2021 年 61 巻 10 号 p. 2613-2619
2021 年 61 巻 10 号 p. 2613-2619
Nitride precipitation during isothermal heating after rapidly cooled from the ferrite phase enriched temperature in duplex stainless steel was investigated to clarify the effect of nitrogen in steel as an alloying element and austenite phase growth. Nitride precipitation is frequently harmful for the properties such as toughness or corrosion resistance in duplex stainless steels. The heat affected zone (HAZ) in the vicinity of fusion boundary in weldments tends to have more remarkable nitride precipitation because of rapid cooling from the ferrite phase enriched temperature. During cooling process the growth of austenite phase occur resulting in the prevention of nitride precipitation.
Employing 25%Cr duplex stainless steels containing various level of nitrogen of 0.1% to 0.3%, the measurement and observation of nitride precipitation were conducted in the specimens isothermally heated at 873 K to 1073 K after rapidly cooled from 1653 K. As results the low nitrogen containing steel had relatively much nitride precipitation than the higher nitrogen containing one because of the less increase in the fraction of austenite phase growth with larger nitrogen solubility. This result can be explained by the decease of oversaturation of nitrogen in ferrite phase by contribution of austenite phase growth during thermal cycle.
Recently duplex stainless steels consisting of ferrite and austenite phase are widely used for various structural materials because of high performance for cost. The high performance in terms of mechanical property and corrosion resistance is obtained by the proper solution treatment without formation of harmful precipitates such as sigma phase or nitride during the manufacturing process in the duplex stainless steel.1,2,3,4,5,6,7,8,9)
However, in the welded portion it is indispensable to be heated at the high temperature where the phase transformation is remarkable, therefore the fraction of austenite phase can be changed to lower in heat affected zone (HAZ) by weld thermal cycles. The HAZ consisting of the small fraction of austenite phase can deteriorate in the mechanical performance and corrosion resistance due to that changing in microstructure. Therefore it is important to obtain the optimum austenite phase fraction in weldments. Many research works10,11,12,13,14,15,16) have been conducted about the microstructure change in HAZ of duplex stainless steels from the view point of chemical composition in steels and welding conditions. The deterioration of performance in the HAZ is mainly caused by the precipitation of harmful phase such as nitride or intermetallic compounds.15,16)
It is known that the dominant factors of nitride precipitation in the HAZ of duplex stainless steel is potential of austenite phase formation and cooling rate. Nitride precipitation phenomena in the HAZ occurs concurrently with the transformation of austenite phase from overcooled ferrite phase.17,18,19) Therefore the analysis by modelling or simulation of this phenomena is not always conducted easily. For example, a conventional software such as TC-PRISMA20) consisting of thermo-dynamic model dealing with multi alloying elements is employed for the simulation of various precipitation. Almost of simulation using such software is usefully applied for the precipitation phenomena in the single phase matrix because of difficulty to apply to concurrent precipitation. Therefore it is important to obtain the experimental findings regarding nitride precipitation with the concurrent decrease or increase in austenite phase during thermal cycles. In this work as the first step to the investigation of nitride precipitation in continuous cooling process in the HAZ, the amount of nitride precipitation during isothermal heating after rapidly cooled from the high temperature where ferrite phase is enriched in duplex stainless steel was experimentally investigated.
The 25%Cr duplex stainless steels containing 0.1% and 0.3% nitrogen listed in Table 1 were used. Those were melted in laboratory, hot forged and hot rolled to 12 mm thick plates in the range of 1373 to 1523 K then those were heat treated by water quench after holding at 1373 K for 1800 s. Those plates were machined to the specimens of 11 mm in thickness, 11 mm in width and 60 mm in length. The specimens were heated at 1653 K for 3 s and cooled rapidly with argon gas to the various test temperatures. And then those are held for various durations at a constant temperature and quenched with argon gas shower. Some of them was cooled to room temperature from 1653 K. The average cooling rate was approximately 100 K/s.
| C | Si | Mn | Ni | Cr | W | Mo | N | |
|---|---|---|---|---|---|---|---|---|
| Steel A | 0.02 | 0.17 | 0.47 | 7.11 | 25.20 | 1.95 | 2.97 | 0.33 |
| Steel B | 0.02 | 0.16 | 0.48 | 7.10 | 25.29 | 1.98 | 3.03 | 0.11 |
The heat treated specimens were evaluated in terms of microstructures observation and measuring the amount of nitride. Microstructure observation was conducted with optical microscope, SEM(Scanning Electron Microscope) and TEM(transmission Electron Microscope) using extraction replica. Measuring the fraction of austenite phase was conducted in the image field of the magnification of 100 using EBSD(Electron Back Scatter Diffraction) analysis. The amount of nitride was measured by the analysis of residue extracted using the chips of steel obtained from the homogenously heated zone of specimens. The chips with about 1 mm2 in cross section and 5 mm in length produced by machining were dissolved in 10%bromine alcohol solution to obtain extracted residue. The amount of N as nitride was measured by analyzing the amount of nitrogen included in the extracted residue. The amount of N as nitride (mass%) was defined as the ratio of the amount of nitrogen included in the extracted residue to the mass of dissolved chips of steel.
Figure 1 shows the amount of nitride as extracted residue from specimens isothermally heated at various temperatures of 873 K to 1073 K for various times after rapidly cooled from 1653 K. The 0.1% nitrogen containing steel had the amount of nitrogen of 0.038% as extracted residue after rapidly cooled to room temperature from 1653 K. After isothermal heating for 10 s to 300 s at the temperature of 873 K to 1023 K, the amount of nitrogen about 0.06% was detected as residue and that amount did not increase remarkably with the increase of isothermal heating time. At the heating temperature of 1073 K the amount of nitrogen as extracted residue rarely increased during isothermal heating and rather decreased after heated for 300 s. The 0.3% nitrogen containing steel had no nitrogen as extracted residue after rapidly cooled to room temperature from 1653 K. After isothermal heating for 10 s to 300 s at the temperature of 873 K to 1073 K, the amount of nitrogen detected was 0.003 to 0.01%, which was remarkably less comparing to that in the 0.1% nitrogen containing steel. By isothermal heating that amount increased gradually.

