2021 Volume 61 Issue 5 Pages 1439-1449
2021 Volume 61 Issue 5 Pages 1439-1449
An excess amount of oxygen originating from hydrogen production is likely to be available as part of the HYBRIT (Hydrogen Breakthrough Ironmaking Technology) initiative, aimed at producing fossil-free steel by replacing coking coal with hydrogen. Oxygen enrichment during magnetite pellet induration can lead to reduced fuel amounts and increased productivity. Induration of magnetite iron ore pellets liberates considerable amounts of heat when magnetite is oxidised to hematite. Elevated oxygen levels in the process gas are expected to promote the oxidation reaction, resulting in increased process efficiency. However, more information is required to enable the transition towards a higher oxygen level process and improved production rate, while maintaining the metallurgical properties of the pellet bed. In this study, interrupted pot furnace experiments were conducted on a magnetite pellet bed (approximately 100 kg) at Luossavaara-Kiirunavaara Aktiebolag to investigate the effect of oxygen levels at approximately 6%, 13%, and 30% O2. Temperature profiles are measured and pellet properties (compression strength, porosity, oxidation degree, microstructures) are analysed at different bed heights. The higher oxygen level (approximately 30% O2) intensifies the oxidation reaction, resulting in increased temperature, oxidation rate and compression strength across the vertical bed height. Three different pellet oxidation profiles are identified, namely, homogenous oxidation across the pellet, complete oxidation of the pellet shell and an unreacted core with a sharp/distinct interface, and partial oxidation of the pellet shell and an unreacted core. A higher oxygen level results in an increased oxidation rate, while the temperature controls the pellet oxidation profile.
Owing to the current transition of Swedish steel production to a hydrogen-based system, led by Hydrogen Breakthrough Ironmaking Technology (HYBRIT), the joint venture between Luossavaara-Kiirunavaara Aktiebolag (LKAB), SSAB and Vattenfall, which aims to replace coking coal with hydrogen, an excess amount of oxygen originating from hydrogen production is likely to be available.1) The excess oxygen could potentially be used for iron ore pellet induration, and thereby improve the process efficiency in terms of fuel consumption and productivity. One of the challenges along this path will be to maintain product quality. The main raw material used in Scandinavian steel production is iron ore pellets. In Sweden, the mining company LKAB produce both blast furnace and direct reduction pellets. Two main methods for pellet production are used, namely, the straight-grate and the grate-kiln processes. In both cases, a large pellet bed is transported through different zones while being exposed to an atmosphere with varying temperatures (room temperature to 1300°C) and a varied oxygen level (approximately 16% to 18% O2 in the pre-heating zone2)). When being transported, the pellets are dried, oxidised and sintered, which is known as induration. Oxidising magnetite ore to hematite3) releases considerable amounts of heat. Promoting this exothermic reaction by increasing the oxygen level, rather than by fuel, would contribute to reducing greenhouse gas emissions from the industry, which is in line with the Paris Climate Agreement signed by the Government of Sweden in 2015.4,5)
The oxidation reaction (Eq. (1)) is highly exothermic,3) releasing approximately 498 kJ/kg of oxidised magnetite,6) and is favoured by an increased temperature and oxygen partial pressure. The surplus energy originating from the exothermic reaction can be utilised in the process and, according to Forsmo,7) accounts for more than two thirds of the total energy demand of the LKAB induration process.
| (1) |
Oxidation of a magnetite pellet, like most gas-solid reactions of porous solids, occurs through the following steps:8,9)
i) Transfer of oxygen molecules from the bulk gas to the pellet surface,
ii) Diffusion of oxygen molecules through the macropores within the pellet, and
iii) Chemical reaction at the surface of the particles, including oxygen adsorption, followed by a transfer of the product by solid-state diffusion.
The oxidation of magnetite pellets and particles has been extensively investigated since the start of the commercial production of iron ore pellets,3,10,11,12,13,14,15,16,17,18,19,20,21,22) and models have been developed and used to describe how oxidation occurs at the single pellet and particle scale.8,15,21,23,24,25) The grain model8) appropriately describes oxidation at the single pellet scale. This model describes the gas-solid reactions of porous solids by combining numerous previously existing models into one general model. The model depicts a pellet as individual particles surrounded by pores (macropores). Gas diffuses via these pores to reach the surface of each particle and the reaction starts. It is assumed that the individual particles are non-porous and that the reaction on a particle scale occurs according to the shrinking core model, also known as the ‘sharp-interface model’.8,23) The reaction at the pellet scale (in this case oxidation) can be described as the result of three different regimes:
i) When solid-state diffusion controls the overall reaction: this leads to the homogenous oxidation of the entire pellet, as pore diffusion is not rate limiting.
ii) When pore diffusion controls the overall reaction: this leads to complete oxidation of the pellet shell, forming a sharp reaction interface oxidation front and an unreacted core, that is, oxidation as described by the shrinking core model.
iii) When both solid-state diffusion and pore diffusion progress at a comparable rate (mixed-control): this leads to a pellet with completely oxidised particles at the pellet shell, partially oxidised particles in the intermediate section and an unreacted core.
