Volume 61, Issue 5
Displaying 1-48 of 48 articles from this issue
- |<
- <
- 1
- >
- >|
Publication Data
-
2021 Volume 61 Issue 5 Pages Cover-
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (433K) -
2021 Volume 61 Issue 5 Pages Editorial-
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (400K) -
2021 Volume 61 Issue 5 Pages Contents-
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (167K)
Review Article
Fundamentals of High Temperature Processes
-
Article type: Review
2021 Volume 61 Issue 5 Pages 1337-1347
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: January 23, 2021Download PDF (924K) Full view HTML
Regular Articles
Fundamentals of High Temperature Processes
-
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1348-1356
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (1918K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1357-1362
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: January 23, 2021Download PDF (760K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1363-1369
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (928K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1370-1378
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: February 28, 2021Download PDF (1832K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1379-1388
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: February 25, 2021Download PDF (2186K) Full view HTML -
Effect of Na Ions on Melt Structure and Viscosity of CaO–SiO2–Na2O by Molecular Dynamics SimulationsArticle type: Regular Article
2021 Volume 61 Issue 5 Pages 1389-1395
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (797K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1396-1403
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (1370K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1404-1411
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (788K) Full view HTML
Ironmaking
-
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1412-1422
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: January 29, 2021Download PDF (1643K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1423-1430
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (1375K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1431-1438
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (1594K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1439-1449
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: February 21, 2021Download PDF (2354K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1450-1458
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: February 19, 2021Download PDF (886K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1459-1468
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (2068K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1469-1478
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: January 28, 2021Download PDF (1059K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1479-1487
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (1298K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1488-1497
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (2388K) Full view HTML
Steelmaking
-
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1498-1505
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: February 24, 2021Download PDF (1057K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1506-1513
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: January 23, 2021Download PDF (1508K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1514-1523
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (1757K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1524-1531
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: February 18, 2021Download PDF (1596K) Full view HTML
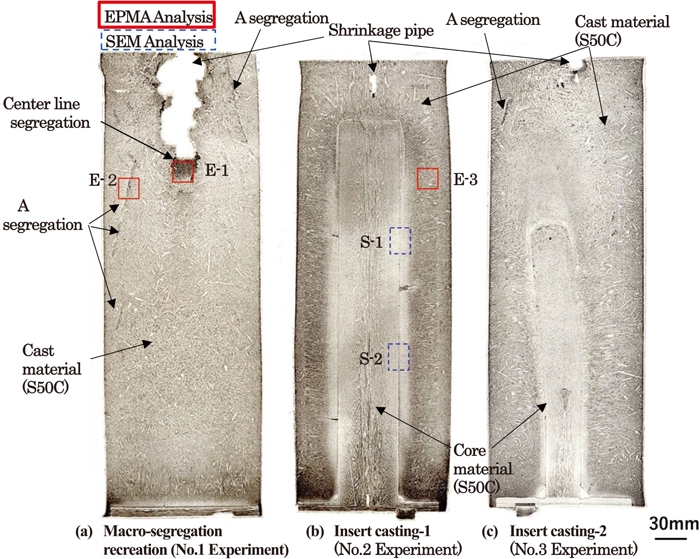
Casting and Solidification
-
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1532-1538
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (1159K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1539-1549
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (616K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1550-1555
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: February 21, 2021Download PDF (1155K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1556-1566
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: March 14, 2021Download PDF (2776K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1567-1578
