2021 Volume 61 Issue 3 Pages 763-772
2021 Volume 61 Issue 3 Pages 763-772
Basic oxygen furnace (BOF) flue gas cleaning system is a crucial flue gas purification device to ensure the cleaner production in steelmaking process. Due to utilization of galvanized steel scrap in steelmaking process, zinc-containing dust collected by the cleaning system forms unremovable high-strength dust agglomerates on the inner wall of evaporative cooler, which can affect the normal operation of cleaning system and seriously hinder the cleaner production in steelmaking process. This issue continuously aggravates as the use of galvanized steel scrap increases, while there are currently no effective control methods. Consequently, how to keep down the zinc-containing dust agglomeration strengthening in the evaporative cooler is a crucial issue for investigation. In this paper, X-ray fluorescence spectrometer, X-ray diffraction, scanning electron microscopy and strength test under high-temperature roasting were carried out to investigate characteristics and strengthening mechanism of dust agglomerates. Based on this, easy implementation of control methods were proposed. The results show that ZnO reacts with Fe2O3 at a high temperature above 600°C to form tightly ZnFe2O4 phase, and chemical adsorption of ZnFe2O4 with BOF dust particles is the primary cause of dust agglomeration strengthening in evaporative coolers. Solid state sintering of low melting point substances containing Na+ and K+ promotes strengthening. Decreasing the temperature in evaporative cooler and controlling the content of low melting point substances in BOF dust are effective measures to reduce dust agglomerates, optimize the environment of cleaning system, and ensure the cleaner production in steelmaking process.
In the metallurgical process of contemporary world, basic oxygen furnace (BOF) steelmaking is the uppermost method. According to World Steel Association, about 70% of the total steel worldwide is produced by BOF, especially, about 90% of the total steel in China.1) In steelmaking, basic oxygen furnaces can generate a large quantity of flue gas solid wastes, referred to as BOF dust, from 10 to 30 kg per metric ton of steel (kg/ts), and the dust concentration is between 150 and 300 g per cubic meter (g/m3).
In 2019, crude steel production was 996 million tons in China, and 1869 million tons worldwide.1) According to average output of BOF dust 20 kg/ts, it can be estimated that in 2018 generation was around 18 million tons in China alone and up to 36 million tons globally. Due to the tiny particle size of BOF dust, it is wafted in the atmosphere, not only pollutes the environment, but also affects human health, causing or exacerbating pulmonary diseases.2,3,4) Therefore, it is particularly vital to select suitable BOF flue gas cleaning equipment to collect these solid wastes.
BOF flue gas cleaning system is an important equipment to ensure the cleaner production in steelmaking process. It is mainly divided into dry and wet systems.5) The dust concentration in BOF wet flue gas cleaning system is generally 100 mg/m3, while BOF dry cleaning system is less than 10 mg/m3. According to an emission standard of air pollutants for steel smelt industry issued by the Ministry of Ecology and Environment of China, the emission concentration limit of particulate matter in BOF flue gas is 50 mg/m3. Obviously, the dry flue gas cleaning system can satisfy the requirements of current steelmaking cleaner production. Furthermore, compared with BOF wet flue gas cleaning system, the dry flue gas cleaning system consumes less electricity and has no secondary pollution.6,7)
As shown in Fig. 1, BOF dry flue gas cleaning system mainly consists of cooling flue, evaporative cooler, electrostatic precipitator and gas cooler, where evaporative cooler plays a significant role. Evaporative cooler sprays water directly into high temperature flue gas, and the water evaporates to atomized water, completes the heat exchange with the flue gas to decrease temperature. Meanwhile, BOF flue gas solid wastes are settled by gravity in evaporative coolers to achieve the first separation of gas and dust. However, the inner wall of evaporative cooler is prone to dust agglomeration, which causes choking of flue gas flow, obstructs the trouble-free operation of BOF dry flue gas cleaning system, and further affects the cleaner production in steelmaking process.