Several process and material parameters influence the oxidation process and, consequently, the pellet properties. It is known that early and homogenous oxidation prior to sintering is advantageous because it increases pellet strength,11) and the oxygen level (pO2) is expected to influence the oxidation kinetics.3,13,15,25) Researchers have investigated the effect of the two major parameters, namely, oxygen level and temperature, on single pellets at isothermal conditions. Edström13) and Papanastassiou and Bitsianes15) concluded that the oxidation rate increases significantly by increasing the oxygen levels at higher temperatures (at 1000°C and 1230°C, respectively). Microscopic investigation of pellets oxidised at temperatures > 1000°C indicate that oxidation of single pellets follows the shrinking core mechanism, that is, the formation of a sharp reaction interface between the oxidised shell and the unreacted magnetite pellet core.15) Whereas, at lower temperatures (600°C and 800°C) and 16%–21% O2, the pellet is more homogenously oxidised although the larger particles are not completely oxidised.2,10,21) Cooke and Ban10) performed isothermal tests for 30 min in air at 100–1200°C, and observed dense magnetite cores in the pellets at 1100°C and 1200°C, which they believed hindered the oxidation rate at these temperatures. Ilmoni and Uggla12) instead emphasised the effect of time duration at each temperature on the oxidation rate.
Pape et al.16) reported that the residence time of the bed in the pre-heating zone is too short for sufficient oxidation. Haas et al.18) then emphasised the importance of identifying the local conditions of the induration processes where the injection of a gas with an increased oxygen level would most contribute to improved production rates. They performed mini-pot experiments, with 2 kg of pellets per bed, simulating the effect of varied oxygen levels (6%, 15%, and 30% O2) on commercial induration. One of their observations from the 30% O2 tests was the effective enhancement of oxidation at approximately 1000°C.
Scale is the key to investigate induration in realistic non-isothermal conditions. The current knowledge on the effect of different oxygen levels on oxidation is based on small-scale experiments. The effect of varied oxygen levels on a large bed scale, such as the resulting oxidation gradient and trends on exothermic heat increase, is thus unknown and well-researched oxidation mechanisms remains to be established from a bed perspective. The aim of this study is to
i) investigate and identify the oxidation mechanisms along a large magnetite pellet bed at various oxygen levels and temperature profiles, and
ii) determine the influence of oxygen partial pressure and temperature at comparable local thermal histories in the bed and across experiments with varying conditions.
We examine magnetite pellets produced at pilot scale at LKAB using an interrupted pot furnace experimental method at an approximate scale of 100 kg of pellets per bed.
We examined the influence of varied oxygen levels on the oxidation of a magnetite pellet bed during induration through interrupted pot furnace experiments. Magnetite concentrate was obtained from LKAB, and Table 1 shows particle size data. The concentrate was mixed with additives (dolomite, limestone and quartzite) and bentonite (as a binder, 0.6 mass%) in a Eirich R09/T pilot scale mixer with a capacity of 240 kg. Green pellets were produced from approximately 200 kg of iron ore concentrate in a batch process pilot-sized disc pelletiser. The desired fraction (10–12.5 mm) of green pellets was continuously collected from roller-screens. The green pellets contained a final moisture content of 8.5 mass%, an average chemical composition of 70.1 mass% Fe (22.1% Fe2+), 0.74 mass% SiO2, 0.91 mass% CaO and 0.67 mass% MgO. The green pellet porosity was on average, considering all experiments, 30.2%. Propane combustion provided the heat of the ingoing gas during the induration, and the oxygen level was determined by the selected amounts of ingoing air, as well as nitrogen or oxygen. Before each experiment, the physical properties of the green pellets (moisture, drop test, compression strength and pellet size distribution) were measured to ensure the desired quality, then a bed of approximately 100 kg green pellets was loaded in the pot.
| Particle size data | ||
|---|---|---|
| < 45 μm | < 63 μm | < 90 μm |
| % | % | % |
| 69.4 | 79.1 | 89.3 |
The total bed height was approximately 45 cm, and thermocouples installed at six different bed heights, as depicted in Fig. 1, continuously measured the temperatures across the vertical direction of the bed. Each thermocouple measured the temperature 5 cm from the furnace wall. We refer to the temperature profile as the measured temperature as a function of time. Figure 1 depicts the positions of the installed thermocouples as a percentage of the total bed height. Thermocouples A, B, C, D, E, and F refer to the measured temperature profiles at 100%, 76%, 51%, 27%, 15%, and 5% of the bed height, respectively. The thermocouple F was complemented with a second thermocouple (F1) at the same bed height, to measure the temperature at the centre of the bed. The hearth layer was at 0% of the bed height (Fig. 1). The three zones of drying and pre-heating (zone 1), varied oxygen level (zone 2) and cooling (zone 3) describes the induration of each experiment.

