2020 Volume 60 Issue 8 Pages 1796-1802
2020 Volume 60 Issue 8 Pages 1796-1802
In high carbon steel, TTT nose temperature rises and upper baninte becomes easy to be formed with quantity of Si addition. Generation of upper bainite is reduced by boron addition. In this study, the influence of boron addition on isothermal transformation behavior in Si-added high carbon steel was clarified. By boron addition, lamellar spacing and growth rate of pearlite doesn’t change, but the nucleation of pealite is reduced. But nucleation of pearlite is promoted when Fe23(C,B)6 precipitates. In the Si-added high carbon steel, upper bainite is often formed with the generated ferrite on prior austenite grain boundary. It is inferred that boron reduces ferrite generation in grain boundary which causes upper bainite formation. It is confirmed that effective existence state of boron is grain boundary segregation.
High-carbon steel wire rods develop a pearlite structure with high work hardening capacity.1) They are used as drawn wire materials for the main cables of bridges, high-strength prestressed concrete (PC) strands and steel cords for tires, among other applications. To strengthen these wire rods, alloying elements are added to their steels to suit specific manufacturing methods. For example, Cr is added to steel cords to increase the drawing strain and refine the lamellar spacing.2) Wires for the main cables of bridges are hot-dip galvanized after drawing. Si is added to inhibit the resultant spheroidization of cementite in the drawn pearlite structure.3) Addition of 0.9% Si made it possible to improve by 200 MPa the strength of the steel wires used to build the Akashi Kaikyo Bridge, the world’s longest suspension bridge at the time of its completion.4)
The addition of these alloying elements, on the other hand, calls for the optimization of conventional heat treating conditions. Heat treatment of the pearlite structure is generally called patenting and consists of austenite solution treatment and isothermal transformation treatment at 500 to 600°C. Cr addition increases the possibility of cementite coming out of solution. Si addition raises the nose temperature of the time-temperature-transformation (TTT) curve and hence increases the optimum transformation temperature. Heat treatment at a temperature below the nose temperature generally forms upper bainite. Actual patenting treatment of wire rods involves heating to the austenite region and immersing in a solvent such as lead. The surface layer and core of the wire rod differ in cooling rate to the solvent temperature. As a result, upper bainite often forms in the surface layer alone. The upper bainite structure has cementite coarsely dispersed, is softer than pearlite, is low in work hardening capacity, and degrades twistability, a wire ductility evaluation index.5) Twistability is evaluated by the number of twists to fracture and by the fracture surface morphology when wires are twisted to fracture. Ochiai et al.5) reported that when an upper bainite structure is present in the surface layer of the wire, the wire is liable to develop longitudinal cracks or delaminations immediately after it is twisted.
Manabe and Yamasaki6) reported that the addition of boron to Si-alloyed high-carbon steel reduces the area fraction of upper bainite. It is well known that the addition of boron to low-carbon steels improves their hardenability. The addition of boron to high-carbon steels is reported to be ineffective for improving hardenability7) and to suppress the formation of second phase ferrite in the pearlite structure.8) The addition of boron is also reported to improve ductility in medium-carbon steels and reduce ductility in high-carbon steels.9,10) Few reports have focused on the behavior of boron in isothermal transformation. In the present study, we focused on and clarified the behavior of boron in Si-alloyed high-carbon steels during patenting.
The experimental steels were vacuum melted. A 0.70mass%C- 1.0mass%Si-1.0mass%Mn base steel was alloyed with Ti (steel A) and with Ti and B (steel B). A 0.87mass%C- 0.90mass%Si- 0.7mass%Mn base steel was used as steel C and was alloyed with B (steel D) and alloyed with Ti to fix as TiN and with B (steel E). The chemical compositions of the five steels A to E are shown in Table 1. Steels A and B were cast into ingots, soaked, hot forged, patented and drawn into wires. Steels C to E were cast into ingots, hot forged into 122 mm square billets and hot rolled into 12 mm diameter rods.
| Steel | C | Si | Mn | P | S | Ti | B* | N* |
|---|---|---|---|---|---|---|---|---|
| A | 0.70 | 1.01 | 1.01 | 0.003 | 0.001 | 0.013 | – | 34 |
| B | 0.70 | 1.01 | 1.00 | 0.003 | 0.001 | 0.013 | 11 | 34 |
