2021 年 61 巻 9 号 p. 2370-2380
2021 年 61 巻 9 号 p. 2370-2380
Demands for cleanliness of high chromium steel have been increasing. In steel refining process, aluminum is usually added in molten steel as a deoxidizing agent. As a result, such inclusions as alumina (Al2O3) and spinel (MgO∙Al2O3) are formed, which cause fatigue failures and surface defects. Therefore, it is important to understand the conditions of the inclusions which form in high chromium steel, and to reduce their harmful effects on steel qualities. In this work, to begin with, thermodynamic conditions of MgO and MgO∙Al2O3 formation in Fe-17mass%Cr molten steel at 1873 K were investigated. The results showed that MgO is more stable in high chromium steel than in plain steel. The boundary of the stable condition of MgO and MgO∙Al2O3 shifts toward higher Al and lower Mg contents in high Cr steel. This cause is judged to be the effect of thermodynamic interaction between Cr and Mg. The interaction parameter of Cr on Mg was estimated to be 0.040 so that the boundary of stable condition of MgO and MgO∙Al2O3 can be explained. Moreover, phase stability diagram of Fe–Cr–Al–Ca–Mg–O system at 1873 K was developed to estimate the effect of chromium on the stable condition of MgO, MgO∙Al2O3 and CaO–MgO–Al2O3(l). Subsequently, the variations of inclusions which formed in Fe-17mass%Cr molten steel were also investigated at 1873 K. The variations of inclusions in molten Fe–Cr steel were reasonably explained by considering the stable conditions of MgO and MgO∙Al2O3 investigated in this work.
High cleanliness of steel products has been requested in order to improve steel properties for various application.1) In general, inclusions form in molten steel as reaction products of oxygen and deoxidizer during secondary refining process. Larger inclusions can be removed by floatation in vacuum degasification process. However, it is difficult to remove smaller inclusions, such as under 1 μm, because it takes much longer time for floatation.2) Al2O3 and MgO∙Al2O3 inclusions which form after Al addition tend to easily aggregate in molten steel, to attach to the inner wall of the nozzle during the solidification process and to cause nozzle clogging.3) In addition, inclusions attached to the inner wall of the nozzle occasionally detach, flow down with molten steel and are trapped by solidified shell, which become defects in steel and cause fatigue failure.3) MgO∙Al2O3 inclusions also cause surface defect, because they have high melting point and hardness. Todoroki et al.3) investigated the causes of surface defects in Fe-18mass%Cr-8mass%Ni stainless steel and reported that 36% of the surface defects were caused by MgO∙Al2O3-based inclusions. Therefore, it is necessary to make the inclusions harmless by controlling their composition. Many studies have been conducted on the formation mechanism and composition control of MgO∙Al2O3 inclusions.4,5,6,7,8) Regarding the composition change of the inclusions in high-Cr steels after aluminum addition, Todoroki et