2019 年 59 巻 1 号 p. 122-128
2019 年 59 巻 1 号 p. 122-128
The modelling of the precipitation and growth behaviour of the sigma phase in super duplex stainless steel was investigated to clarify the quantitative effects of chemistry. The alloying elements of Cr and Mo useful for improving of corrosion resistance can promote sigma phase precipitation but that phase does not always grow rapidly. Therefore it is meaningful to clarify the effect of alloying elements on the kinetics of sigma phase growth to improve the trade-off relation between the corrosion resistance and avoiding harmful phase precipitation.
A physical model regarding sigma phase growth rate as a function of temperature and the parameters regarding the amount of allying elements in the steel was suggested on the basis of the traditional nucleation theory during isothermal heating process. It was confirmed that the physical model suggested in this work explains the experimental results of the effects of temperature and alloying elements of CR, MO and Won the growth rate of the sigma phase.
Duplex stainless steels are widely applied to the various structures because they exhibit high cost performance. These steels have the potential to obtain a high corrosion resistance, high strength and high toughness economically because they have lower nickel content in comparison with that in austenitic stainless steels. In later years, super duplex stainless steels improved in terms of corrosion resistance due to the increase in the amount of chromium, molybdenum, tungsten1,2,3,4) and nitrogen have been developed. The increase in the amount of these elements promotes the harmful intermetallic such as sigma phase. Therefore the prevention of this phase formation is an important subject in the welding, fabrication, and manufacturing process.
Many research works have focused on sigma phase precipitation in duplex stainless steel applicable to the heat treatment, hot work or welding process. In many research works focusing on the precipitation kinetics of the sigma phase, the growth properties were determined by using an experimental method and in steels of various chemistries.5,6,7,8,9,10,11,12,13,14,15,16,17,18) It was reported that the growth of sigma phase accords to the J-M-A-K (Johnson-Mehl-Avrami-Kolmogolov)19,20,21) or A-R (Austin-Rickett)22) equation. In most of the aforementioned studies, it was clarified that the above equations were applicable to explain the experimental results concerning the dependence of temperature on growth properties. However, the effect of each alloying elements on the growth property has not been systematically explained. Those findings do not always have sufficient extensibility because the influence mechanism of alloying elements on sigma phase precipitation have not been sufficiently clarified. For the duplex stainless steel consisting of other chemistry it is necessary to determine the growth rate by using experimental method individually. So far as the effects of molybdenum and nickel the effects on the growth rate of the sigma phase has already been suggested with a physical model proposed by the authors.23)
In this work the precipitation and growth of sigma phase in duplex stainless steel was investigated to clarify systematically the quantitative effects of chromium and tungsten, which has not investigated in the previous work, on the growth rate in isothermal heating process and to propose a new kinetic model for explaining the precipitation behaviour.
Duplex stainless steels consisting of 23/28.5%Cr, 2/4%Mo, 7%Ni and 0.3%N listed in Table 1 were melt with a laboratory electric furnace. Those steels were fabricated to the 12 mm thick plates by hot forging and hot rolling followed by solution treatment. Solution treatment is performed at 1323 K for 1800 s and quenched by water. The specimens were machined to be 11 mm thick, 11 mm wide and 60 mm long and heated at various constant temperatures ranging from 1073 K to 1273 K during various time followed by quenching with helium gas. In addition the long term heating for 360 ks at each temperature same as the above tests in order to the equilibrium fraction of sigma phase. After the heat treatment the specimens were polished and electrochemically etched in 10% oxalic acid and 10N KOH aqueous solution in cross section for microstructure observation. As an example Fig. 1 shows the optical microstructure and binarized image of 25%Cr-7%Ni-3%Mo-0.3%N steel heated at 1173 K for 1000 s.Area fraction of sigma phase etched as black phase was measured with an image analyser.