Schematic illustration of the pot furnace and position of thermocouples A, B, C, D, E, and F as a percentage of total bed height. (Online version in color.)
In zone 1, the bed was first dried (up draft, UD) with air, until thermocouple A reached 150°C, then the bed was further dried (down draft, DD) for 120 s by air at 180°C. The combination of UD and DD was chosen to optimise the drying and avoid condensation at lower bed heights. After drying, the bed was pre-heated (DD) gradually from 500°C to 800°C for 240 s, and then the ingoing gas temperature was held at 800°C (DD) for 300 s. During pre-heating, the ingoing gas oxygen level was kept as low as possible (approximately 3%). After zone 1, the temperature measured at the top of the bed was approximately 800°C (at 100% bed height), whereas the corresponding temperature at the bottom of the bed was approximately 170°C (at 5% bed height). A separate pre-experiment determined the oxidation degree and moisture at the end of zone 1. Table 2 summarises the initial conditions before the start of zone 2 (example data from the pre-experiment) and shows the oxidation degree gradient in the bed. The pellets at the bottom of the bed exhibited properties similar to green pellets, whereas the pellets at the top of the bed had begun to oxidise (approximately 28% and 14% oxidation degree at thermocouple A and B, respectively).
| Initial conditions | Bed height | Temperature | Degree of oxidation | Moisture |
|---|---|---|---|---|
| Thermocouple | % | °C | % | wt.% |
| A | 100 | 800 | 28 | 0 |
| B | 76 | 610 | 14 | 0 |
| C | 51 | 375 | 4 | 0 |
| D | 27 | 205 | 1 | 0 |
| E | 15 | 190 | Not applicable | 0 |
| F | 5 | 170 | Not applicable | 0 |
| Hearth layer | 0 |
Gas with a predefined oxygen level and constant temperature was introduced (DD) in zone 2 for 725 s at a constant gas flow of 250 kg/h. After zone 2, the experiment was interrupted by cooling (UD) in zone 3 with nitrogen gas until thermocouple A reached 200°C, and then cooling continued (UD) with air. The gas direction (UD) during cooling was chosen to preserve the conditions at lower bed heights to the greatest extent by avoiding the additional heat transfer from upper bed heights associated with using down draft direction. Three separate experiments were conducted where the gas temperature was set to 800°C and the oxygen level to approximately 6%, 13% and 30% O2, respectively. Whereas, one experiment was performed at gas temperature of 1100°C and an oxygen level of approximately 6% O2. Each experiment was replicated once or twice, that is, one replicate for the oxygen level at approximately 6% and 13% O2, respectively, and two replicates at approximately 30% O2. Figure 2 illustrates the ingoing gas temperatures during the experiments, and Table 3 shows an overview of the experiments. The three oxygen levels were chosen based on the current design specifications of the equipment, which allowed for approximately 6% and 30% O2 as the lower and higher level, respectively, when the ingoing gas flow rate is at 250 kg/h. Reported oxygen levels during pre-heating in commercial induration processes (approximately 16–18% O2)2) motivated the choice of the oxygen level of approximately 13% O2, the slightly lower level was preferred because it allowed for the investigation of a greater variation.

Overview of the ingoing gas temperature during the experiments. Zone 1: 240 s with an ingoing gas at 500–800°C (DD) and then 300 s at 800°C (DD). Zone 2: varied oxygen level for 725 s and ingoing gas temperature at 800°C or 1100°C (DD). Zone 3: cooling (UD). (Online version in color.)
| Overview of experiments | |||
|---|---|---|---|
| No. of experiments | Oxygen level of the ingoing gas (DD) | Temperature of the ing. gas (DD) | Gas flow rate of the ing. gas (DD) |
| % O2 | °C | kg/h | |
| 2 | ≈ 6 | 800 | 250 |
| 2 | ≈ 13 | 800 | 250 |
| 3 | ≈ 30 | 800 | 250 |
| 3 | ≈ 6 | 1100 | 250 |
Figure 3 shows the measured temperature profile (X-Y plot) at 27% of the bed height (thermocouple D) in the pellet bed from the experiment with an ingoing gas at 800°C and approximately 30% O2 in zone 2. Temperature profiles and a selection of parameters are used to describe the local thermal histories at different bed heights, and Fig. 3 and Table 4 provides an example. MaxT refers to the maximum measured temperature in zone 2, dT/dt400-MaxT refers to the heating rate from 400°C to MaxT in zone 2 and the time duration (Δt1000°C, Δt1100°C) refers to the approximate time that the measured temperatures were at or above either 1000°C or 1100°C (zone 2 and zone 3).