Effects of temperature and time on amount of N as nitride measured with extracted residue in a) 25Cr-7Ni-3Mo-2W-0.1N and b) 25Cr-7Ni-3Mo-2W-0.3N steel isothermally heated after rapid cooled from 1653 K.
Figures 2 and 3 show the optical micrographs in specimens isothermally heated at various temperatures for 30 s after rapidly cooled from 1653 K in the steels containing 0.1% nitrogen and 0.3% nitrogen respectively. In the steel containing 0.1% nitrogen, very fine areas etched black in the ferrite phase matrix were observed, of which density was high in the case of the heating temperature of 923 K and 973 K. However, in the 0.3% nitrogen containing steel very fine size of areas etched black were rarely observed.

Optical micrograph of 25Cr-7Ni-3Mo-2W-0.1N steel heated at various temperatures for 30 s after rapid cooled from 1653 K.

Optical micrograph of 25Cr-7Ni-3Mo-2W-0.3N steel heated at various temperatures for 30 s after rapid cooled from 1653 K.
Examples of EBSD image of specimens isothermally heated at 1073 K after rapidly cooled from 1653 K in the 0.1% nitrogen containing steel are shown in Fig. 4. Fine austenite phase increased during isothermal heating in the ferrite phase matrix as well as in the ferrite grain boundary. The very fine areas etched black in optical micrograph were assumed to be consisting austenite phase and deeply etched ferrite around the nitrides. The SEM micrographs of those specimens are shown in Fig. 5. Some white looking fine particles because of large Cr content observed in the micrographs were considered as chromium nitrides. Though fine particles looking white considered as nitride were observed intragranularly both in the containing 0.1% nitrogen and 0.3% nitrogen steel, the particle density in the steel containing 0.1% nitrogen was high relatively. Figure 6 shows an example of TEM image and analysis of diffraction pattern in extraction replica from a specimen isothermally heated described above. From the results those fine particles were identified as Cr2N.