| C | Si | Mn | Ni | Cr | Mo | W | N | α (%) | Teq (K) | |
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 0.021 | 0.31 | 0.51 | 7.0 | 23.0 | 2.96 | 2.10 | 0.31 | 42 | 1331 |
| W1 | 0.020 | 0.30 | 0.51 | 7.0 | 25.2 | 2.97 | 2.16 | 0.31 | 44 | 1339 |
| C2 | 0.020 | 0.31 | 0.52 | 7.0 | 26.3 | 3.06 | 2.19 | 0.27 | 49 | 1346 |
| C3 | 0.021 | 0.32 | 0.51 | 6.9 | 27.1 | 3.04 | 2.18 | 0.29 | 55 | 1347 |
| C4 | 0.020 | 0.33 | 0.52 | 7.0 | 28.6 | 3.07 | 2.21 | 0.31 | 63 | 1354 |
| M1 | 0.017 | 0.33 | 0.51 | 7.0 | 25.0 | 2.94 | – | 0.25 | 47 | 1284 |
| M2 | 0.018 | 0.31 | 0.51 | 7.0 | 25.0 | 3.42 | – | 0.25 | 51 | 1299 |
| M3 | 0.018 | 0.31 | 0.51 | 7.0 | 25.1 | 3.91 | – | 0.29 | 52 | 1314 |
| M4 | 0.020 | 0.31 | 0.50 | 7.0 | 25.2 | 1.99 | 2.19 | 0.26 | 42 | 1309 |
| M5 | 0.028 | 0.30 | 0.50 | 6.9 | 25.0 | 3.90 | 2.20 | 0.29 | 50 | 1367 |
| W2 | 0.021 | 0.30 | 0.50 | 7.0 | 25.1 | 2.97 | 1.18 | 0.27 | 42 | 1305 |
| W3 | 0.021 | 0.32 | 0.50 | 7.1 | 25.2 | 2.99 | 3.16 | 0.26 | 46 | 1365 |
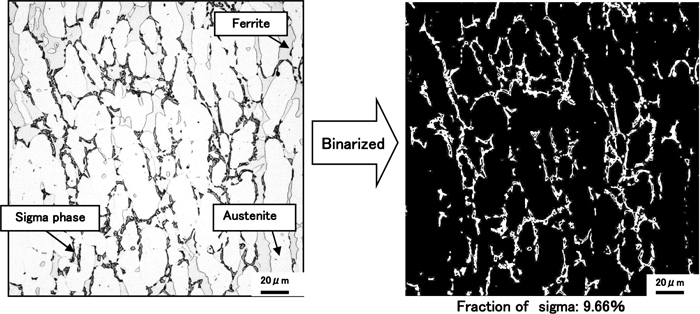
Example of sigma phase precipitation in 25Cr-7Ni-3Mo-0.3N steel heated at 1173 K for 1100s s and binarized image to measure area fraction by image analyser.
The following kinetic model based on the classical nucleation theory and diffusion controlled spherical growth model19,20,21,22,23,24) including the impingement effect was applied.
The nucleation rate is obtained by the product of f1 and f2, where f1 is the density of critical nuclei and f2 is the transition frequency from embryo to nuclei.
| (1) |
| (2) |
Here Nx is number of atoms to determine the growth rate, V is the molar volume, σ is the interface energy, Δgc is the energy barrier of nucleation (=4πrc2σ/3), k is Boltzmann constant, D is the diffusion constant, ra is the lattice constant, ΔH is the enthalpy of transformation of intermetallic, Teq is the solvus temperature of the intermetallic phase, ΔT is the degree of undercooling (=Teq−T), and Cp is the concentration in the intermetallic phase of the dominant atom to determine the growth rate and Co is concentration in the steel of the dominant atom to determine the growth rate, and rc is the radius of critical nuclei (=2σV/(Cp ΔH)·Teq/ΔT).
The extend volume of sigma phase which spherically glows is given as Eq. (3). The fraction of sigma phase is given as Eq. (4) using the Johnson-Mehl-Avrami-Kolmogolov (JMAK) equation19,20,21) for constant nucleation rate.
| (3) |
| (4) |
It was reported that in the 25%Cr duplex steels the main intermetallic to be formed in the vicinity of 1123 K is mainly sigma phase regardless of the tungsten addition.12,13) However, it was reported that in the 25%Cr duplex steel including 2% tungsten nucleation as the quasi equilibrium phase of the tungsten enriched chi phase (Cr12Fe36 (Mo,W)2,8) and the nucleation rate was considered to be controlled by the diffusion of tungsten atom.2) Therefore the nucleation phenomena of the quasi equilibrium phase was applied to this simplified model by modifying the term of diffusion constant in the Eq. (5) as effective diffusion constant defined as the following Eq. (6).
