2019 年 59 巻 8 号 p. 1437-1439
2019 年 59 巻 8 号 p. 1437-1439
Hypercoal (HPC) is examined as a caking additive to the mixture of strongly coking coal and non-slightly coking coal. Samples were coked, then their strength and crystallinity of the carbon structure by Raman spectra were measured. Hypercoal addition increased strength of coke, and a good correlation was observed between the mechanical strength of coke and the Raman parameter defined by the ratio of D3-band to G-band. Results suggest that mutual melting between blended coal and HPC brought strong carbonaceous structure with highly crystallized. The Raman parameter can predict the coke strength to some extent.
It has been investigated to mix various coals for making coke to suppress the use of strongly caking coal. In that case, a certain amount or more of strongly caking coal is required. So, an enhancement of caking property by adding caking material to weakly caking coal is a promising method. To evaluate such coke obtained by mixed caking, several parameters are important. As for the macroscopic structure, the mechanical strength of coke and the melting and softening properties to a plastic phase is important. On the other hand, chemical structures of coke can be also evaluated by Raman spectroscopy in microscopic aspects. We have investigated the relationship between the macroscopic structure including caking property and strength, and the microscopic structure of coke by Raman spectroscopy. So far, we have found that hypercoal (HPC) showed the most good nature among various binders,1) for which shows large number of polycyclic naphthenes or aromatic compounds, low ash content, high softening and melting property. HPC is produced by a solvent extraction of coal and sequential drying, and it includes much polycyclic naphthenes and aromatics, less ash, and has high softening and melting property.2,3,4) So HPC is a good additive for coke production. In this work, we evaluate the structure of coal mixture with HPC under various coking conditions using Raman spectra and strength index. Raman spectra are very informative for evaluating the microstructure of carbonaceous materials,5,6,7) so we used Raman observation for the evaluation of coke crystallinity.
For coke sample preparation, equal amounts of semi-anthracite Coppabella coal were mixed with Tarrawonga coal or Glencore coal, they have low carbonization degree. Proximate and ultimate analyses for these coals are summarized in Table 1. Then certain amount of HPC was added 10% as a binder. The particle size of coal was < 1 mm, and that of HPC was < 0.15 mm in this case. Samples are treated under three conditions with an apparatus as shown in Fig. 1;
| Proximate analysis [wt%, d.b.] | Ultimate analysis [wt%, d.a.f] | |||||||
|---|---|---|---|---|---|---|---|---|
| Coal name | Ash | V.M. | F.C. | C | H | N | S | O (by diff.) |
| TARRAWONGA | 6.2 | 37.5 | 56.3 | 83.5 | 5.1 | 2.0 | 0.4 | 9.0 |
| GLENCORE | 9.3 | 37.8 | 52.9 | 82.8 | 5.7 | 1.9 | 0.7 | 8.9 |
| HPC | 1.6 | 46.5 | 52.0 | 87.8 | 4.9 | 1.6 | 0.7 | 5.1 |
V.M.; Volatile matter, F.C.; Fixed carbon.
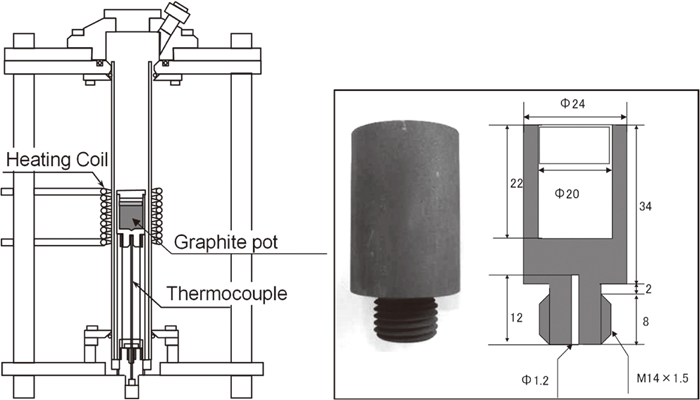
Coking apparatus.
A) Packing density of 750 kg m−3 with a heating rate of 3 K min−1 to be 1173 K,
B) Packing density of 1000 kg m−3 with a heating rate of 3 K min−1 to be 1173 K,
C) Packing density of 1000 kg m−3 with a heating rate of 50 K min−1 to be 1173 K.