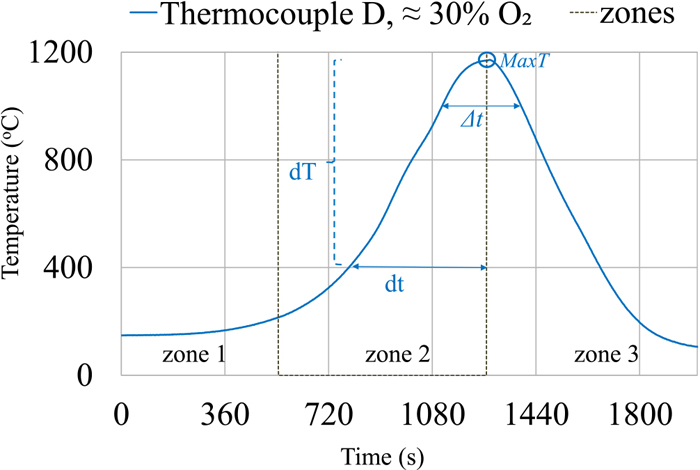
Example of a temperature profile in a pellet bed (at 27% height) from experiment with an ingoing gas at 800°C and approximately 30% O2. (Online version in color.)
| Parameters (average data) describing thermal history as function of bed height | |||||
|---|---|---|---|---|---|
| Bed height | Initial Temp. | MaxT | dT/dt400-MaxT | Δt1000°C | Δt1100°C |
| °C | °C | °C/min | min | min | |
| 27% (D) | 205 | 1180 | 100 | 5 | 3 |
Batches of pellet samples (approximately 4 kg pellets) were collected at the same bed heights as the installed thermocouples (A–F). To obtain representative sub-samples, the samples were manually sieved to 10.0–12.5 mm according to ISO standard 4700: 201526) and then divided using a riffle-splitter. Pellet samples for microscopic investigation were collected randomly from one of the sub-samples. Due to sampling errors, only one data point at 51% bed height from the experiment with approximately 13% O2 in zone 2 was obtained. Wet-chemical titration with a K2Cr2O7 standard solution determined the concentration of ferrous ion (Fe2+), and using Eq. (2), the oxidation degree was calculated based on the analysed Fe2+ for the green and indurated pellets from each experiment. The cold compression strength (CCS) was measured in accordance with ISO 4700: 2015,26) however, when the CCS was below 100 daN complementary measurements using the LKAB pellet multi-press (PMP) instrument27) were performed. Pycnometry, using an Accupyc II 1340 and a GeoPyc 1360 instrument (Micromeritics Inc., USA), determined the porosities of green and indurated pellets.
| (2) |
Manually grinding each pellet to the pellet centre prepared the pellet samples for microscopic investigation. The pellet halves were then mounted in epoxy resin, and then vacuum impregnated. After hardening, the samples were ground and then polished down to a 1 μm fineness, using alcohol-based diamond suspensions. All steps of the sample preparation were conducted using alcohol. A light optical microscope, Leica (DM 6000M), equipped with a Leica camera (DFC 295) and a motorised movable stage enabled the pellet microstructural examination. Using the mosaic feature of the image analysis software, Leica Application Suite LAS V4.5.0 Multistep, enabled the capture of images of the entire pellet cross section at a magnification of ×100. These individual images with a size of 2048 × 1536 pixels were collected automatically and stitched together producing a mosaic picture of approximately 20000 × 20000 pixels (14.3 × 14.3 mm). Additionally, we manually collected individual images at a larger magnification (×200).