Schematic diagram of BOF dry flue gas cleaning system. (Online version in color.)
Currently, the reason for dust agglomeration on the inner wall of most evaporative coolers is that the fine BOF dust can flows back with flue gas under the complex flow field inside the cooler.8,9) These dust agglomerates can be eliminated via improving flow field conditions in the evaporative cooler and regularly scouring them with water cannons attached to BOF dry flue gas cleaning system. Nevertheless, in recent years, some steel plants in China have found that dust agglomerates were formed with high zinc content in evaporative coolers of dry flue gas cleaning systems, after extensive use of galvanized steel scrap in steelmaking process. This kind of dust agglomerate is rocky, high hardness, difficult to fall off, and cannot be cleaned with water cannons. It not only affects BOF production, but also causes cleaning systems to clog and not operate normally, further seriously hinders the cleaner production in steelmaking process.
Agglomeration problem caused by the enrichment of zinc-containing substances has been investigated in metallurgy fields. In blast furnaces, zinc vapor enriched in the brick-lined pores of refractory materials forms ZnO agglomeration, which expands the volume of brick lining and causes destruction.10) Therefore, zinc loading limits are generally set at 100–150 g per ton liquid iron in blast furnaces.11) In rotary hearth furnaces, the enriched Zn and ZnO agglomeration block the flues, resulting in low production efficiency of rotary hearth furnace. Nevertheless, this problem has not been solved for a long time, and restricts the development of rotary hearth technology. In electric furnaces, although the zinc content in dust is high, usually between 14–40%,12,13) because the electric furnace dust cleaning system adopts bag filter,14) the dust is difficult to form agglomerates and the equipment is easy to clean, therefore, there is no zinc-containing dust agglomerates in the electric furnace dust cleaning system.
If zinc concentration is controlled in basic oxygen furnaces as in blast furnaces, it will inevitably affect the utilization of galvanized steel scrap as an excellent recycling resource. Recently, especially in China, with the transformation of consumption structure, automobile and household appliance industries are growing rapidly, and the output of galvanized sheet is increasing at a high speed, resulting higher utilization rate of galvanized steel scrap in steelmaking plants and an increase of zinc content in BOF flue gas solid wastes. Therefore, the strengthening problem of dust agglomerates in evaporative coolers is gradually rising. In the future, with the increase in the use of galvanized steel scrap, this problem certainly will aggravate, unfortunately, there are currently no other effective controls for it.
Hence, in order to control the dust agglomeration of zinc-containing dust in the evaporative cooler of BOF dry flue gas cleaning system and realize the cleaner production of the steelmaking process, clarifying the characteristics and strengthening mechanism of the dust agglomerates are crucial issues for investigation. In this paper, we first analyzed the dust agglomerate samples via X-ray fluorescence spectrometer (XRF), X-ray diffraction (XRD) and scanning electron microscopy (SEM) to clarify the composition of the main substances that affect the agglomeration strengthening; secondly, through the high-temperature roasting experiment of zinc-containing BOF dust, the influence of zinc-containing substances on dust agglomerates was explained; finally, the strengthening mechanism of dust agglomerates was analyzed, and the methods for keeping down high-strength dust agglomerates in BOF dry flue gas cleaning system was proposed. This study provides a theoretical basis for dust agglomeration research in BOF dry flue gas cleaning systems. It is of positive significance for improving dust agglomeration, ensuring the cleaner production in steelmaking process and promoting environmental protection development of steel enterprises.
Dust agglomerates were produced by the accumulation of BOF flue gas solid wastes in evaporative coolers, so the solid wastes were first analyzed. BOF flue gas solid wastes used in this research was provided by Shanghai Meishan, which was divided into coarse dust and fine dust. The average particle size of coarse dust is 50–70 μm, and the fine dust is 30–50 μm.