Example of EBSD analysis of austenite phase in 25Cr-7Ni-3Mo-2W-0.1N steel heated at 1073 K for various duration after rapid cooled from 1653 K. (Online version in color.)
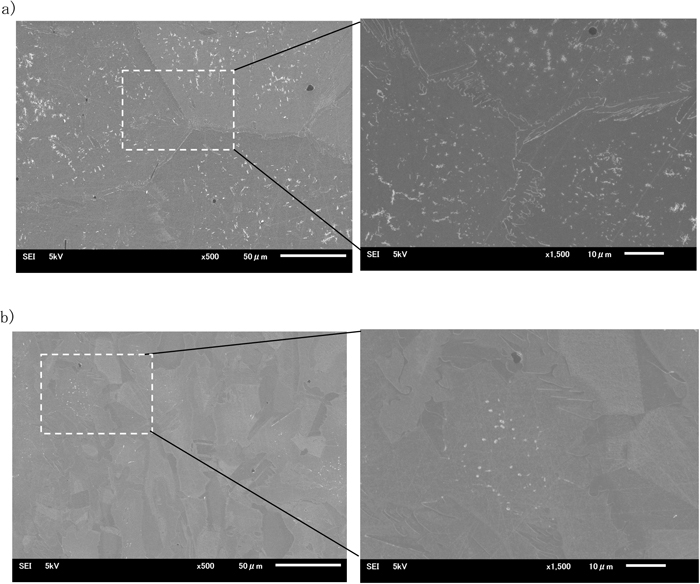
SEM micrograph of a) 25Cr-7Ni-3Mo-2W-0.1N and b) 25Cr-7Ni-3Mo-2W-0.1N steel heated at 973 K for 300 s after rapid cooled from 1653 K.

Precipitates identified as Cr2N in TEM micrograph with extraction replica of microstructures of 25Cr-7Ni-3Mo-2W-0.1N steel heated at 973 K for 300 s after rapid cooled from 1653 K. (Online version in color.)
Fractions of austenite phase measured by EBSD were shown in Fig. 7. The curves shown in the figure are the fraction of austenite phase obtained using the model in the previous work.21) The 0.3% nitrogen containing steel had large fraction of austenite phase over 50% immediately after cooled from 1653 K. The fraction of austenite phase increased slowly during isothermal heating. In contrast the 0.1% nitrogen steel had almost no fraction of austenite phase after cooled from 1653 K. However that fraction increased during isothermal heating at 1073 K and that was close to 20% after heated for 100 s.

Fraction of austenite phase in 25Cr-7Ni-3Mo-2W-0.1N steel heated at 1073 K or 973 K for various duration after rapid cooled from 1653 K.
Effect of nitrogen containing in steel as an alloying element on the nitride precipitation in specimens rapidly cooled from high temperature of 1653 K was discussed from the view point of roll of austenite phase on the reduction of oversaturation of nitrogen in ferrite phase.
Phase diagram calculated by using the thermodynamic data (2017a, database:TCFe8)23) is shown in Fig. 8. That is the cross section of the phase diagram of N and Fe-25%Cr-7%Ni-3%Mo-2%W. That indicates nitride in 0.1% nitrogen containing steel can precipitate below 1150 K in equilibrium condition with containing austenite phase fraction of about 50%. However the microstructure rapidly cooled from 1653 K consisted of almost single phase of ferrite. Therefore another phase diagram of ferritic stainless steel shown in Fig. 9 was referred as quasi equilibrium, which was also calculated by using the thermodynamic data (2017a, database:TCFe8).23) That is the cross section of the phase diagram of N and Fe-25%Cr-3%Mo-2%W without containing Ni. In the 0.1% nitrogen containing steel the amount of nitrogen of 0.038% was detected as extracted residue after rapidly cooled to room temperature from 1653 K. The microstructure of which matrix consists of single phase of ferrite can have nitride precipitation at high temperature of 1410 K. The higher temperature of nitride precipitation gives the larger supercooling resulting in high nucleation frequency in the temperature range with large diffusion rate of Cr atom, which determined growth rate of nitride. So that much of nitride was considered to precipitate after rapidly cooled to room temperature from 1653 K. From this consideration rather oversaturation of nitrogen in ferrite phase can be reduced immediately after rapidly cooled to 873 K to 1073 K from 1653 K before the initiation of isothermal heating. However oversaturation of nitrogen in ferrite phase was be dissolved completely so that during isothermal heating the amount of nitride increased in concert with the reduction of retained oversaturation. Much of nitride precipitated comparing to that in 0.3% nitrogen containing steel due to smaller fraction of austenite phase which has larger solubility of nitrogen. The amount of nitride rather decreased by isothermal heating at 1073 K for long duration of 300 s. Regarding that it has been reported that in the structure after rapidly cooled from high temperature reducing of nitride can occur during isothermal heating accompanied by the growth of austenite phase.17) In the case of this work once much of nitrogen discharged as nitride due to single phase of ferrite immediately after rapidly cooled and after isothermal heating for long duration of 300 s the discharged nitrogen can be absorbed into austenite phase resulting in the reduction of nitride because of sufficiently growth of austenite phase during heating at 1073 K.