The growth rate constant kp is explained as the following equation from the Eqs. of (1), (2), (3), (4).
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
The above model indicating the growth rate constant kp at any temperature T is determined by the diffusion constant and the parameters Teq, ϕ and Koo which are the functions of the alloying elements of steels. The value of Teq was calculated using thermodynamic data base of THERMOCALC.
The measurement of the sigma phase fraction were conducted using all the steels of this work to clarify the effects of alloying elements of chromium, molybdenum and tungsten on the growth properties of the sigma phase during a constant temperature. The experimental results regarding the area fraction of the sigma phase measured by image analyser in the various steels employed were plotted against the heating time in Figs. 2, 3, 4, 5 as the example at 1173 K where the vertical axis is shown as log(−log(1−X)) obtained from Eq. (4). In all the results obtained within the early stage of precipitation corresponding to the value of log(−log(1−X)) lower than −1, the value of log(−log(1−X)) increased almost proportionally to the logarithm of heating time with the inclination of 2.5. The results confirmed that the sigma phase growth just in the early stage occurred according to the Eq. (4). The values of the intercept obtained from the linear relation of those experimental plots correspond to the growth rate constant kp in the Eq. (4). The effects of the alloying elements of chromium, molybdenum, and tungsten on the growth rate constant kp at various temperatures are shown Figs. of 6, 7, 8, 9 respectively. As Figs. of 6, 7, 8 shows the growth rate was accelerated by the increase of molybdenum or chromium content. On the contrary as Fig. 9 shows, the growth rate was not always monotonically accelerated by the increase of tungsten. In the temperature range lower than 1123 K the growth rate was reduced by the increase of tungsten within 2%, while the growth rate was accelerated by the increase of tungsten at the heating temperature of 1223 K or with the addition of tungsten of 3%.
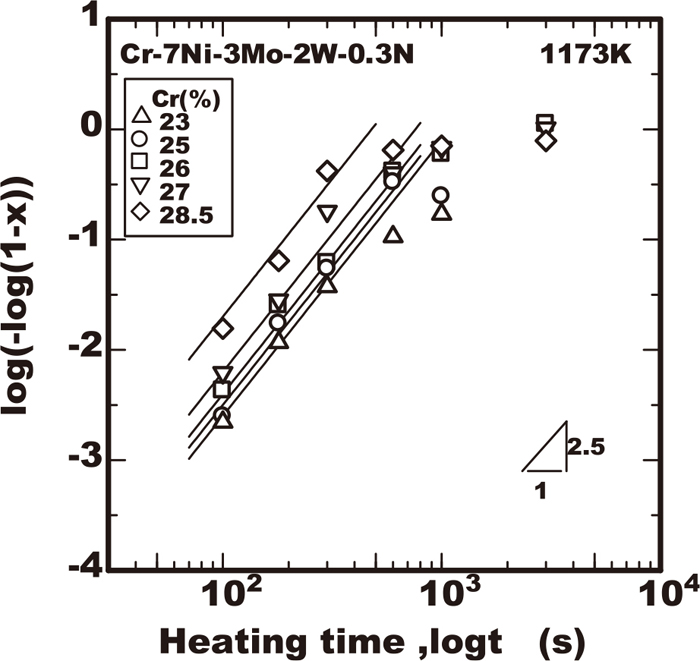
Effect of Cr on sigma phase growth at 1173K in Cr-7Ni-3Mo-2W-0.3N steels.