The chemical composition (Table 1) of BOF dust were measured by X-ray fluorescence spectrometer (Lab Center XRF-1800 manufactured by Shimadzu), and the phase components (Fig. 2) of BOF dust was measured by using a Shimadzu diffractometer with an image plate detector and Cu Kα radiation (1.5406 Å), diffraction patterns were collected between 10° ≤ 2θ ≤ 90° at a rate of 1°/min. It can be seen from Table 1 and Fig. 2 that in addition to a large amount of Fe and Fe oxide, there is scant amounts of Zn in BOF dust.
| Component | Fe | Ca | Si | Mg | Zn | Na | K | Cl |
|---|---|---|---|---|---|---|---|---|
| BOF coarse dust | 38.79 | 18.53 | 1.44 | 4.30 | 2.42 | 0.73 | 0.63 | 0.58 |
| BOF fine dust | 56.09 | 5.76 | 1.13 | 1.13 | 3.58 | – | 0.67 | 0.44 |

XRD diffractograms of (a) coarse dust and (b) fine dust.
Dust agglomerate samples were taken from the evaporative cooler of BOF dry flue gas cleaning system at Shanghai Meishan. The temperature in evaporative cooler gradually decreased from top to bottom, so the dust agglomerates in the upper part (JL-1), the middle part (JL-2) and the bottom part (JL-3) were separately selected (Fig. 3).

Dust agglomerate samples at different positions in the evaporative cooler of BOF dry flue gas cleaning system. (Online version in color.)
Dust agglomerate samples were crushed and subsequently ground using an agate mortar and passed through a 74 μm sieve prior to XRF and XRD analysis, respectively. The chemical composition and phase components of dust agglomerate samples are shown in Table 2 and Fig. 4.
| Component | Fe | Zn | K | Cl | Na | Ca | Mn | Si |
|---|---|---|---|---|---|---|---|---|
| JL-1 | 53.27 | 7.83 | 2.75 | 3.17 | 2.32 | 1.34 | 0.98 | 0.67 |
| JL-2 | 55.18 | 6.31 | 2.42 | 2.93 | 2.78 | 2.76 | 0.76 | 0.72 |
| JL-3 | 64.63 | 5.92 | 2.47 | 1.39 | 2.14 | 1.70 | 0.81 | 0.68 |

XRD diffractograms of dust agglomerate samples. (a) JL-1 sample; (b) JL-2 sample; (c) JL-3 sample.
As can be seen from Table 2, in addition to Fe, there are a certain amount of Zn, K, Na, and Cl in the dust agglomerates. Compared with the chemical composition of BOF dust, the content of Zn, K, Na and Cl in the dust agglomerates is enriched.
From Fig. 4, one can see that the phase composition of dust agglomerates is related to the temperature in evaporative coolers. In the high temperature zones of evaporative cooler upper and middle parts, the main phases of JL-1 and JL-2 samples are ZnFe2O4 and Fe-containing substances; in the low temperature zone of evaporative cooler bottom part, the main components of JL-3 sample are ZnO and Fe-containing substances. The difference between the two is reflected in the Zinc phases. Compared with the low temperature zones, the formation of ZnFe2O4 in the high temperature zones may involve more complex physical and chemical reactions, so it is necessary to focus on the analysis in the high temperature zones.
2.2. Methods 2.2.1. Analysis of Dust Agglomerate SamplesThe dried dust agglomerate samples were sliced, polished, and gold sprayed for microstructure analysis using SEM (VEGA3 SBH manufactured by Tescan, tungsten filament, 30 KV, 3.0 nm).
2.2.2. High Temperature Roasting ExperimentVisible from the above analysis results, in addition to the normal Fe volatiles, there are also Zn, K, Na, Cl elements enriched in BOF flue gas solid wastes, which should be ZnO, KCl, NaCl according to the material source of BOF dust.