Phase diagram of Fe-25Cr-7Ni-3Mo-2W-0.02C-N system calculated using thermodynamic data (2017a, database: TCFe8). (Online version in color.)

Phase diagram of Fe-25Cr-3Mo-2W-0.02C-N system calculated using thermodynamic data (2017a, database: TCFe8). (Online version in color.)
In the 0.3% nitrogen containing steel the microstructure is considered not to change to single phase of ferrite even heated at 1653 K phase by referring the phase diagram shown in Fig. 8. Therefore it was understood after rapidly cooled to room temperature duplex microstructure including rather austenite phase fraction of 50% was obtained. Though nitride can precipitate blow 1260 K in equilibrium condition by referring the phase diagram shown in Fig. 8, almost of nitrogen can be included in the austenite phase which has large solubility of nitrogen so that it is considered oversaturation of nitrogen in ferrite phase can be small resulting in no nitride precipitation after rapidly cooled to room temperature. It was considered there was no nitride precipitation at the initiation of isothermal heating at 873 K to 1073 K immediately after rapidly cooled from 1653 K. However due to the slightly retained oversaturation of nitrogen in ferrite phase nitride precipitation can continue during isothermal heating at 873 K to 1073 K. But the amount of nitride was considered to be much less comparing to that in the 0.1% nitrogen containing steel by contribution of much austenite phase resulting in reduction of oversaturation of nitrogen in ferrite phase.
The following results were obtained by the investigation regarding the nitride precipitation during isothermal heating after rapidly cooled from ferrite temperature (1653 K) in the 25%Cr duplex stainless steels containing 0.1% and 0.3% nitrogen.
(1) In the 0.1% nitrogen containing steel much of nitride precipitation of Cr2N was observed in the heating condition especially remarkable below 1023 K. In the 0.3% nitrogen containing steel nitride precipitation was also observe but the amount was about one tenth of that in 0.1% nitrogen steel.
(2) In the 0.1% nitrogen steel the microstructure was almost single phase of ferrite phase immediately after rapidly cooled from 1653 K and the fraction of austenite phase slowly increased during isothermal heating. In the 0.3% nitrogen steel the microstructure had austenite phase of about 50% immediately after rapidly cooled and the fraction of austenite phase slowly increased during isothermal heating.
(3) The dominant factor on the difference of nitride precipitation between the steels containing 0.1% and 0.3% nitrogen was considered to be amount of austenite. In the 0.1% nitrogen steel almost of nitrogen can be included in ferrite phase resulting in remarkable oversaturation due to no existence of austenite phase so that nitride precipitation can be caused at high temperature of about 1400 K in rapid cooling process. In the 0.3% nitrogen steel nitrogen precipitation can be less due to lower equilibrium temperature of 1260 K and smaller oversaturation of nitrogen by contribution of sufficient austenite phase.