| C | 0.86 | 0.90 | 0.72 | 0.008 | 0.008 | – | – | 44 |
| D | 0.86 | 0.91 | 0.75 | 0.007 | 0.008 | – | 18 | 43 |
| E | 0.86 | 0.89 | 0.74 | 0.008 | 0.008 | 0.011 | 20 | 40 |
Formaster test specimens were cut out from steels A and B, heated to 950°C and held there for 180 s, held in a temperature range of 500 to 675°C until the end of transformation, and quenched with helium gas to room temperature on a fully automatic transformation point measuring and recording machine (Formaster testing machine). Their TTT curves were then measured. To investigate the effect of boron on pearlite transformation at temperatures above the TTT nose, we quenched the specimens during transformation at temperatures above the nose to freeze their microstructure. The specimens were then etched in a picral solution and observed with a field emission scanning electron microscope (FE-SEM) to check the growth rate of pearlite nodules and the formation of pearlite nuclei. Using an electron backscatter diffractometer (EBSD), pearlite blocks were defined as grain boundaries separated with an orientation angle difference of 9°11) and the grain size was calculated by the intercept method. Pearlite block grains were counted by changing the area to be measured with pearlite transformation temperature so that 30 or more pearlite block grains were contained. Concerning transformation at temperatures below the TTT nose, specimens were quenched in the middle of transformation at 525°C to freeze their microstructure. The specimens were then observed with the FE-SEM and EBSD to study the formation behavior of upper bainite and the effect of boron. The existence state of effective boron was also studied. In the experimental steels, boron is considered to exist as intergranularly segregated B, BN and Fe23(C, B)6. Regarding intergranularly segregated B, the distribution of boron in steel B was studied by the alpha ray track etching (ATE) method. The nuclei of boron atoms react with neutrons to form lithium nuclei and helium nuclei. The helium nuclei react with the film pasted to the observation surface of the specimen. The ATE method uses this reaction. ATE images were observed as described below. A methyl acetate solution was applied to an acetyl cellulose film. The acetyl cellulose film was pasted to the observation surface of the specimen. The specimen was neutron irradiated in the JRR-3 facility of the Japan Atomic Energy Agency (JAEA). The irradiated film was etched in a 2.5 N NaOH aqueous solution. The etched surface of the specimen was vapor coated with gold and observed with an optical microscope. Concerning the precipitation of BN, Yano et al.12) reported a precipitation nose at 850°C. Concerning the precipitation of Fe23(C,B)6, a precipitation nose at 650 to 800°C is reported by R. A. Grange and J. B. Mitchel13) and by Yamamoto et al.14) Specimens of steels C to E were heated to 1100°C for 180 s, held at 850°C for 0 to 30 s to precipitate BN, and isothermally transformed at 525°C. Specimens of steels C and E were heated to 950°C for 180 s and held at 675°C for 3 s to precipitate Fe23(C,B)6, and isothermally transformed at 525°C. The isothermally transformed microstructures of these specimens were observed. The area fraction of upper bainite was obtained to check the existence state of effective boron. The precipitates were identified with a JEOL JEM-2100F field emission transmission electron microscope (FE-TEM), an energy dispersive x-ray spectrometer (EDS) and a field emission electron probe microanalyzer (FE-EPMA). Five FE-SEM micrographs of areas of about 10000 μm2 were taken at 2000X from each specimen. Portions judged to be those of upper bainite were marked on the micrographs and the mark-bordered portions were averaged to obtain the area fraction of upper bainite.
Figure 1 shows the TTT diagram of steels A and B. The nose temperature of pearlite transformation can be seen to be about 625°C. The upper bainite area fraction in a temperature range of 600°C or less and below the nose was calculated and compared with the upper bainite area fraction obtained when steel A was isothermally transformed at 500°C. The results are shown in Fig. 2. The microstructural observation results of steels A and B isothermally transformed at 525°C are shown in Fig. 3. The characteristics of isothermal transformation in boron-alloyed steels B, D and E are as follows;
• The boron addition raises the transformation start and end temperatures in the temperature range below near the nose temperature.
• The boron addition accelerates the transformation in a temperature range above the nose temperature (675°C and above).
• The boron addition restricts the formation of bainite in a temperature range below the nose temperature.