When the oxidising gas comes into contact with the bed, the magnetite pellets begin to oxidise and exothermic heat is released. When the gas is introduced continuously from above (DD), the pellets at the top of the pellet bed maintain a similar temperature to the ingoing gas. The continuous gas flow transports the heat generated by oxidation at the top to the lower levels of the bed. Therefore, the pellet layers on the bottom experience a significantly warmer gas than the top layer; this creates different conditions for oxidation. Oxidation of the subsequent layers thus generates more heat, which transports further down the bed and so on. The varying temperature of the gas flowing through the bed, in combination with a varied initial pellet temperature, generates different temperature profiles in the bed. As a result, oxidation of the pellets at different bed heights progresses differently. Figure 4 shows simplified illustrations of the resulting oxidation degree throughout the pellet beds from the experiments with gas at 800°C and O2 levels of approximately 6%, 13% and 30%, whereas, Table 5 lists the corresponding oxidation degree at each bed height (A–F). The light grey and black colours represent hematite and magnetite, respectively. Table 6 shows the achieved maximum temperature (MaxT) and heating rate (dT/dt400-MaxT) for each bed height. As expected, the oxygen level at approximately 30% O2 generates the bed with the highest oxidation degree, whereas the lowest is generated at approximately 6% O2. As the representative pellet cross sections in Table 5 shows, non-oxidised pellet cores increase closer to the bottom of each pot. In the experiment with an ingoing gas at 800°C and approximately 6% O2, the highest oxidation degree is found at the top of the bed (at 100% of the bed height). Whereas, the corresponding values for oxygen levels at approximately 13% and 30% O2 are found at 76% and at 51% of the bed height, respectively. In the bed from the experiment with approximately 30% O2, the macrostructures of pellets from the top of the bed (at 100% bed height) indicate complete oxidation; however, the oxidation degree is less than at lower bed heights (Table 5). Unlike at the top of the bed, more of the exothermic heat accumulates in the middle and lower parts of the bed, meaning that these pellets experience higher temperatures (> 1100°C) in combination with the higher oxygen level of approximately 30% O2 (Table 6).

Schematic illustration of pellet beds from the experiments with the ingoing gas at 800°C and different oxygen levels in zone 2. The light grey and black colours represent hematite and unoxidised magnetite, respectively. This illustration does not describe the real dimensions of the pellets or the pot furnace.
| Parameters (average data) describing thermal history as function of bed height | ||||||||
|---|---|---|---|---|---|---|---|---|
| % O2 of the ing. gas in zone 2 | ≈ 6% O2 | ≈ 13% O2 | ≈ 30% O2 | ≈ 6% O2 | ||||
| Temp. of the ing. gas in zone 2 | 800°C | 800°C | 800°C | 1100°C | ||||
| Bed height | MaxT | dT/dt400-MaxT | MaxT | dT/dt400-MaxT | MaxT | dT/dt400-MaxT | MaxT | dT/dt400-MaxT |
| % | °C | °C/min | °C | °C/min | °C | °C/min | °C | °C/min |
| 100 (A) | 812 | Not applicable | 819 | Not applicable | 841 | Not applicable | 1121 | Not applicable |
| 76 (B) | 916 | Not applicable | 964 | Not applicable | 1053 | Not applicable | 1191 | Not applicable |
| 51 (C) | 969 | 50 | 1057 | 61 | 1140 | 93 | 1199 | 70 |
| 27 (D) | 822 | 58 | 977 | 78 | 1180 | 100 | 1060 | 76 |
| 15 (E) | 758 | 59 | 862 | 75 | 1154 | 117 | 855 | 69 |
| 5 (F) | 697 | 58 | 816 | 80 | 1115 | 128 | 730 | 65 |
Figure 5 illustrates the effect of increasing the ingoing gas temperature, as opposed to increasing the oxygen level, on the bed oxidation profile. The figure shows the resulting oxidation degree as a function of the bed height when the ingoing gas temperature is either 1100°C or 800°C and the oxygen level is approximately 6% O2. The oxidation degree increases at the top of the bed resulting from the higher gas temperature, whereas at 5–76% of the bed height, the oxidation degree is similar regardless of the variation of the ingoing gas temperature. As shown in Table 6, the pellets at 15–100% of the bed height reaches higher maximum temperatures when the ingoing gas temperature is higher, while the thermal history at 5% bed height is comparable for both of the ingoing gas temperatures. At 15–27% of the bed height, the heating rates in the bed exposed to the higher gas temperature and approximate 6% O2 level are comparable to the heating rates at the same bed heights as when the ingoing gas is at 800°C and approximately 13% O2 (Table 6).

Oxidation degree as a function of the bed height when the ingoing gas temperature is either 1100°C or 800°C and the oxygen level is approximately 6% O2 in zone 2. (Online version in color.)