al.9,10) and Liu et al.11) studied that the composition change of the inclusions in high-Cr steels after aluminum addition to the Fe-18msss%Cr-8mass%Ni alloy and Fe-11mass%Cr alloy, respectively. The experimental conditions and the results of their experiments are summarized in Table 1. The inclusion which firstly form after aluminum addition is Al2O3 in both studies. Subsequently, the composition of inclusion changes to MgO or CaO–MgO–Al2O3 via MgO∙Al2O3. Todoroki et al.9,10) reported that the inclusions changed in the order of Al2O3, MgO∙Al2O3 and MgO when 0.05 to 0.3 mass% Al was added, and the inclusion further changed to CaO–MgO–Al2O3 at higher Al concentration. On the other hand, in case slag includes 10 mass% SiO2, the change of inclusions stopped after Al2O3 changed to MgO∙Al2O3. Furthermore, Todoroki et al. discussed the change of inclusions based on calculated phase stability diagrams and stated that the relationship between the change of inclusions and the composition of molten steel was reasonably explained.9,10) Liu et al.11) reported that the inclusions changed in the order of Al2O3, MgO∙Al2O3 and MgO when 0.25 mass% Al was added. The composition of inclusion further changed to CaO–MgO–Al2O3 when 0.75 or 2.5 mass% Al was added, and the CaO content of inclusions increased with an increase of Al concentration added in the alloy. Liu et al.11) also discussed the change of inclusions based on phase stability diagrams. However, they indicated the difficulty to know the change of the inclusions from the phase stability diagrams because thermodynamic data on the deoxidation equilibria of Mg and Ca are different among researchers.11) Therefore, in order to discuss the change of inclusions, it is important to know the phase stability region of each compound, such as Al2O3, MgO∙Al2O3, MgO or CaO–MgO–Al2O3, in equilibrium with high-Cr steel accurately in the phase stability diagram. Jo et al.12) draw phase stability diagrams for the MgO–Al2O3 system by measuring the interaction parameters of Mg on Cr and Mg on Al in molten Fe-18mass% Cr steel. However, there are few reports of direct measurements of equilibrium relationships between such compounds. Accordingly, in this work, we investigated the composition of molten Fe-17mass%Cr alloy in equilibrium with CaO–MgO–Al2O3 slag doubly saturated with MgO and MgO∙Al2O3 at 1873 K, and the boundary of stable condition of MgO and MgO∙Al2O3 is clarified. Moreover, we investigated the variation of inclusions formed in molten Fe-17mass%Cr steel after aluminum (and calcium) addition at 1873 K. The variation of inclusions formed in molten Fe–Cr steel including the results of Todoroki et al.9,10) and Liu et al.11) are discussed based on the boundary of stable condition of MgO and MgO∙Al2O3 investigated in this work.
| Author | Steel type | Crucible | Slag | Al addition mass% | Time (min) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 5 | 10 | 30 | 60 | 90 | 120 | |||||
| Todoroki et al.9,10) (1823 K) | Fe-18Cr-8Ni (500 g) | MgO | CaO–MgO– Al2O3–F | 0.05 | CrOx | Al2O3 | Al2O3 + MgO·Al2O3 | \ | MgO·Al2O3 | MgO·Al2O3 + MgO | MgO | |