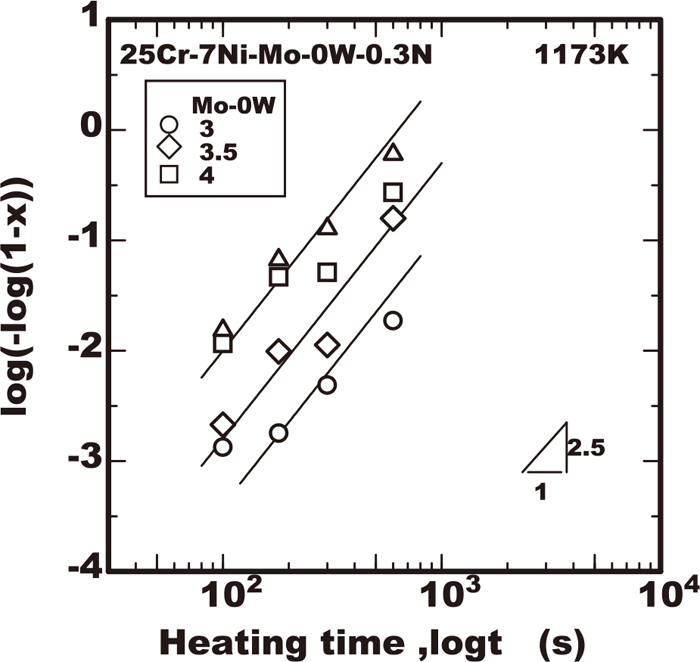
Effect of Mo on sigma phase growth at 1173 K in 25Cr-7Ni-Mo-0W-0.3N steels.

Effect of Mo on sigma phase growth at 1173 K in 25Cr-7Ni-Mo-2W-0.3N steels.

Effect of W on sigma phase growth at 1173 K in 25Cr-7Ni-3Mo-W-0.3N steels.

Effect of Cr on sigma phase growth rate constant in Cr-7Ni-3Mo-2W-0.3N steels.

Effect of Mo on sigma phase growth rate constant in 25Cr-7Ni-3Mo-0W-0.3N steels.
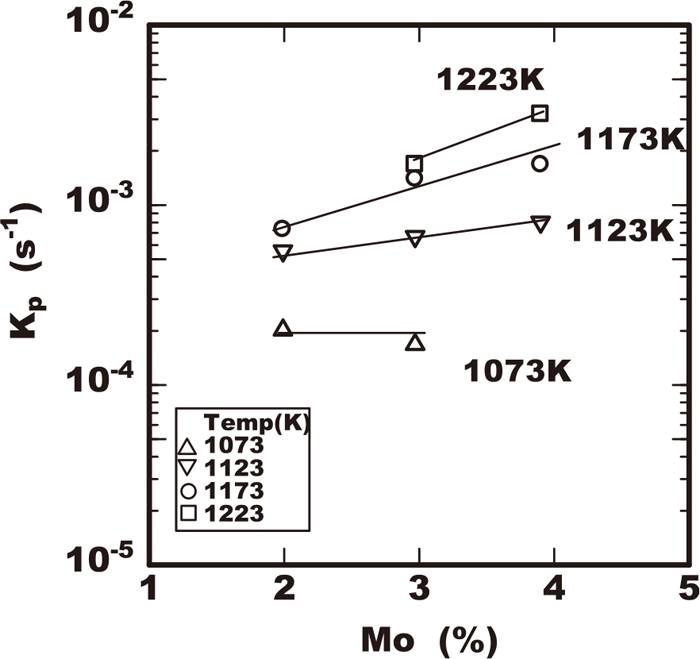
Effect of Mo on sigma phase growth rate constant in 25Cr-7Ni-3Mo-2W-0.3N steels.

Effect of W on sigma phase growth rate constant in 25Cr-7Ni-3Mo-W-0.3N steels.
The values of growth rate constants of kp are plotted against the heating temperature tested as shown in Figs. 10, 11, 12, 13. Each figure shows the effects of the alloying elements of chromium, molybdenum, or tungsten on the growth rate constant kp respectively. All the growth rate constants of kp had the maximum value for the temperature. The temperature for the maximum value of kp was the higher in the steel including the higher molybdenum, tungsten or chromium content. The maximum value of kp increased in the steel including the higher molybdenum or chromium content. On the contrary the increase of the maximum kp was not accelerated remarkably by the increase of tungsten within 2% as shown in Fig. 13.

Effect of heating temperature on sigma phase growth rate constant in Cr-7Ni-3Mo-2W-0.3N steels.