The microstructural investigation of individual pellets from different bed heights shows that the oxidation, in terms of degree and mechanisms, varies significantly from one pellet layer (bed height) to another. Based solely on the oxidation profiles, all the obtained microstructures are categorised into three types:
3.2.1. Type 1The macrostructures of Type 1 pellets indicate complete and homogenous oxidation. Although the microstructures confirm the homogenous oxidation, it also shows that particles larger than approximately 20 μm are only partially oxidised (Fig. 6). Type 1 pellets are found at the top of the bed from the experiment with an ingoing gas at 800°C and approximately 30% O2. Table 6 lists the thermal history of typical Type 1 pellets (see layer A, at 100% bed height, from experiments with an ingoing gas at 800°C and approximately 30% O2 in zone 2).

Macro- and microstructure of reference pellets categorised as Type 1. The images at ×200 corresponds to the white markings in the pellet cross section image. Oxidised particles appear light grey, while unoxidised magnetite appear dark in the microstructure images.
The macrostructures of Type 2 pellets shows unreacted magnetite cores (Fig. 7). The microstructures reveal an oxide layer (hematite) consisting of completely oxidised particles, and shows that the oxidation front has grown topo-chemically from the shell into the pellet core, with a sharp/distinct interface at the reaction front. The microstructures also show bridges between particles throughout the pellet cross section. Type 2 pellets are found in the middle or lower part of a pellet bed, where the measured maximum temperature (MaxT) is above approximately 1150°C, for example, in the bed from the experiment with the ingoing gas at 800°C and approximately 30% O2 at 27% of the bed height (Fig. 7). Table 6 lists the thermal history of typical Type 2 pellets (see layer D, at 27% bed height, from experiments with an ingoing gas at 800°C and approximately 30% O2 in zone 2).

Macro- and microstructures of reference pellets categorised as Type 2. The images at ×200 corresponds to the white markings in the pellet cross section image. Oxidised particles appear light grey, while unoxidised magnetite appear dark in the microstructure images.
The macro- and microstructures of Type 3 pellets is a combination of Type 1 and Type 2. Macroscale visual observations show an oxidised pellet shell combined with an unreacted magnetite core. The microstructure reveals that the particles at the shell are only partially oxidised. There is a variation in the appearance of the microstructures within this group, resulting from how far the reaction front has progressed on a macro- and microscopic scale. Figure 8 depicts the oxidation variation at an earlier and a later stage. In the earlier stage of oxidation, the particles larger than approximately 20 μm are only partially oxidised and in the later stage of oxidation, the particles larger than approximately 100 μm are only partially oxidised. The oxidation front in the former case has not progressed as far as in the latter case (Fig. 8). Type 3 pellets are observed in all bed heights where the measured maximum temperature (MaxT) is below approximately 1150°C, including at the top layer (at 100% bed height) of the bed from the experiment with an ingoing gas at 800°C and oxygen levels at approximately 6% or 13% O2. Table 6 lists examples of thermal histories of the typical Type 3 pellets, see layer E, at 15% bed height, from experiments with an ingoing gas at 800°C and approximately 13% or 30% O2 in zone 2.

Macro- and microstructures of reference pellets categorised as Type 3. The images at ×200 corresponds to the white markings in the pellet cross section image. Oxidised particles appear light grey, while unoxidised magnetite appear dark in the microstructure images.
The current experimental setup enables the possibility of viewing each bed height (A–F) with its local conditions as a separate experiment, and thus enables the comparison of these pellet layers between themselves. This approach allows the influence of temperature and oxidation degree on pellet quality to be delineated, specifically, the oxidation degree and the CCS in an early stage of the pellet induration resulting from the interrupted experiments. The two comparisons below exemplify and illustrate this dependency:
3.3.1. Pellet Oxidation at Different Thermal Histories and Similar Oxygen LevelsThe first comparison concerns pellets with different thermal histories (MaxT, heating rates and Δt1100°C), but a similar oxygen level (approximately 30% O2). In this case, the pellets (C-0.30 and D-0.30) originate from the same bed (ingoing gas at 800°C in zone 2). C-0.30 pellets refers to pellets from 51% of the bed height with an initial temperature of approximately 375°C, whereas D-0.30 pellets refers to pellets from 27% bed height with an initial temperature of approximately 205°C. Figure 9 depicts the temperature profiles of the C-0.30 and D-0.30 pellets, and Table 7 summarises the data describing the thermal histories and pellet properties (oxidation degree, cold compression strength and porosity). The D-0.30 pellets experience a higher maximum temperature and a slightly faster heating rate than the C-0.30 pellets. The microscopic investigation shows complete oxidation of C-0.30 pellets, and that of the D-0.30 pellets shows Type 2 microstructures. The oxidation degree is similar for both the C-0.30 and D-0.30 pellets, and the D-0.30 pellets exhibit higher average cold compression strength.