Isothermal Transformation Diagram.

Relationship between Bainite fraction and isothermal transformation temperature.

Microstructure transformed at 525 degrees.
The effect of the isothermal transformation temperature above the nose temperature on the pearlite block size (PBS) of steels A and B is shown in Fig. 4. The effect of the isothermal transformation temperature above the nose temperature on the minimum lamellar spacing of steels A and B is shown in Fig. 5. The PBS of non-boron-alloyed steel A increases with increasing transformation temperature. The PBS of boron-alloyed steel B increases at transformation temperatures up to 625°C and decreases at transformation temperatures of 625°C to 650°C. The minimum lamellar spacing changes little, irrespective of whether or not the steel is alloyed with boron. Steels A and B were quenched during isothermal transformation at 625°C and 650°C, respectively, and the change with time in maximum pearlite nodule size was investigated. The results are shown in Fig. 6. The slope of each curve showing the growth rate of pearlite changes little. This means that the growth rate of pearlite does not change, irrespective of whether or not the steel is alloyed with boron. At 625°C, the holding time agrees with the maximum pearlite nodule size, irrespective of whether or not the steel is alloyed with boron. At 650°C, the growth rate of pearlite nodules is almost the same for both steels A and B, but the holding time at which the same maximum pearlite nodule size is achieved is shorter for boron-alloyed steel B. The reason that the boron addition increased the PBS at and below 625°C is considered to be because the pearlite transformation nucleation frequency was retarded. The reason that the boron addition decreased the PBS at and above 650°C is considered to be because the nucleation sites that accelerate pearlite transformation formed and increased.
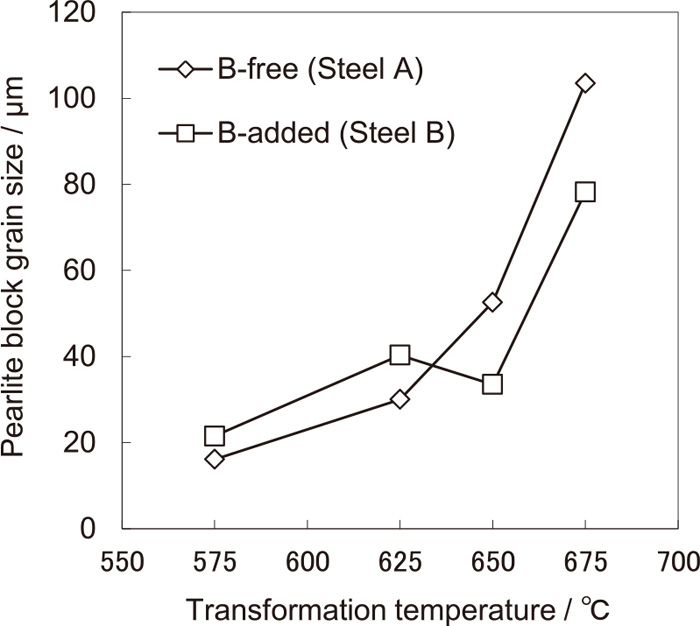
Relationship between PBS and isothermal transformation temperature.

Relationship between minimum lamellar spacing and isothermal transformation temperature.