In order to investigate whether ZnO, KCl and NaCl are the main factors for dust agglomeration strengthening, the cylindrical sample strength of BOF dust with different contents of ZnO, KCl and NaCl was tested by simulating the internal environment of evaporative cooler, and the scheme is shown in Table 3. In order to show the experimental results more clearly, compared with the actual situation, the content of ZnO, KCl, and NaCl was increased in the experiment.
| Sample number | Constituents | Total | ||||
|---|---|---|---|---|---|---|
| Coarse dust | Fine dust | NaCl | KCl | ZnO | ||
| S0 | 25 | 75 | – | – | – | 100 |
| S1 | 23 | 73 | 4 | – | – | 100 |
| S2 | 23 | 73 | – | 4 | – | 100 |
| S3 | 21 | 71 | 4 | 4 | – | 100 |
| S4 | 23 | 73 | – | – | 4 | 100 |
| S5 | 19 | 69 | 4 | 4 | 4 | 100 |
The raw materials were mixed with water and poured into a cylindrical mold having a diameter of 20 mm and a height of 15 mm to form briquette samples. Then, a manual hydraulic press was used to briquet with a pressure of 1 MPa and a molding time of 0.5 s. The specific sample preparation process is shown in Fig. 5. After the samples were formed, they were kept in a muffle furnace heated to 900°C for 2 h, and air-cooled to room temperature for compressive strength measuring.

Schematic diagram of sample preparation process. (Online version in color.)
Furthermore, it can be seen from XRD diffractograms in Fig. 4 that the phase composition is various for dust agglomerate samples at different temperatures. Therefore, the study conducted a high temperature roasting experiment on S5 samples in a muffle furnace at 500°C, 600°C, 700°C, 800°C, 900°C, and 1000°C for 2 h, simulating the effect of the temperature in evaporative cooler on dust agglomerate strength. The samples under different temperatures were air-cooled to room temperature for compressive strength testing and XRD pattern analysis.
SEM morphologies of JL-2 sample in evaporative cooler middle part are shown in Fig. 6. The profile of JL-2 sample in Fig. 6(a) is superimposed and hierarchical. The compact and loose layers of uniform thickness have periodic interphase distribution. This distribution may be related to cyclical production of basic oxygen furnaces.

SEM morphologies of JL-2 dust agglomerate sample. (Online version in color.)
It can be seen from Fig. 6(b) that the substances in each compact layer are closely connected, so there must be a connecting phase which bond the internal substances of compact layer to each other. In terms of macroscopic structure, the high strength of the entire dust agglomerate is inseparable from the connecting phase. Therefore, the connecting phase must be analyzed. As can be seen from a partial enlarged view of the compact layer in Fig. 6(c), the layer is structurally tightness and has blocky crystals distributed.
The SEM-mapping analysis shown in Fig. 7 is performed on the square region. The positions of Na and Cl elements are highly coincident with the position of the blocky crystal, so it can be confirmed that the crystal is NaCl. Combining the energy dispersive spectrometer (EDS) analysis result for the square region in Table 4, and the XRD diffractograms in Fig. 4, it is found that in the square region, NaCl is embedded in Fe oxide, and ZnFe2O4 is dispersed in Fe oxide.