Representative temperature profiles (X-Y plot) of C-0.30 (pellets at 51% bed height exposed to approximately 30% O2 in zone 2) and D-0.30 (pellets at 27% bed height exposed to approximately 30% O2 in zone 2).
The second comparison concerns pellets with comparable thermal histories (MaxT and Δt1100°C), but different oxygen levels (approximately 6% and 30% O2). The previous section describes the D-0.30 pellets. The other pellets, C-0.06, refers to pellets at 51% of the bed height from a bed exposed to a gas at 1100°C and approximately 6% O2. The initial temperature of the C-0.06 pellets is approximately 375°C. Figure 10 depicts the temperature profiles of the C-0.06 and D-0.30 pellets, and Table 7 summarises the data describing the thermal histories and pellet properties. The microscopic investigation shows that both the C-0.06 and D-0.30 pellets have Type 2 microstructures, regardless of the oxygen level variation of the ingoing gas, and despite the higher initial temperature of the C-0.06 pellets. For the D-0.30 pellets, the oxidation front has progressed further, the oxidation degree is higher and the average cold compression strength is also higher. In the current experiments, this is true for all pellets with comparable thermal histories and different oxygen levels.

Representative temperature profiles (X-Y plot) of C-0.06 (pellets at 51% bed height exposed to approximately 6% O2 in zone 2) and D-0.30 (pellets at 27% bed height exposed to approximately 30% O2 in zone 2).
One common trend for all pellet beds from the current experiments is the variation of local conditions (thermal histories), leading to corresponding variations in the rate-controlling steps and oxidation mechanisms. As previously described, the pellets are categorised into three groups, according to the obtained macro- and microstructure images (Type 1, 2 and 3). Generally, the resulting types are consistent with the oxidation mechanisms described by the grain model by Szekely et al.8) and confirms the observations from single pellet oxidation experiments performed by other researchers.2,15,21) Figure 11 shows schematic representations of Type I, II and III oxidation mechanisms. The oxidation profile corresponding to homogeneous oxidation through the entire pellet, Type I, is likely to occur in pellets exposed to relatively low temperatures at the top of the bed and with a higher oxygen level. Under these conditions, solid-state diffusion is plausibly the rate-controlling step and increasing the temperature would significantly promote oxidation. Further down in the bed, Type II oxidation emerges when the temperature is high enough (above approximately 1150°C), and neither the chemical reaction at the interface nor solid-state diffusion are the rate controlling steps. Instead, oxidation is controlled by the available oxygen at the oxidation front (that is, gas pore diffusion), and would thus improve from an increased oxygen level. The third case, Type III oxidation occurs in situations where the rate of oxygen pore diffusion and solid-state diffusion are comparable. When oxidation is a mixed rate-controlled process, oxidation would be improved by increasing the temperature and/or the oxygen level. The oxygen level of the ingoing gas influences the oxidation profile throughout the bed. The increased oxygen level not only implies an increase in available oxygen, but it also means that the release and accumulation of exothermic heat at a certain bed height determines the thermal history of those pellets. The more efficient heat transportation to the lower parts of the bed, resulting from the higher oxygen level, indicates the potential for more effective oxidation throughout the bed within a shorter period of time. The faster bed scale oxidation confirms the potential to increase the production rate by increasing the oxygen level, which is in line with the previous findings from small-scale (2 kg pot) experiments by Haas et al.18) The scale of the current experiments (approximately 100 kg pellets) allows us to observe the significant heat increase and faster oxidation that occurs at the lower bed heights because of the higher oxygen level. The variation in oxidation degree and mechanisms along the vertical direction of the bed, due to the varying local conditions, is another outcome from the results of the current experiments. To further evaluate the effect of varied oxygen levels on large bed scale, higher oxygen levels (>30% O2) and its influence on the resulting bed temperature profiles is of high interest to investigate in the future.

Schematic sketch of the categorised types of oxidation profiles. The white and black colours represent hematite and unoxidised magnetite, respectively. This illustration does not describe the real particle geometry or particle size distribution.
To exemplify the type of oxidation profiles that occur when the oxidation is not the main source of heat, the comparison is extended to include a bed at a higher ingoing gas temperature (1100°C) and a low oxygen level (approximately 6% O2). When increasing the gas temperature alone, the oxidation degree at the top of the bed increases, whereas the higher gas temperature does not affect the oxidation degree significantly at the lower bed heights. In the durations of the current experiments, only half of the bed reaches the temperature of the ingoing gas. One reason for this could be that the amount of heat associated with the gas temperature is lower than the amount of theoretical heat release from the oxidation reaction. As the experimental setup allows for efficient heat transfer, most of the available heat associated with the ingoing down draft gas is probably transferred to the upper parts of the bed. Other than for comparative purposes, there is no benefit from oxidation at higher gas temperatures (≥ 1100°C), in combination with a lower oxygen level, as it involves the risk of increasing the temperature in the upper part of the bed for an extended amount of time without sufficient access to oxygen.