Pearlite growth rate.
To clarify factors for the change in pearlite nucleation with the boron addition at high temperatures, we conducted the microstructural observation of boron-alloyed steel B. Figure 7 shows the optical micrographs of specimens quenched when the pearlite transformation started at each isothermal transformation temperature. As the temperature rose, precipitates came to be observed on untransformed prior austenite grain boundaries. When the microstructure of a steel B specimen was observed with the SEM early in the 650°C transformation, the nucleation of ferrite from grain boundary precipitates was observed as shown in Fig. 8. To identify the grain boundary precipitates, an additional steel B specimen was heated, held at 675°C for 3 s, quenched, microstructurally observed, and analyzed for elements with the FE-EPMA. The precipitates were identified by the extraction replica method and by the TEM. The FE-EPMA results are shown in Fig. 9 and the TEM electron beam diffraction results are shown in Fig. 10. Carbon and boron were detected in the precipitate positions in the SEM micrograph. The grain boundary precipitate was identified as Fe23(C,B)6. Fe23(C,B)6 precipitates in a plate form on the grain boundaries, has a Kurdjumov-Sachs (K-S) relationship with austenite on either side, and does not necessarily have an orientation relationship with austenite on the other side.15) The possibility may be considered that ferrite preferentially formed in low-carbon regions created by the precipitation of Fe23(C,B)6 and at the incoherent interface side without orientation relationship and became pearlite nuclei.

Microstructure quenched at transformation initial (Steel B).

Initial microstructure transformed at 650 degrees (Steel B).

Elemental map of boron and carbon (Steel B). (Online version in color.)

TEM image and electron diffraction of Fe23(C,B)6.
As described above, the boron addition promoted pearlite transformation at high temperatures. This may be because Fe23(C,B)6, precipitated during isothermal holding, grew and acted as nucleation sites for pearlite.
3.3. Effect of Boron on Upper Bainite Formation below Nose TemperatureFigure 11 shows the initial microstructure transformed on the prior austenite grain boundaries when steel A was quenched during the 525°C isothermal transformation. Upper bainite and pearlite form in competition with each other. Some upper bainite was seen to have formed singly. As shown in Fig. 12, bainite was observed to have formed together with ferrite on the prior austenite grain boundaries. This grain boundary ferrite was observed less in boron-alloyed steel B than in non-boron-alloyed steel A. When the inverse pole figure (IPF) maps of bainite (BCC) were taken at 40 nm steps by using the EBSD, it was found that grain boundary ferrite and bainite were formed in the same orientations.

Initial microstructure transformed at 525 degrees (Steel A).

Initial microstructure of bainite and IPF map. (Online version in color.)
We checked the orientation relationship between grain boundary ferrite and prior austenite and the orientation relationship between grain boundary upper bainite and prior austenite. That is to say, we checked the orientation relationship between ferrite and upper bainite across the austenite grain boundaries and retained austenite near ferrite and upper bainite. The pole figures of upper bainite and ferrite are shown in Fig. 13. The orientation relationship among ferrite, upper bainite and austenite is schematically illustrated in Fig. 14. It was found that ferrite grew in a K-S orientation relationship with austenite, that upper bainite had a nearly K-S orientation relationship with austenite, and that ferrite had a nearly K-S orientation relationship with austenite on either side of the grain boundary. Martensite and austenite are generally known to have a K-S orientation relationship. Sakai et al.16) reported that when ferrite formed in a low-carbon ferrite-martensite dual-phase steel, the orientation relationship between ferrite and martensite approached a K-S orientation relationship. Accordingly, the nucleation of upper bainite in Si-alloyed high-carbon steels is considered to accelerate in the presence of ferrite that satisfies the K-S orientation relationship with austenite.

Pole figure of ferrite, upper bainite and austenite. (Online version in color.)