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: February 17, 2021Download PDF (3127K) Full view HTML
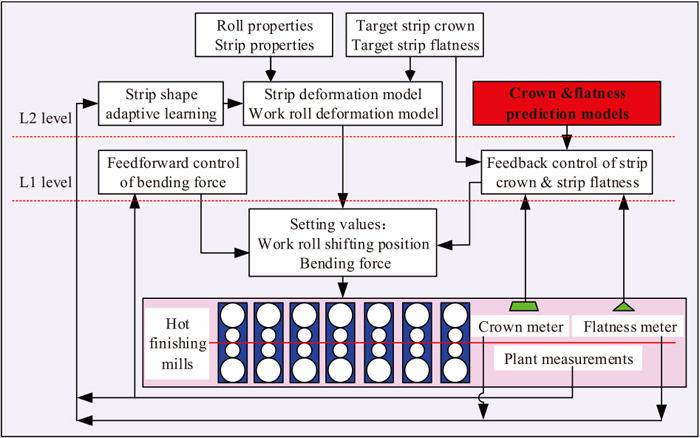
Instrumentation, Control and System Engineering
-
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1579-1583
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (420K) Full view HTML
Chemical and Physical Analysis
-
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1584-1593
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: February 14, 2021Download PDF (1333K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1594-1602
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (2287K) Full view HTML
Forming Processing and Thermomechanical Treatment
-
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1603-1613
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (2314K) Full view HTML
Welding and Joining
-
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1614-1622
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (2679K) Full view HTML
Surface Treatment and Corrosion
-
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1623-1632
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: February 25, 2021Download PDF (1607K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1633-1640
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (1490K) Full view HTML
Transformations and Microstructures
-
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1641-1649
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (1975K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1650-1659
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (2087K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1660-1668
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (1465K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1669-1678
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (2095K) Full view HTML
Mechanical Properties
-
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1679-1687
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: February 28, 2021Download PDF (1797K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1688-1697
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: February 24, 2021Download PDF (2657K) Full view HTML
Physical Properties
-
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1698-1707
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (1131K) Full view HTML -
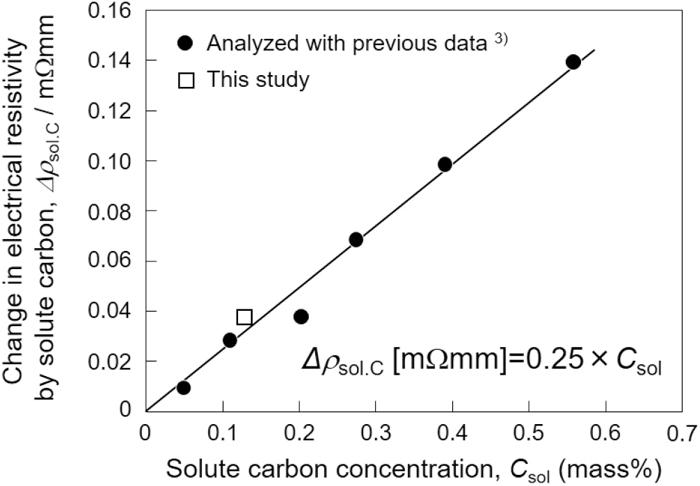
Estimation of Solute Carbon Concentration by Electrical Resistivity Measurement in Martensitic SteelArticle type: Regular Article
2021 Volume 61 Issue 5 Pages 1708-1715
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Download PDF (1099K) Full view HTML
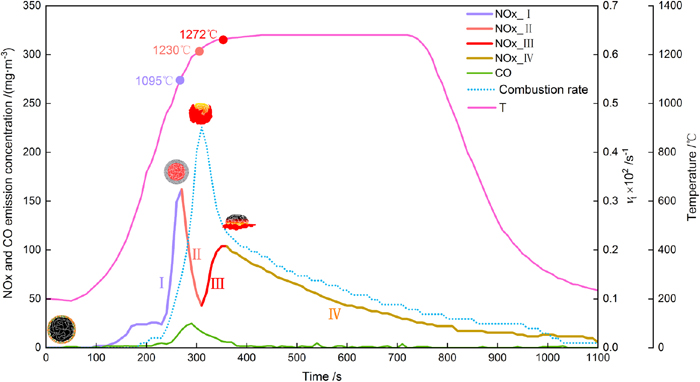
Social and Environmental Engineering
-
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1716-1724
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: February 27, 2021Download PDF (3017K) Full view HTML -
Article type: Regular Article
2021 Volume 61 Issue 5 Pages 1725-1735
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: February 21, 2021Download PDF (1619K) Full view HTML
Note
Mechanical Properties
-
Article type: Note
2021 Volume 61 Issue 5 Pages 1736-1738
Published: May 15, 2021
Released on J-STAGE: May 15, 2021
Advance online publication: January 21, 2021Download PDF (515K) Full view HTML
- |<
- <
- 1
- >
- >|


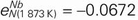 ,
, 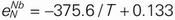 . In the liquid Fe–Cr–Nb and Fe–Ni–Nb systems, the second-order cross-interaction parameters of chromium or nickel with niobium on nitrogen were determined as follows:
. In the liquid Fe–Cr–Nb and Fe–Ni–Nb systems, the second-order cross-interaction parameters of chromium or nickel with niobium on nitrogen were determined as follows: 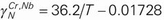 ,
, 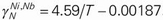 . Furthermore, a more accurate nitrogen solubility prediction model for the liquid Fe–Cr–Ni–Nb system was established based on the existing thermodynamic parameters and the interaction parameters obtained in this study.
. Furthermore, a more accurate nitrogen solubility prediction model for the liquid Fe–Cr–Ni–Nb system was established based on the existing thermodynamic parameters and the interaction parameters obtained in this study.