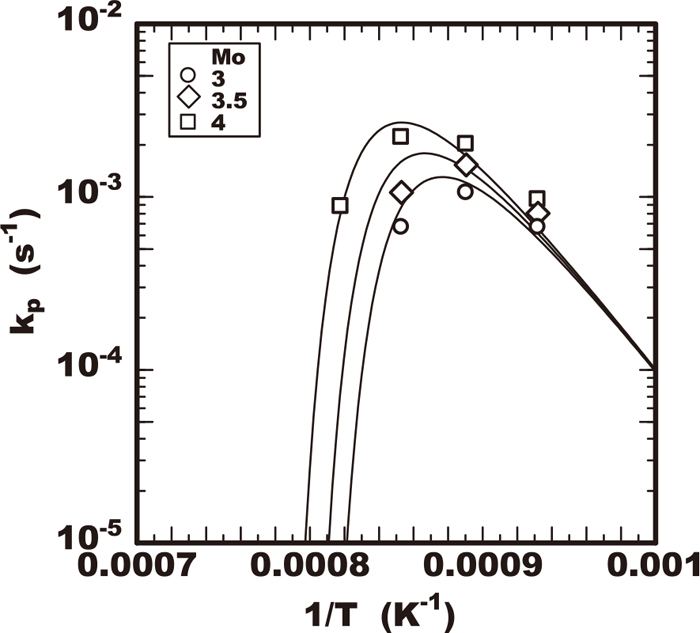
Effect of heating temperature on sigma phase growth rate constant in 25Cr-7Ni-Mo-0W-0.3N steels.
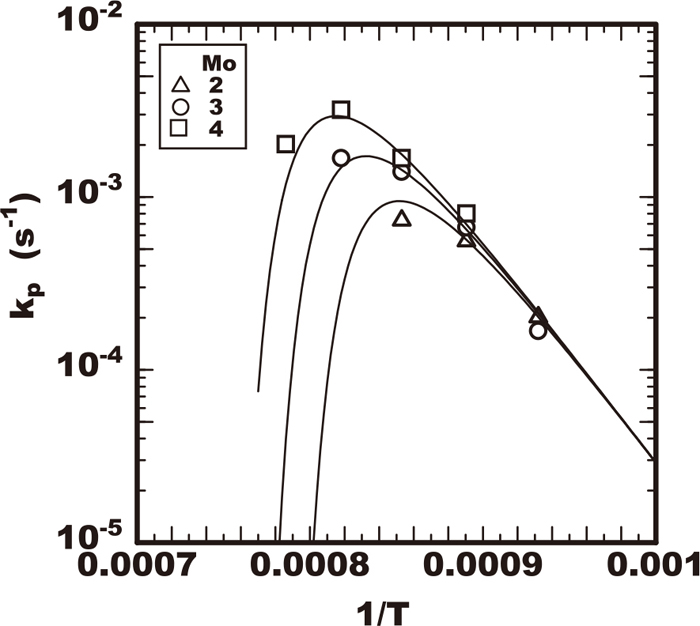
Effect of heating temperature on sigma phase growth rate constant in 25Cr-7Ni-Mo-2W-0.3N steels.

Effect of heating temperature on sigma phase growth rate constant in 25Cr-7Ni-3Mo-W-0.3N steels.
By applying the Eq. (5) to each experimental results in Figs. of 10, 11, 12, 13, the optimum values of ϕ and Koo for each steel tested are summarized in Tables 2, 3, 4. The calculated results by Eq. (5) using these values of ϕ and Koo were drawn with the curves of slid or dot lines in Figs. of 10, 11, 12, 13. Comparing the experimental plots and those curves, the calculated results were confirmed to be good fit with experimental ones. As results the effects of temperature on the growth rate constant of kp were described using Eq. (5) and the effects of alloying elements on kp were solely determined with the just four parameters of Teq, Deff, Koo and ϕ which are included in this equation. The results confirmed that this kinetic model by the Eqs. (4) and (5) is applicable to describe the sigma phase growth property at least within the early stage of precipitation.