| 0.3 | CrOx | Al2O3 | Al2O3 + MgO·Al2O3 | MgO·Al2O3 + MgO | MgO | |||||||
| 0.5 | CrOx | Al2O3 + MgO·Al2O3 | MgO·Al2O3 | MgO | MgO·Al2O3 + MgO | CaO–MgO– Al2O3 | – | |||||
| 1.0 | CrOx | Al2O3 + MgO·Al2O3 | MgO·Al2O3 | MgO | CaO–MgO–Al2O3 | – | ||||||
| CaO–MgO– Al2O3–SiO2–F | 0.05 | CrOx | Al2O3 | Al2O3 + MgO·Al2O3 | MgO·Al2O3 | |||||||
| 0.1 | CrOx | Al2O3 | Al2O3 | MgO·Al2O3 | ||||||||
| 0.3 | CrOx | Al2O3 | Al2O3 + MgO·Al2O3 | MgO·Al2O3 | – | |||||||
| Liu et al.11) (1873 K) | Fe-11Cr (140 g) | MgO | CaO–MgO–Al2O3 (CaO and MgO Saturation) | 0.25 | Al2O3 | \ | MgO·Al2O3 + MgO | MgO | ||||
| 0.75 | \ | \ | \ | \ | MgO | \ | MgO + CaO–MgO– Al2O3 | |||||
| 2.5 | Al2O3 + MgO·Al2O3 | \ | MgO·Al2O3 + MgO | MgO·Al2O3 + MgO +CaO–MgO– Al2O3 | MgO·Al2O3 + MgO | CaO + MgO + CaO–MgO –Al2O3 | \ | CaO–MgO– Al2O3 | ||||
The schematic illustrations of the experimental setup and the sample arrangement are shown in Figs. 1(a) and 1(b). The experiments were conducted using an electric resistance furnace with a mullite tube (60 mm o.d., 52 mm i.d., 1000 mm height). Firstly, an electrolytic iron (>99.99%) and a regent grade of chromium (> 99.9%) were put in an alumina crucible and were inductively heated in an argon. After melting, aluminum was added, and the Fe-17mass% Cr-(0.01 to 3.7)mass%Al alloy was prepared. CaO was made by heating reagent grade of CaCO3 (98.5%) at 1323 K for 720 min. Reagent grade of Al2O3 and MgO (98%) and the CaO powder were mixed in a ratio of 34 mass%CaO-14 mass% MgO-52 mass% Al2O3 and were pressed to prepare the slag tablets. The slag composition was determined from the doubly saturated composition with MgO and MgO∙Al2O3, which is shown in Fig. 2. Similarly, MgO∙Al2O3 tablets were prepared by mixing MgO and Al2O3 at the ratio of 30 mass%MgO-70 mass% Al2O3 and by being pressed. 50 g of the Fe–Cr–Al alloy, 9 g of the slag tablet and 4 g of the spinel tablet were put in a MgO crucible (32 mm o.d., 26 mm i.d., 80 mm height), and the MgO crucible was put in a carbon crucible (44 mm o.d., 36 mm i.d., 125 mm height). The carbon crucible was inserted in the electrical resistance furnace at room temperature. The sample was heated to 1873 K at a heating rate of 10 K/min in an argon and was held for 720 min. After the equilibrium was attained, the sample was withdrawn from the furnace and was cooled in water. The aluminum content of the alloy was analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES). The magnesium and calcium contents were also analyzed by ICP-AES and by glow discharge mass spectrometry (GD-MS). The oxygen content was analyzed using an oxygen analyzer by an inert gas fusion infrared absorption method.
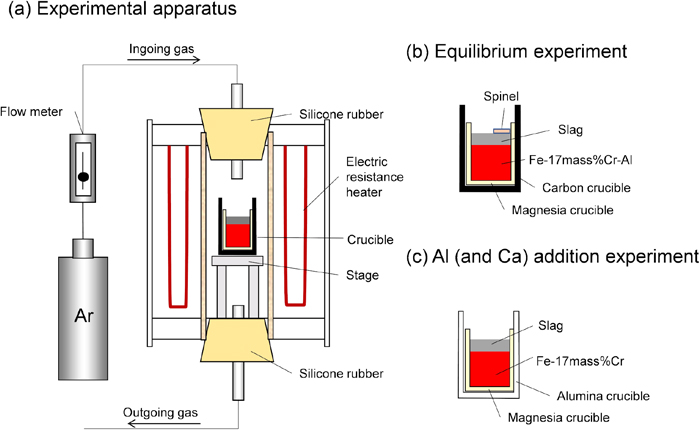
Schematic illustration of (a) experimental apparatus, (b) sample arrangement for the equilibrium experiment, and (c) sample arrangement for the Al (and Ca) addition experiment. (Online version in color.)

Composition of the slag employed in this work.