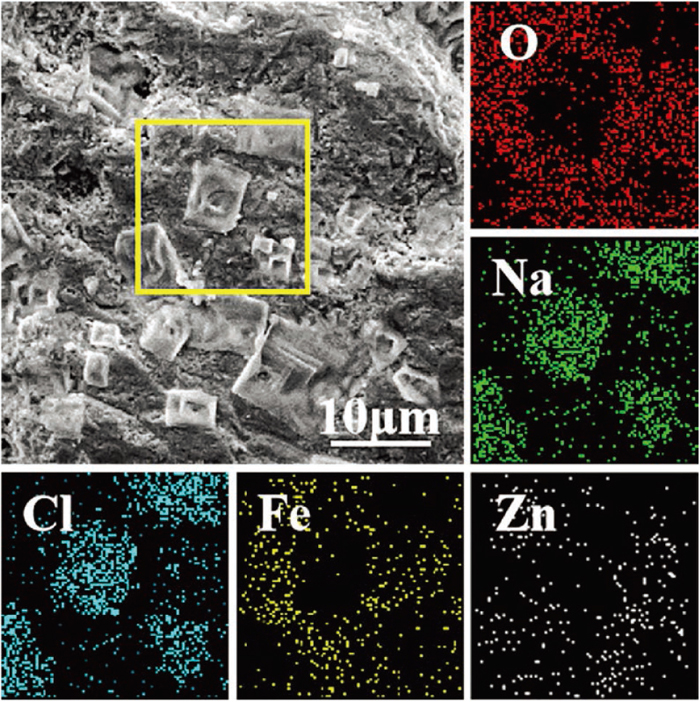
SEM-mapping images of JL-2 dust agglomerate sample. (Online version in color.)
| Component | Fe | Zn | Cl | Na | O |
|---|---|---|---|---|---|
| Mass percent | 38.23 | 13.89 | 14.18 | 11.55 | 22.14 |
The experiment was carried out according the scheme listed in Table 3, and the results are shown in Fig. 8. S0 sample without any added additives has the lowest compressive strength of only 268 N, and the sample strength increases slightly by adding NaCl and KCl (S1, S2, and S3 samples). On the other hand, the compressive strength of S4 and S5 sample containing ZnO is significantly increased, and S5 sample with the addition of NaCl, KCl and ZnO has the highest strength of 706 N.

Sample compressive strength with different raw materials after roasting at 900°C. (Online version in color.)
It can be seen that ZnO has the dominant influence on the sample compressive strength, and the strength of S5 sample is increased by 4 to 5 times relative to S0 sample. Although the samples containing NaCl and KCl have a small increase in strength compared with the sample containing ZnO, but comparing S0, S1, S2 and S3 samples, it can be seen that the strength of S0 sample is 268 N, while that of S3 sample reaches 340 N, the strength is increased by about 30%, which has a certain influence on the dust aggregate strength.
The compressive strength of S5 sample after roasting at different temperatures for 2 h is shown in Fig. 9. One can see that the sample strength increases with rising of temperature, and the growth trend of the strength is steeper between 600 and 700°C.

Relationship between temperature and compressive strength of S5 sample. (Online version in color.)
Furthermore, it can be seen from XRD diffractograms of S5 sample at different temperatures in Fig. 10 that ZnFe2O4 phase exists in the samples with a roasting temperature exceeding 600°C, and the peak of ZnFe2O4 phase increases with rising temperature. The results illustrate the effect of temperature on dust agglomerate phases and strength. The higher the temperature, the more the ZnFe2O4 phase and the higher the strength. The formation of ZnFe2O4 phase is directly related to dust agglomerate strength. Therefore, the formation process of ZnFe2O4 phase deserves further study.

XRD diffractograms of S5 sample at (a) 500°C, (b) 600°C, (c) 700°C, (d) 800°C, (e) 900°C and (f) 1000°C roasting temperatures.
Thermodynamic conditions vary widely in BOF dry flue gas cleaning systems due to changes in temperature and flue constituents (Fig. 11). The temperature in evaporative coolers is generally in the range of 150 to 1000°C, and in this temperature range, Fe and Fe oxides in dust agglomerates can not be sintered to increase the strength.15) Therefore, it is necessary to analyze the influence of the substances containing Zn2+, Na+ and K+ on dust agglomerate strength.

Local temperature and flue constituents of BOF dry flue gas cleaning system. (Online version in color.)
A certain amount of zinc-containing substances were found from the composition and morphologies of dust agglomerates. Meanwhile, in the high-temperature roasting experiment, zinc-containing substances greatly improved the dust agglomerate strength. Therefore, the transformation process of the zinc-containing substances was analyzed.