| W | 0 | 2 | ||||
|---|---|---|---|---|---|---|
| Mo | 3 | 3.5 | 4 | 2 | 3 | 4 |
| Teq (K) | 1284 | 1299 | 1305 | 1309 | 1339 | 1367 |
| Do (m2/s) | 2.4 × 10−4 | 2.23 × 10−4 | ||||
| Q (kJ/mol) | 239 | 242 | ||||
| ϕ (J/mol) | 250 | 200 | ||||
| Koo (1/s) | 7.0E+ 11 | 2.8E+ 11 | ||||
| W | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Teq (K) | 1284 | 1314 | 1339 | 1365 |
| Do (m2/s) | 2.4 × 10−4 | 2.23 × 10−4 | ||
| Q (kJ/mol) | 239 | 242 | ||
| ϕ (J/mol) | 250 | 200 | ||
| Koo (1/s) | 7.0E + 11 | 7.0E + 11 | 2.8E + 11 | 3.0E + 11 |
| Cr | 23 | 25 | 26 | 27 | 28.5 |
|---|---|---|---|---|---|
| Teq (K) | 1331 | 1339 | 1346 | 1347 | 1354 |
| Do (m2/s) | 2.23 × 10–4 | ||||
| Q (kJ/mol) | 242 | ||||
| ϕ (J/mol) | 190 | 200 | 250 | 310 | 350 |
| Koo (1/s) | 2.1E + 11 | 2.8E + 11 | 4.4E + 11 | 8.8E + 11 | 1.4E + 12 |
Focusing on the effect of alloying element on the values of ϕ and Koo, the steels including various molybdenum content had same values of ϕ and Koo as shown in Table 2 so far the amount of other alloying elements is same. Regarding the effect of tungsten, the steel including tungsten lower than 1% had the same values of ϕ and Koo as shown in Table 3. Regarding the effect of chromium, the values of ϕ and Koo was different among the steel containing various level of chromium as shown in Table 4. Figure 14 shows a comparison between the growth rate constants by the experiment and the calculated values by applying the Eq. (5) using the parameters in Tables 2, 3, 4. The calculated values almost matched the experimental results therefore the validity of this kinetic model proposed is confirmed.
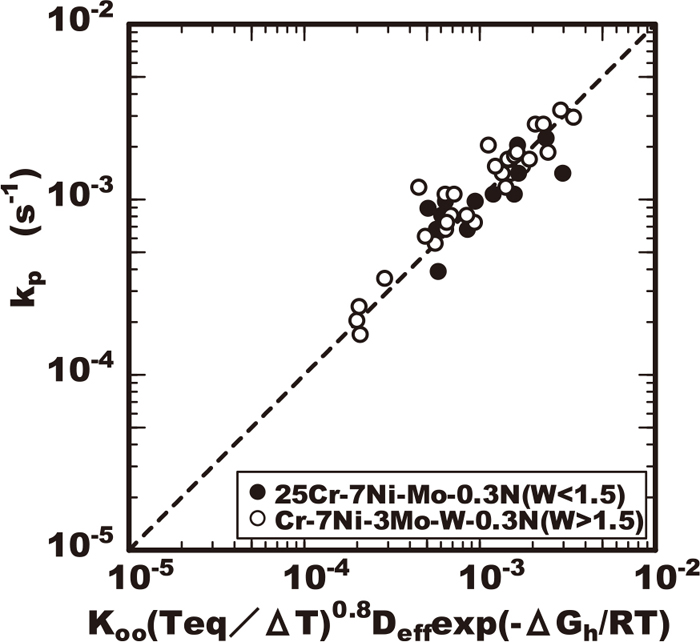
Comparison of calculated value and all experimental data of growth rate constant kp.
The growth rate constant is described by the kinetic model of Eq. (5) therefore the analysis of the parameters of Koo, ϕ, Teq and Deff included in that equation is considered to be effective for understanding the effect of each alloying element on the sigma phase growth. As shown in the Eqs. (8) and (9) the parameters Koo and ϕ are a function of the enthalpy ΔH of sigma phase transformation and both of the parameters increase with the decrease of the enthalpy ΔH. The parameter Koo is also a function of the dominant atom content C0. Therefore the influence mechanism of each alloying element can be understood by considering the effect of each element on the parameters of ΔH and C0.
At first considering the effect of molybdenum on the acceleration of the growth, the parameters of Koo and ϕ were constant in the steel even varying in the molybdenum content so far as the chromium and tungsten contents were the same. The dominant atom content C0 corresponds to the constant chromium content of 25% in those steels. Due to the constant chromium and nickel in those steels, the enthalpy ΔH of sigma phase transformation is almost constant. It is understandable that is the reason the parameter of Koo was constant among these steels. Therefore it should be considered the reason why molybdenum promotes the sigma phase growth is due to raising the solvus Teq of the sigma phase. In case of the higher Teq, the larger overcooling is given resulting in larger driving force in the temperature range with higher diffusion rate of dominant atom in growth of sigma phase.
