
| 720–750 | O–Ti–O deformation in sheet units | [29] |
| 790–830 | Ti–O stretching vibrations in | [11], [29], [30] |
| 840–860 | [27] | |
| 860–880 | Ti–O stretching vibrations in | [29] |
| 900–920 | [27] | |
| 960–980 | [27] | |
| 1010–1040 | [27], [30] |
Usually, it is considered that the changes in the relative contents and conversions of various structural units affect the degree of polymerization of molten slag.33) The abundance of coexisting structural units in the molten slag can be deduced from the areas of corresponding Raman bands. Such as, the mole fractions of
| (1) |
The variations of A1/A2, A3/A2 and A0/A2 as a function of CaO/SiO2 are shown in Fig. 7. A1/A2 and A3/A2 continuously decrease with the increase of CaO/SiO2 from 0.20 to 0.80. Besides, A0/A2 increases. It demonstrates that the depolymerizations occur within the silicate network with increasing the CaO/SiO2. The depolymerization mechanism can be expressed with the reaction

Area ratios of corresponding Raman bands as a function of CaO/SiO2.
The activation energy for viscous flow (Eη) is an important viscous characteristic of molten slag. It can express the frictional resistance for viscous flow and the variations of Eη further indicate the changes of slag structure.14) Unless the characteristics of main viscous units in slag change, the value of Eη is expected to be constant for a certain molten slag.17) In addition, the Eη can also describe the fluctuations of viscosity in response to a change of temperature. Higher Eη suggests a stronger temperature dependence of viscosity.
In this study, the Eη of experimental titanium-bearing slags was evaluated with the Arrhenius type equation and viscosity data above the TBr of slag. The Arrhenius type equation is shown as follows.35)
| (2) |
After taking the natural logarithm, the Arrhenius type equation is re-arranged as Eq. (3). It indicates that
| (3) |
Based on the experimental data above the TBr of slag, the fitting results of

Linear fitting between lnη and T−1 (a) and analyses of Eη for various slags.
(1) When the CaO/SiO2 is increased from 0.20 to 0.80, the viscosity, break point temperature and activation energy for viscous flow of CaO-SiO2-11.00wt%MgO-11.00wt%Al2O3-43.00wt%TiO2 systems decrease.
(2) FTIR and Raman spectroscopy results reveal that the complex viscous units in experimental slag are gradually depolymerized with the increase of CaO/SiO2. The slag structure is simplified and the frictional resistance for viscous flow in the molten slag decreases, improving the fluidity of slag.
(3) With an increase of CaO/SiO2, the relative amounts of low melting point complicated pyroxene in slag increase. The meltability of slag is improved, facilitating the decrease of break point temperature of slag.
The authors gratefully acknowledge financial support by National Natural Science Foundation of China (Grant No. 51574067) and Fundamental Research Funds for the Central Universities (N172503016).