The same experimental apparatus as the equilibrium experiment is used. The schematic illustration of sample arrangement is shown in Fig. 1(c). The Fe-17mass% Cr alloy was preliminary made. Reagent grade of Al2O3 and MgO and the CaO powder were mixed in a ratio of 53 mass%CaO-10 mass% MgO-37 mass% Al2O3 and were pressed to prepare the slag tablets. The slag composition was determined from the doubly saturated composition with CaO and MgO, which is shown in Fig. 2. The experimental procedure is summarized in Fig. 3. 100 g of Fe-17mass%Cr alloy and 10 g of slag tablet were put in the MgO crucible, and it was put in an alumina crucible (46 mm o.d., 36 mm i.d., 130 mm height). The alumina crucible was inserted in the electrical resistance furnace at room temperature. The sample was heated to 1873 K at a heating rate of 10 K/min in an argon and was held for 30 min. In aluminum addition experiments, after the sample was melted, 0.5 gram of aluminum was added to the metal sample from the top of the furnace using an iron wire. The time when aluminum was added was defined as the starting time (time, t = 0). After 10 or 30 min were passed from the addition of Al, the crucible was withdrawn from the furnace and was cooled in an argon stream. These experiments are called Al addition experiments, 1 (after 10 min from Al addition) and 2 (after 30 min from Al addition). In Ca addition experiments, after the same experiments were conducted as Al addition experiments, 0.5 g of calcium was added. These experiments are called Ca addition experiments, 1 (Ca is added after 10 min from Al addition) and 2 (Ca is added after 30 min from Al addition). After 30 min were passed from the addition of calcium, the crucible was withdrawn from the furnace and was cooled in an argon stream. The composition of the alloy was analyzed using ICP-AES. The morphology and composition of the inclusions observed in the sample were analyzed using scanning electron microscope and energy dispersive x-ray spectroscopy (SEM-EDS).

Procedure of the Al (and Ca) addition experiments. (Online version in color.)
The composition of the Fe-17mass%Cr alloy samples after the equilibrium experiments is shown in Table 2. The dependences of Ca or Mg contents on Al content of the alloy samples are shown in Figs. 4(a) and 4(b), respectively. The Mg content increases with increasing Al content of the alloy and is in the range of 1 to 26 mass ppm. It is found the Ca content also increases with an increase of Al content of the alloy.
| No. | Al | Mg | Ca | O |
|---|---|---|---|---|
| 1 | 0.024 | 0.00017 | 0.00011 | 0.00028 |
| 2 | 0.021 | 0.00013 | 0.000050 | 0.00022 |
| 3 | 0.012 | 0.00011 | 0.00019 | 0.00018 |
| 4 | 0.010 | 0.00013 | 0.00061 | 0.00016 |
| 5 | 0.0080 | 0.000090 | 0.00017 | 0.00021 |
| 6 | 0.20 | 0.00077 | 0.0011 | 0.0018 |
| 7 | 0.077 | 0.00066 | 0.0016 | 0.00023 |
| 8 | 0.047 | 0.00053 | 0.0021 | 0.00026 |
| 9 | 0.45 | 0.0013 | 0.0018 | 0.0014 |
| 10 | 1.5 | 0.0022 | 0.0025 | 0.0018 |
| 11 | 2.5 | 0.0026 | 0.0016 | 0.0018 |

Composition of Fe-17mass%Cr steel in equilibrium with CaO–MgO–Al2O3 slag doubly saturated with MgO and MgO∙Al2O3.
Ten inclusions in each sample were randomly chosen and their morphology was observed. The composition of each inclusion was also analyzed. The representatives of the inclusions are shown in Fig. 5. The average composition of each inclusion from (a) to (j) are also shown in Fig. 5. Major inclusions observed in Al addition experiments 1 and 2 were rounded, with few corners. It is found that the inclusions tend to become more rounded with time. On the other hand, in Ca addition experiments 1 and 2, smaller inclusions with various shape are observed. The combined inclusions or the inclusions that may be newly formed around the inclusions are also observed. The size of the inclusions observed in each experiment is shown in Fig. 6. The size of the inclusions is ranging from 4.2 to 7.2 μm in the Al addition experiment 1, from 0.6 to 4.7 μm in the Al addition experiment 2 and from 2.0 to 4.7 μm in the Ca addition experiments 1 and 2. The size of the inclusions seems to become smaller with time from the results of Al addition experiments 1 and 2. On the other hand, no significant differences were identified in the size of the inclusions between the Ca addition experiments 1 and 2.

Typical morphology and composition of inclusions observed in the Al (and Ca) addition experiments.

Variation of inclusion size of the Al (and Ca) addition experiments.