The composition of BOF flue gas solid wastes contains zinc due to the addition of galvanized steel scrap during steelmaking. Zinc in the galvanized steel scrap is mainly in the form of elemental Zn and ZnO.16,17) ZnO has a very high melting point (1975°C) and boiling point (2360°C), so it exists as a solid in basic oxygen furnace. Since the density of ZnO (5.606 g/cm3) is less than liquid steel (7.0 g/cm3), it will be suspended on the surface of liquid steel, and brought into the slag-metal-gas three-phase emulsified slag via a large amount of CO gas generated by carbon-oxygen reaction. In the emulsified slag, ZnO may react with CO to form elemental Zn. Elemental Zn has a lower melting point (419.53°C) and boiling point (907°C), it will enter the cleaning system with BOF flue gas after vaporization in steelmaking process. The chemical reactions of zinc-containing substances that may occur in BOF dry flue gas cleaning systems are shown in Eqs. (1), (2), (3), (4), (5).
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
In order to determine whether these chemical reactions occurred in the cleaning systems, Factsage 6.4 software was used for thermodynamic analysis. Figure 12 shows the relationship between the Gibbs free energy ΔrGTθ of different reactions and temperatures. It can be seen from Fig. 12 that the Gibbs free energy of Eq. (1) is greater than zero in the temperature range of BOF dry flue gas cleaning system, indicating that ZnO cannot be reduced to elemental Zn by CO. Therefore, ZnO in basic oxygen furnace will remain in the slag, and elemental Zn will enter the cleaning systems as zinc vapor at a high temperature.

Relationship between Gibbs free energy ΔrGTθ and temperature in different chemical reactions of Zn-containing substances in BOF dry flue gas cleaning system. (Online version in color.)
After zinc vapor formed under the high temperature enters the cleaning system with flue gas, and its transformation process is shown in Fig. 13:

Transformation process of Zn-containing substances in BOF dry flue gas cleaning system. (Online version in color.)
i) Gaseous zinc atoms collide and and coagulate due to Brownian motion. Therefore, both gaseous zinc atoms and agglomerated solid zinc particles are present in the zinc vapor.18,19)
ii) From BOF outlet to cooling flue, gaseous zinc atoms react rapidly with O2 to form ZnO (Eq. (2)) under BOF oxygen blowing,20) and ZnO grows by heterogeneous nucleation on the surface of solid zinc particles.21) Because of the compactness of ZnO layer, most of zinc atoms are not completely oxidized and still remain in elementary substance.
iii) After entering the evaporative cooler, the temperature of gaseous zinc atoms drops below the boiling point, molten liquid zinc begins to encapsulate ZnO and solid zinc particles.
iv) The liquid zinc containing ZnO adheres to the surrounding BOF dust particles, causing ZnO to contact with Fe2O3 in BOF dust. It can be seen from the thermodynamic analysis in Fig. 12 that ZnO can react with Fe2O3 to form ZnFe2O4.
From the perspective of dynamics, due to the tiny bonding strength between ZnO and Fe2O3 particles, the particles can only vibrate in a small range at the equilibrium position, so the solid phase reaction rate is tardy, and ZnFe2O4 cannot be formed in a short time. However, under the high temperature, the solid particles acquire the energy necessary to participate in the chemical reaction and then can diffuse to other solid surfaces in contact, resulting in solid phase reaction between ZnO and Fe2O3.
Previous literature22) has indicated that ZnO begins to react with Fe2O3 to form ZnFe2O4 above 600°C, and the reaction rate accelerates with the rising of temperature. The XRD diffractograms in Fig. 10 also demonstrates this conclusion. Since ZnFe2O4 belongs to equiaxed crystal system in lattice structure, the host or support structure is oxygen ion, chemical bonds between the ions are strong, and atomic stacking is tight, so the physical and chemical properties of ZnFe2O4 are extremely stable.23,24) After the formation of ZnFe2O4 with exceedingly high strength, it chemically bonds with the surrounding BOF dust particles to form a dense agglomerate, which increases the dust agglomerate strength.