Obtained sample was evaluated by Raman spectroscopy using NRS 4500 (JASCO), using 532 nm laser, 10 s observation for 5 times, in the range of 500–4000 cm−1, and the same observation was conducted for three times to confirm the reproducibility. An autograph (AG-I 50 kN, Shimadzu Corp.) was used for evaluation of the strength of carbonized tablet. One sample was used for each measurement. The fixed indenter was forced by the rate for the 1 mm/min to the side of the cylindrical tablet. The obtained maximum test power when the tablet was destroyed, was recorded as the strength.
In general, spectra as shown in Fig. 2 can be obtained when observing low quality carbon by Raman spectroscopy. It can be deconvoluted into several peaks, and these peaks reflect various structural features of coal. So, we deconvoluted the Raman spectra, and define the amorphous carbon intensity by the D3-band and the structured carbon intensity by the G-band, as shown in this Fig. 2, then the intensity of D3-band was divided by the intensity of G-band, and call it as a Raman parameter8,9,10) in this work (i.e. ID3/IG).1) Table 2 summarizes the Raman parameters and its strength in the case of Tarrawonga coal, and photographs for the sample shape are shown in Fig. 3. As for the Tarrawonga coal, the sample heated with 3 K min−1 without HPC did not become molded, and it was impossible to measure the strength due to its powdery morphology. On the other hand, the sample heated by a rapid ramping rate with 50 K min−1 showed a molded form, and its strength was 21.7 kgf, and the Raman parameter was 0.47, very low value; i.e. highly ordered structure. By adding HPC to the Tarrawonga coal, both of samples heated by two conditions were molded, and the Raman parameter showed smaller values as shown in Table 2, so the HPC addition plays an important role for forming ordered strong carbon structure.
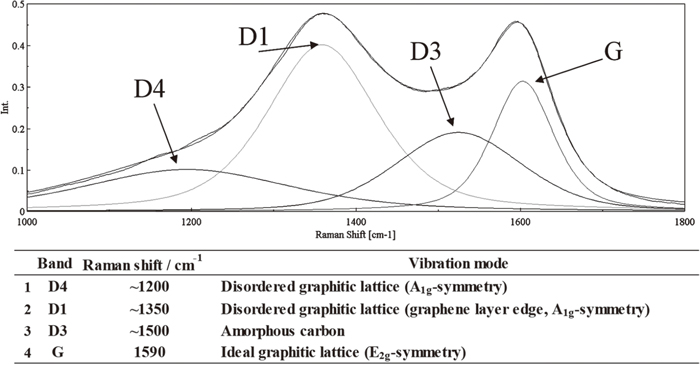
General Raman spectra of coke and deconvoluted peak assignments.
| Sample name | HPC addition | Heating condition | Packing density/ kg m−3 | Strength/ kgf | Raman parameter ID3/IG |
|---|---|---|---|---|---|
| T-A) | No | 3 K/min | 750 | n.d. | 0.52 |
| T-B) | No | 3 K/min | 1000 | n.d. | 0.54 |
| T-C) | No | 50 K/min | 1000 | 21.7 | 0.47 |
| T-HPC-A) | Yes | 3 K/min | 750 | n.d. | 0.55 |
| T-HPC-B) | Yes | 3 K/min | 1000 | 5.5 | 0.52 |
| T-HPC-C) | Yes | 50 K/min | 1000 | 37.5 | 0.48 |
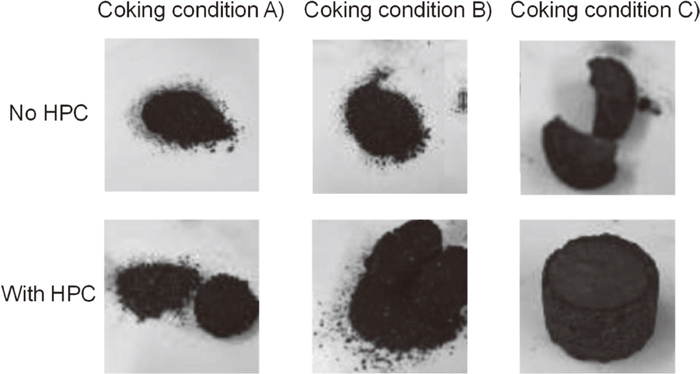
Photographs of Tarrawonga coal samples under various pretreatment conditions.