In addition to the influence of oxygen level on the oxidation profiles, which is the primary focus in this study, this work also provides insight into the influence on pellet strength (CCS) during the initial stages of pellet induration. The thermal histories of pellets at comparable oxidation degrees affects their strength. The influence of increased temperature and duration at higher temperature on strength is expected and related to sintering that becomes significant at 1200°C.7) In the current experiments, in earlier stages of the induration, when pellets experience comparable thermal histories (MaxT and Δt1100°C) but different oxygen levels, higher strength (CCS) is correlated to higher oxidation degree. The comparison of the C-0.06 and D-0.30 pellets (Fig. 10 and Table 7) exemplifies this trend. Bridging between particles during oxidation is known to influence pellet quality at lower temperatures,11,28) and this study highlights the extended importance of oxidation at higher temperatures. The complete correlation between temperature, oxidation and strength at varied oxygen levels is complex and remains to be understood in detail.
The variation of microstructures in a bed exposed to a higher oxygen level (approximately 30% O2), shows that pellets at the bottom of a bed oxidise according to different mechanisms than pellets at the top of the same bed. The difference in oxidation mechanisms is mainly due to the variation in temperature, whereas the oxygen level affects the oxidation rate. Oxidation at the top of the bed is promoted by an increase in temperature or possibly by reducing the flow rate of the ingoing gas. In contrast, higher oxygen levels promote the oxidation of pellets at the lower bed heights. It is likely that a lower gas flow rate would generate a bed with a higher oxidation degree in the upper part of the bed, as it would allow a greater accumulation of heat in this part of the bed than in the present case. Similarly, increasing the ingoing gas flow rate would likely transport the exothermic heat further down into the bed. Another variant would be to increase the ingoing gas temperature and oxygen level simultaneously, which could significantly improve the pellet strength. However, we recommend caution in this case because substantially high temperatures could lead to sintering before complete oxidation, resulting in inferior pellet quality. In addition, the oxidation rate is also highly dependent on particle size distribution. Smaller particles will oxidise faster8,29,30) and the distribution will affect particle packing within the pellet and hence porosity.15) Thus, particle size distribution is another relevant parameter to consider in future studies. From a bed scale perspective, besides material properties, the choice of increased oxygen level in combination with the ingoing gas temperature and flow rate will always constitute a balancing act, and the findings from this study can serve as a starting point.
In this study, we investigate the influence of varied oxygen levels (approximately 6%, 13% and 30% O2) and ingoing gas temperature (800°C and 1100°C) on the induration of a magnetite pellet bed in a pot furnace. Experiments with the specific material and experimental settings described in this article resulted in the observation of the following trends.
• Increasing the oxygen level results in a higher oxidation degree of the entire bed compared to beds exposed to lower oxygen levels.
• The local bed conditions generate pellets with three types of oxidation profiles, namely the homogenous oxidation of the entire pellet, an unreacted core with a distinct/sharp interface and a completely oxidised shell, and an unreacted core with a partially oxidised pellet shell.
• Pellets experiencing comparable thermal histories show a similar oxidation mechanism, independent of the oxygen level in the gas. Thus, the oxygen partial pressure will only affect the extent of the oxidation.
• Increasing the oxygen level of the ingoing gas increases the pellet bed temperature and the oxidation degree at the lower bed heights more effectively than solely increasing the ingoing gas temperature.
• The higher oxygen level (approximately 30% O2) leads to an increase in the oxidation degree at temperatures above 1100°C, as well as increased strength (CCS) in the earlier stages of pellet induration.
This work has been conducted as part of the HYBRIT research project RP1. We gratefully acknowledge financial support from the Swedish Energy Agency. HYBRIT (Hydrogen Breakthrough Ironmaking Technology) is a joint initiative of the three companies SSAB, LKAB and Vattenfall with the aim of developing the world’s first fossil-free ore-based steelmaking route.
The authors would like to acknowledge the support and guidance by the personnel at LKAB. In particular, Staffan Hedvall, Jörgen Rosenqvist and the team at the Pot furnace laboratory – Pernilla Nordin, Emma Nordström, Ebba Granström, Elisabeth KurthsDotter, Johan Forss, and Marie-Ann Helin.
Ida Christin Eriksson, illustrator, is kindly acknowledged for assistance with the artwork in Fig. 4.