Crystallographic orientation relationship between ferrite, upper bainite and ausitenite.
Since the boron addition suppresses the formation of grain boundary ferrite, the mechanism whereby the boron addition reduces the formation of upper bainite is considered to result from the suppression of the formation of grain boundary ferrite, a precursor phase.
3.4. Existence State of Boron Affecting Formation of Upper BainiteThree steel B specimens (a), (b) and (c) were prepared for observation by the ATE method. Specimen (a) was solution treated at 1150°C to put boron into solution and quenched. Specimen (b) was solution treated at 1150°C to put boron into solution, isothermally held at 690°C for 200 s without pearlite transformation, and quenched. Specimen (c) was solution treated at 1150°C to put boron into solution, isothermally held at 475°C for 200 s without pearlite transformation, and quenched. The three specimens were then processed and observed by the ATE method. The ATE micrographs obtained are shown in Fig. 15. The dark spots indicate the positions of boron. It can be seen that specimen (a) has boron segregated on the prior austenite grain boundaries. The darkening of the grain boundaries was more conspicuous in specimen (b) isothermally held at 690°C near the precipitation nose for 200 s than in specimen (c) isothermally held at 475°C for 200 s. Since holding above 675°C is estimated to cause the precipitation of Fe23(C,B)6 as described in Section 3.1, the darkening of the grain boundaries in specimen (b) isothermally held at 690°C may be ascribed to the precipitation of Fe23(C,B)6. The existence state of boron in specimen (c) solution heat treated at 475°C and then quenched may be mainly ascribed to the grain boundary segregation of boron. This consequently indicated the possibility that the existence state of boron, effective in suppressing the formation of upper bainite, is its grain boundary segregation. To clarify whether the existence state of boron, effective in suppressing the formation of upper bainite, is grain boundary segregated boron, BN, or Fe23(C,B)6, we examined the change in the area fraction of upper bainite after isothermal transformation with the aforementioned boron precipitation heat treatment.

ATE image (Steel B).
Steel C, D and E specimens were austenitized, held at 850°C to precipitate BN, and isothermally transformed at 525°C. Figure 16 shows the effect of the isothermal holding time on the area fraction of upper bainite. Steel C and E specimens were austenitized, held at 675°C to precipitate Fe23(C,B)6, and isothermally transformed at 525°C. Figure 17 shows the effect of the isothermal holding time on the area fraction of upper bainite. The area fraction of upper bainite in the steel C specimens isothermally transformed at 525°C without isothermal holding at 850°C or 675°C is taken as unity in Figs. 16 and 17. The area fraction of upper bainite was high in the non-boron-alloyed steel C specimens, regardless of whether or not they underwent BN or Fe23(C,B)6 precipitation treatment. The area fraction of upper bainite increased with the BN precipitation treatment in the specimens of boron-alloyed steel D. The area fraction of upper bainite did not change with the BN precipitation treatment but increased with the Fe23(C,B)6 precipitation treatment in the specimens of steel E alloyed with boron and with titanium to fix N as TiN. We thus found that the precipitation of boron, as BN or Fe23(C,B)6, reduces its effectiveness in suppressing the formation of upper bainite and that the grain boundary segregation of boron is effective in suppressing the formation of upper bainite.

Effect of 850°C immersed time on upper bainite fraction.

Effect of 675°C immersing treatment on upper bainite fraction. (Steel C, Steel E).
We investigated the effect of boron addition to Si-alloyed high-carbon steels on their isothermal transformation behavior and obtained the following conclusions:
• When Fe23(C,B)6 precipitates at high temperatures above 650°C, it acts as nuclei for pearlite formation and promotes pearlite transformation. When Fe23(C,B)6 precipitates less below 625°C, it suppresses the pearlite transformation. The boron addition does not affect the growth rate of pearlite and the lamellar spacing.
• The boron addition suppresses the upper bainite transformation below the nose temperature. Often with Si-alloyed high-carbon steels, ferrite first forms on prior austenite grain boundaries and upper bainite then forms with ferrite as a precursor phase. Boron has a high possibility of suppressing the formation of ferrite.
• The existence state of boron effective in suppressing the formation of upper bainite is its grain boundary segregation. When boron precipitates as BN, its effectiveness diminishes.