The composition of ten inclusions which are randomly chosen in each sample is plotted on the phase diagram of CaO–MgO–Al2O3 ternary system, and the variation of the composition with Al (and Ca) addition is shown in Fig. 7. The composition of the inclusions in Al addition experiment 1 is plotted in Fig. 7(a) and is mostly found in Al2O3 side between Al2O3 and MgO∙Al2O3. On the other hand, almost all of the composition of the inclusions found in Al addition experiment 2 is MgO, which is plotted in Fig. 7(b). This fact indicates that the Al2O3 and/or MgO∙Al2O3 inclusions firstly form and change to MgO with time in Al addition experiments.

Composition of the inclusions observed in (a) Al addition experiment 1, (b) Al addition experiment 2, (c) Ca addition experiment 1, and (d) Ca addition experiment 2.
In Ca addition experiment 1, Ca was considered to be added when Al2O3 and MgO∙Al2O3 inclusions formed in molten alloy from the results of Al addition experiment 1. It is found that CaO–MgO–Al2O3, MgO and Al2O3 are observed from the composition of the inclusions plotted in Fig. 7(c). This fact indicates that the Ca and Mg contents of the inclusions are increased by adding Ca and by being held for 30 min.
In Ca addition experiment 2, Ca was considered to be added when MgO inclusion formed in molten alloy from the results of Al addition experiment 2. It is found that MgO, CaO–MgO–Al2O3, Al2O3 and MgO∙Al2O3 are observed from the composition of the inclusions plotted in Fig. 7(d). The addition of Ca increased both of the Ca and Al contents of inclusions. Compared to Ca addition experiments 1, the inclusions with lower Al content are found in Ca addition experiment 2.
From the composition of each inclusion, the observed inclusions are classified into Al2O3, MgO∙Al2O3, MgO and CaO–MgO–Al2O3. The ratio of each inclusion observed in Al (and Ca) addition experiments is shown in Fig. 8. The major inclusions were Al2O3 and MgO in the Al addition experiments 1 and 2, and CaO–MgO–Al2O3 and MgO in the Ca addition experiments 1 and 2, respectively. From the results of the Al and Ca addition experiments 1, it is found that the major inclusion is changed from Al2O3 to CaO–MgO–Al2O3 by Ca addition. On the other hand, from the results of the Al and Ca addition experiments 2, it is also found that the major inclusion is still MgO although all of 4 kinds of inclusions are observed after calcium addition. The variation of inclusions in this work is summarized in Fig. 9. The Al2O3 and MgO∙Al2O3 inclusions firstly form by Al addition. The Al content of the inclusions decreases, and the inclusions change to MgO with time. The Ca content of inclusions increases and some of the CaO–MgO–Al2O3 inclusions are observed by Ca addition. It is also found that the MgO inclusion tends to increase with time by Al (and Ca) addition in this work.

The ratio of inclusions observed in the Al (and Ca) addition experiments.

Summary of the variation of inclusions observed in this work. (Online version in color.)