The unoxidized liquid zinc reacts in CO/CO2 atmosphere of evaporative cooler to form ZnO (Eq. (6)), and then continues to react with the adsorbed Fe2O3 to form ZnFe2O4, thereby further increasing the dust agglomerate strength.
| (6) |
In addition, the presence of ZnFe2O4 in XRD diffractograms (Figs. 4(a) and 4(b)) demonstrates the occurrence of these reactions in the evaporative cooler with high temperature. Nevertheless, ZnO is found in Fig. 4(c), indicating that the temperature in evaporative cooler bottom part is lower, which is not conducive to the formation of ZnFe2O4. Since the liquid zinc adheres to BOF dust particles, the dust particles are enriched on the inner wall of evaporative cooler. Due to the high fluidity of liquid zinc, dust particles are wetted or wrapped after contact with it, and the new phase ZnO formed becomes a binder phase between the dust particles.
It is concluded that due to the formation of ZnFe2O4 binder phase which is extremely stable in physical and chemical properties, dense in atomic stacking, and strong in chemical bonds between ions, the dust agglomerates in the upper and middle high temperature zones of evaporative cooler are high in compactness and is not easy to fall off under gravity. While the dust agglomeration strengthening in the bottom low temperature zone is caused by the formation of the new binder phase ZnO, but ZnO binder phase is different from the chemical adsorption generated by ZnFe2O4 phase, and the agglomerate strength is relatively weak.
3.3.2. Effect of Substances Containing Na+ and K+ on Dust AgglomerationThe main elements of dust agglomerates in Table 1 contain Na, K, and Cl, and NaCl crystals are found from SEM-mapping analysis in Fig. 7. Furthermore, it can be seen from the high temperature roasting experiment shown in Fig. 8 that NaCl and KCl have a certain degree of improvement on dust agglomerate strength.
Both NaCl and KCl are low melting point materials. The melting point and boiling point of NaCl are 801°C and 1412°C, respectively, and KCl is 773°C and 1420°C, respectively. In BOF production process, due to the addition of various excipients, it will contain Na, K, Cl impurities. Under the high temperature, Na, K, and Cl impurities will volatilize in the form of NaCl and KCl gases, and then entering the cleaning system along with the flue gas. After reaching the cooling flue, NaCl and KCl gases are liquefied into suspended droplets, and entrain the surrounding dust particles into the evaporative cooler. Under the thrust of atomized water in the evaporative cooler, NaCl and KCl adhere to the inner wall. Because of the low temperature, the liquid NaCl and KCl solidify into solid phases, and at this temperature, solid state sintering of NaCl and KCl occurs,25) which increases the dust agglomerate strength.
3.3.3. Dust Agglomeration Strengthening MechanismBased on the above investigations, the dust agglomeration strengthening mechanism in the evaporative cooler can be illustrated by Fig. 14. After BOF flue gas solid wastes generated in steelmaking process enter the evaporative cooler, they move to the inner wall of evaporative cooler under the lateral thrust of atomized water. The molten liquid zinc containing ZnO is first adsorbed on the inner wall of evaporative cooler, and ZnO reacts with Fe2O3 in dust particles to form a dense ZnFe2O4 phase. ZnFe2O4 chemically bonds with the surrounding dust particles to form a dense aggregate, which leads to an increase in the agglomerate strength, and the aggregates continue to accumulate to form compacted layers. If low melting point substances such as NaCl and KCl are present in BOF dust, they exist in a solid form under the temperature condition of evaporative cooler, and the dust agglomerate strength is increased by solid state sintering.

Schematic diagram of dust agglomeration strengthening in evaporative coolers. (Online version in color.)