Next considering the effect of chromium, the value of the enthalpy ΔH can be reduced by the increase of chromium content because of the increase of sigma phase stability. Consequently the values of the parameters K00 and ϕ increase. The increase of Koo promotes the precipitation. In addition chromium also raised the solvus Teq of the sigma phase therefore the growth rate is accelerated by the same reason as molybdenum. At the same time the value of ϕ increases by the addition of chromium and the increase of militate as the prevention factor of precipitation. The higher chromium containing steels have the higher dominant atom content of Co naturally. Therefore it was understood the parameters of Koo and ϕ increased as the effect of chromium.
Considering the effect of tungsten not always to accelerate the growth monotonically, the parameters of Koo and ϕ were separated with the two cases between the steels with tungsten less than 1% and with those higher than 2%. It is considered the nucleation phase is the sigma phase in steels lower tungsten than 1% though in steels over 2% tungsten the nucleation one is the chi phase.23) In case of lower tungsten the behaviour of tungsten is the same as that of molybdenum that gives no influence on the value of the parameters of Koo and ϕ but that raises the value of Teq. On the contrary in case of tungsten over 2% the parameters of Koo and ϕ were reduced with the increase of the tungsten content. The difference in the two cases is considered to be caused by the difference of the dominant atom due to the difference in the kind of nucleation phase. As mentioned above in the 2% tungsten steel the nucleation occurs as a quasi-equilibrium chi phase. The quasi-equilibrium phase nucleates prior to the sigma phase due to the lower interfacial energy of the chi phase and the matrix than that of the sigma.2) The parameter of ϕ is the function of interfacial energy as shown in Eq. (8), therefore the value of ϕ is reduced by a tungsten addition than 2% because the nucleation phase becomes the chi phase with the lower interfacial energy. The parameter of Koo is the function of Co of concentration of dominant atom as shown in Eq. (9). In the case of the chi phase nucleation the dominant atom in the diffusion process is tungsten, therefore the value of Co corresponding to the tungsten content of 2 to 3% is relatively lower in comparison with the case that Co corresponds to the chromium content of 25%. It is understood that is the reason the value of Koo was about a half time lower than that of low tungsten steels as summarized in Table 2 with the sigma phase as the nucleation phase. In a comparison between high and low tungsten steels regarding heating temperature, the sigma phase precipitation in lower temperature side is delayed by tungsten. The reason is explained by the decrease in the value of Koo due to lower concentration of the dominant element of tungsten in the nucleation. And the acceleration by tungsten in the higher temperature side is explained by that delaying effect mentioned above is cancelled by raising Teq.
Finally on comparing molybdenum and tungsten as alloying elements they both were found to contribute toward improving the pitting corrosion resistance of duplex stainless steels. The addition is indispensable to obtaining enough high properties required for purpose. On the contrary both of those elements promote the precipitation of the harmful phase such as the sigma phase. However from the quantification of the effects of those elements on the sigma phase precipitation using the kinetic model proposed it was indicated that the optimum addition with the combination of molybdenum and tungsten is useful for the alloy design of super duplex stainless steels with high pitting corrosion resistance reducing the sigma phase precipitation risk.
The following conclusions were obtained from the investigation regarding the sigma phase precipitation and the growth rate of 23/28%Cr-7%Ni-2/4%Mo-0/3%W-0.3%N duplex stainless steels in this work.
(1) The sigma phase precipitation is accelerated by the increase in the molybdenum or chromium content in the steels containing the same amount of tungsten.
(2) It was confirmed in the early stage the sigma phase grows according to the kinetic model based on the classical nucleation theory and diffusion controlled spherical growth model with the constant nucleation rate.
(3) The acceleration of the sigma phase by molybdenum is due to the increase the solvus of sigma phase.
(4) The sigma phase precipitation in lower temperature side is delayed by tungsten addition though that effect is cancelled in the higher temperature side.