Thermodynamic conditions of MgO and MgO∙Al2O3 formation in molten Fe-17mass%Cr are discussed. When Fe-17mass%Cr alloy is equilibrated with CaO–MgO–Al2O3 slag, Ca, Mg and Al in iron are in equilibrium with the slag components as Eqs. (1), (3) and (5), and the equilibrium constants are expressed by Eqs. (2), (4) and (6), respectively.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
The effect of Cr is taken into account by introducing the first-order interaction parameters with each element, j (j: Mg, Al, Ca and O). The activity of each component in slag in equilibrium with MgO and MgO∙Al2O3 is determined to be aCaO=8.622×10−2, aAl2O3 = 5.486×10−2, aMgO = 1 from literature.13) By substituting Eqs. (10), (11), (12), (13) into Eqs. (7), (8), (9), the molten steel composition when slag is doubly saturated with MgO and MgO∙Al2O3 can be calculated. The mass concentration of chromium, [%Cr], is fixed at 0 or 17 mass%. The remaining variables are [%Al], [%Mg], [%Ca] and [%O]. This allows us to obtain [%Mg], [%Ca] and [%O] for arbitrary [%Al] by solving Eqs. (7), (8), (9) simultaneously by a numerical method. The values of the equilibrium constants and the first- and second-order interaction parameters used in this work are shown in Tables 3 and 4, respectively. The calculated composition of molten Fe-(0, 17) mass%Cr alloy in equilibrium with CaO–MgO–Al2O3 slag doubly saturated with MgO and MgO∙Al2O3 at 1873 K is shown in Fig. 10. The present experimental results (equilibrium experiments) are also shown in the figure. It is found that MgO is more stable in high chromium steel than in plain steel. Several values of interaction parameter of Cr on Mg are reported in literature and are shown in Table 5. Accordingly, in the case of Fe-17mass%Cr, the equilibrium lines in Fig. 10 are described by using each interaction parameter,

The composition of molten pure iron and Fe-17mass%Cr alloy in equilibrium with CaO–MgO–Al2O3 slag doubly saturated with MgO and MgO∙Al2O3 at 1873 K.

Calculated composition of molten Fe-17mass%Cr alloy in equilibrium with CaO–MgO–Al2O3 slag doubly saturated with MgO and MgO∙Al2O3 at 1873 K. (Online version in color.)
To be noticed, oxygen content takes a minimum value at about 0.16 mass%Al and increases with an increase of aluminum content due to the interaction between Al and O. At the same time, the magnesium content also increases due to the interaction between Mg and O. Therefore, the deviation of the calculated line and the experimental results is considered to be related with the effect of the increase of oxygen content.
Subsequently, the variation of inclusions formed in molten high Cr steel is discussed. In this work, Al2O3 and MgO∙Al2O3 inclusions were observed at 10 min after Al addition. Then, MgO inclusion was observed at 30 min. On the other hand, CaO–MgO–Al2O3 is a major inclusion after adding Ca at the time when the formed inclusions are Al2O3 and MgO∙Al2O3. When Ca is added after the formed inclusion is changed to MgO, the inclusion is unchanged, and MgO remains as it is. The Al, Mg, O, and Ca contents of Fe-17mass% alloy after the Al and Ca addition experiments are shown in Table 6. The phase stability diagram of Fe–Cr–Al–Ca–Mg–O system at 1873 K was developed to estimate the effect of chromium on the stable condition of MgO, MgO∙Al2O3 and CaO–MgO–Al2O3(l). The experimental results of this work are plotted on the phase stability diagram of Fe-17mass%Cr-Al-Ca-Mg-O system in Fig. 12. The activity data of slag components are found in the literature.13) According to Todoroki et al.,9,10) Al content of molten Fe–Cr steel rapidly increases when Al is added, and Al2O3 forms by a reaction expressed as Eq. (14).
| (14) |
| (15) |
| (16) |
| (17) |
| Al mass% | Mg mass ppm | Ca mass ppm | O mass ppm | Inclusion | |
|---|---|---|---|---|---|
| Al addition experiment 1 | 0.33 | 3 | 31 | 8.4 | Al2O3 +MgO·Al2O3 |
| Al addition experiment 2 | – | – | – | – | MgO |
| Ca addition experiment 1 | 0.38 | 32 | 107 | 7.1 | Al2O3 + MgO + CaO–MgO–Al2O3 |
| Ca addition experiment 2 | 0.12 | 69 | 130 | 2.1 | Al2O3 + MgO·Al2O3 + MgO + CaO–MgO–Al2O3 |

Variation of inclusions observed in Fe-17mass%Cr with Al (and Ca) addition. (S: MgO∙Al2O3, M: MgO, L: CaO–MgO– Al2O3). (Online version in color.)