Since BOF production is cyclical, when the new solid wastes enter the evaporative cooler, they will adhere to the original dust agglomerate layers. However, the surface of original layers are rough, and the temperature and composition of new and old layers are also different, resulting in the generation of loose layers. New compacted layers are formed on the loose layers, thereby circulating and the dust agglomerate layers are continuously thickened.
3.4. Control of Dust Agglomeration StrengtheningVisible from the above analysis, the main reason for dust agglomeration strengthening in evaporative coolers is that ZnO and Fe2O3 form a dense ZnFe2O4 binder phase through solid phase reaction. Therefore, controlling the formation of ZnFe2O4 is the key to avoiding dust agglomeration strengthening.
The initial solid phase reaction temperature of ZnO and Fe2O3 to form ZnFe2O4 is 600°C, and the reaction rate increases with the rising of temperature, while the temperature of BOF flue gas solid wastes can reach 800–1000°C when entering evaporative coolers. Therefore, the temperature in evaporative coolers can be lowered without affecting the flue gas cleaning effect, thereby reducing the solid phase reaction rate, decreasing the generation of ZnFe2O4, making it impossible to consolidate with BOF dust particles, and avoiding dust agglomeration strengthening.
Furthermore, solid NaCl and KCl will sinter in evaporative coolers. The solid state sintering of NaCl and KCl is mainly carried out by evaporation-viscoaggregation,26,27) and the sintering kinetics formula can be expressed as:
| (7) |
It can be seen from Eq. (7) that as the sintering temperature T elevates, the surface vapor pressure ρ0 will increase to promote sintering. Therefore, the sintering strength of NaCl and KCl can be lowered by keeping down the temperature in evaporative coolers. In addition, it is necessary to strictly control the content of Na+ and K+ contained in basic oxygen furnaces, and to reduce the formation of low melting point substances from the source.
The research carried out experiments using the dry flue gas cleaning system of 150 t BOF in Shanghai Meishan to reduce the inlet temperature of evaporative cooler to less than 850°C, and control Na+ and K+ content in the early stage of steelmaking to be less than 0.5%, respectively. Through a whole year of production application, the dust agglomerates in the evaporative cooler were significant reduced, and the cleaning system was not affected by dust agglomeration strengthening.
Consequently, the control methods proposed based on the dust agglomeration strengthening are validated to be effective. The methods ensure the cleaner production in steelmaking process and can be used for long-term industrial applications.
This paper presents the characteristics of zinc-containing dust agglomerates in the evaporative cooler of BOF flue gas cleaning system agglomerates and the strengthening mechanism, as well as proposes effective control methods. From the results obtained in this paper the following conclusions can be drawn:
• The high strength dust agglomerates in the evaporative cooler of BOF dry flue gas cleaning system consist of compact and loose layers, and the compact layers contain ZnFe2O4, NaCl and KCl.
• After ZnO reacts with Fe2O3 at a high temperature above 600°C to form tightly ZnFe2O4 phase, chemical adsorption of ZnFe2O4 with BOF dust particles is the primary cause of dust agglomeration strengthening in evaporative coolers. Solid state sintering of low melting point substances containing Na+ and K+ promotes dust agglomeration strengthening.
• Decreasing the temperature in evaporative coolers, reducing the amount of ZnFe2O4 produced, and controlling the content of low melting point substances containing Na+ and K+ in BOF flue gas solid wastes can effectively improve dust agglomeration in evaporative coolers, optimize the environment of cleaning system, and ensure the cleaner production in steelmaking process.
The authors wish to thank National Natural Science Foundation of China (Grant No. 51574050) for supporting this research. The authors wish to express their gratitude to the industrial experimental site provided by Shanghai Meishan Iron and Steel Co., Ltd., the technical assistance provided by Jianguo Hong and Wenhua Yan, and the workers involved in industrial experiments.