On the other hand, Glencore coal can be molded even without HPC as shown in Fig. 4, but the strength was low without HPC as shown in Table 3. By adding HPC to Glencore coal, the structure transformed to a very high strength, and the Raman parameter was also very low; namely highly ordered carbon was observed.
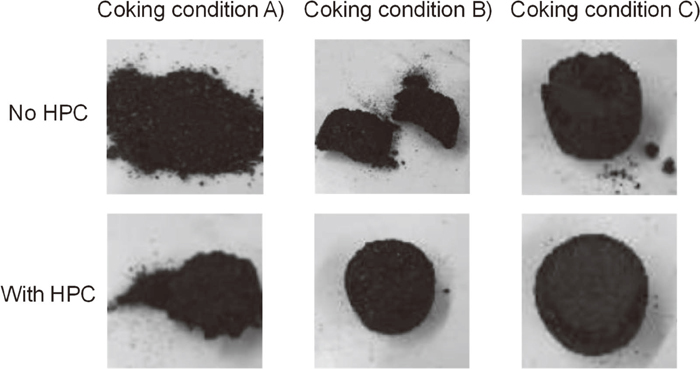
Photographs of Glencore coal samples under various pretreatment conditions.
| Sample name | HPC addition | Heating condition | Packing density/ kg m−3 | Strength/ kgf | Raman parameter ID3/IG |
|---|---|---|---|---|---|
| G-A) | No | 3 K/min | 750 | n.d. | 0.58 |
| G-B) | No | 3 K/min | 1000 | 16.6 | 0.56 |
| G-C) | No | 50 K/min | 1000 | 34.1 | 0.52 |
| G-HPC-A) | Yes | 3 K/min | 750 | 5.5 | 0.55 |
| G-HPC-B) | Yes | 3 K/min | 1000 | 68.0 | 0.50 |
| G-HPC-C) | Yes | 50 K/min | 1000 | 61.5 | 0.48 |
From these data, we summarize the relationship between the Raman parameter and the strength of coke in Fig. 5. Even though the Raman parameter represents microscopic structure of carbon, the strength of coke (which is macroscopic value) has strong relationship to the Raman parameter. So the strength of coke can be predicted in a certain range by Raman observation without any destruction of the coke. In this work, the sample has almost no porous structure, so the effect of pore formation is ignored in this case, so further investigation on the effect of pore formation on the strength and structure, is expected.
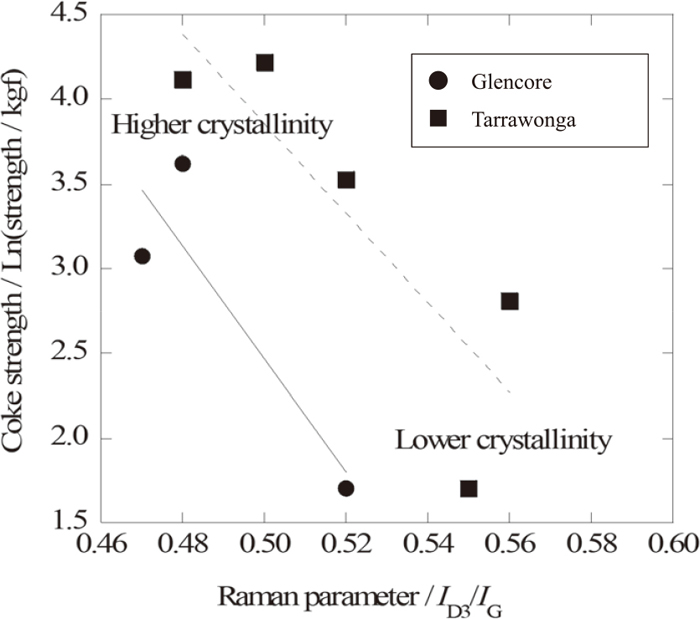
Relationship between the strength of coke and the Raman parameters.
Coking low grade coal with HPC as a binder was conducted under various conditions, and resultant coke was evaluated by Raman observation. Results suggest that mutual melting between blended coal and HPC brought strong carbonaceous structure with highly crystallized. The Raman parameter ID3/IG can predict the coke strength to some extent without any destruction of the coke, if the sample has low porosity.