Todoroki et al.9,10) reported the variation of inclusions observed in Fe-18mass%Cr-8mass%Ni with 0.3 mass% Al addition. The results are shown in Fig. 13. The boundary of the stable condition of MgO and MgO∙Al2O3 for Fe-18mass%Cr-8mass%Ni is described in the figure base on the investigation in this work. In the case of silica free slag, the alloy composition once enters the MgO∙Al2O3 stable region, and MgO∙Al2O3 is observed together with Al2O3. Afterwards, the composition of the alloy moves to higher Mg and lower Al contents, and returns to MgO stable region. Correspondingly, the observed inclusion changes to MgO. On the other hand, in the case of slag containing SiO2, the alloy composition stays in MgO∙Al2O3 stable region, therefore, the variation of inclusions is stopped after Al2O3 changes to MgO∙Al2O3. The boundary of the stable condition of MgO and MgO∙Al2O3 obtained in this work can reasonably explain their results. Liu et al.11) also reported the variation of inclusions observed in Fe-11mass%Cr by the addition of 0.25, 0.75 and 2.5mass%Al. The results are shown in Fig. 14. The composition of the alloy with 0.25%Al addition starts from MgO∙Al2O3 stable region. The inclusion firstly observed is Al2O3. Then, the composition moves to higher Mg content and enters to MgO stable region. Correspondingly, the observed inclusion changes to MgO. The variation of inclusions to MgO is in good agreement with the stable condition of MgO, except that Al2O3 is firstly observed in the MgO∙Al2O3 stable region. On the other hand, in the case of 0.75 and 2.5 mass% Al addition, it is found there are many plots between the extrapolation of solid and dashed lines (that is, calculated and experimentally measured lines), where MgO is mainly observed. This fact means that the boundary of the stable condition of MgO and MgO∙Al2O3 experimentally measured in this work is valid, and the calculation curve deviates upward in Fig. 14 and seems to contain some errors at higher aluminum concentration. From the above discussion, it can be concluded that the variation of inclusions in molten Fe–Cr steel can be reasonably explained by the stable conditions of MgO and MgO∙Al2O3 investigated in this work.


Variation of inclusions observed in Fe-11mass%Cr11) with Al addition. (A: Al2O3, S: MgO∙Al2O3, M: MgO, L: CaO–MgO–Al2O3, C: CaO). (Online version in color.)
Thermodynamic conditions of MgO and MgO∙Al2O3 formation and variation of inclusions which formed in Fe-17mass%Cr molten steel at 1873 K were investigated. The conclusions are summarized as follows.
(1) MgO is more stable in high chromium steel than in plain steel. The boundary of the stable condition of MgO and MgO∙Al2O3 shifts toward higher Al and lower Mg contents in high Cr steel. This cause is judged to be the effect of thermodynamic interaction between Cr and Mg. The interaction parameter of Cr on Mg was estimated to be 0.040 so that the boundary of stable condition of MgO and MgO∙Al2O3 can be explained.
(2) Phase stability diagram of Fe–Cr–Al–Ca–Mg–O system at 1873 K was developed, which can be used to estimate the effect of chromium on the stable condition of MgO, MgO∙Al2O3 and CaO–MgO–Al2O3(l).
(3) The calculated boundary of the stable condition of MgO and MgO∙Al2O3 deviates from the experimental results at more than 0.2 mass%Al. The variations of inclusions in molten Fe–Cr steel were reasonably explained by considering the stable conditions of MgO and MgO∙Al2O3 experimentally measured in this work.
This study was carried out with the support and advice of the Iron and Steel Institute of Japan, “Clean Cr steel production by slag, inclusion control” research group. We would like to express our deepest gratitude to the Iron and Steel Institute of Japan and its study group. We would also like to thank Dr. Hisashi Yamana, Mr. Toshiaki Takashima and Mr.Takahiro Onoe of Nippon Koshuha Steel CO., LTD. for their help of chemical analysis and helpful discussion, and Prof. Yoshinao Kobayashi of Tokyo Institute of Technology for his help in conducting this work.