2022 Volume 62 Issue 4 Pages 632-641
2022 Volume 62 Issue 4 Pages 632-641
The present work investigates the manufacture of high basicity (CaO/SiO2 = 1.45) magnesium containing fluxed pellets with hydrated lime used as binder and fluxing agent. The fired pellets and lump ores could constitute a binary burden for blast furnace with lower carbon emission. Both of the green and fired pellets with high basicity possessed good physical and chemical properties, and its metallurgical performances were better than the fired pellets in which bentonite and limestone were used as binder and fluxing agent, respectively. The optical microscopy, SEM/EDS and thermodynamic calculation were employed to clarify the difference between limestone fluxed pellets and hydrated lime fluxed pellets. The effect of basicity on the properties of green and indurated pellets was also studied. The results found that basicity and decomposition characteristics of the fluxing agents had great influence on the mineral composition of the fired pellets. Larger amounts of high-melting phases like MgFe2O4, strong bonding phases such as CaFe2O4 and good reducibility phases like SFCA could be generated in the hydrated lime fluxed pellets with high basicity during induration process, thus its metallurgical performances being improved.
As one of the most important iron ore agglomeration processes, pelletizing possesses some irreplaceable advantages, such as low energy consumption and less exhaust gases and harmful dusts emission compared to the sintering.1) Moreover, the metallurgical performances and physical strength of iron ore pellets are better than sinter.2) Therefore, the proportion of pellets in blast furnace burden has been gradually increased in recent years.3)
The process of pelletization mainly includes the raw materials preparation, balling, drying and induration of green pellets. Besides the process conditions, the ingredients of the pellet feed also have significant influence on the quality of green and indurated pellets. The pellet feed mainly comprises the iron ore concentrates, binder and suitable additives like anthracite (or coke breeze) and fluxing agents. In general, the types of pellets can be mainly divided into acid, fluxed and basic pellets according to their components.
Usually, the blast furnace burden comprises fired pellets, sinter and lump ores. The sinter and pellets have become the dominant blast furnace burden as the quality of the remaining lump ore deposits has decreased in recent years.3) Therefore, in order to reduce the flue gas and carbon dioxide emission in sintering process, higher ratio of the fired pellets is required. The acid pellets with the basicity below 0.4 possess the characteristics of high iron grade and cold compressive strength, but low softening and melting temperature.4) Hence, the research and application of basic pellets with better metallurgical performances has been rapidly developed in recent years.5) As for the fluxed pellets, some fluxing agents can effectively improve the pellet quality, especially the metallurgical performances, and the fluxed pellets are well-accepted by the downstream reduction processes.6) The oxides (CaO, MgO and SiO2) originating from fluxing agents, which include the limestone, dolomite, calcined lime and olivine, play a crucial role in improving the fired pellet quality.7) The CaO can improve the strength of the fired pellets with the formation of bonding phases (CaOx·Fe2O3 and SFCA) in induration process.8) The MgO can increase the softening point and decrease the reduction swelling index of the fired pellets with the generation of some high melting phases in the induration process of magnesium containing pellets.9,10)
In the production process of fluxed pellets, the type of iron ore and the binder have great influence on the pelletization process and pellet quality. In the induration process of magnetite ore pellets, the magnetite will be oxidized and transformed into hematite. The exothermic oxidizing reaction and the high activity of newly generated hematite are benefit for the crystallization and solidification reactions in the pellets.11) In contrast, the solidification is hard to take place in the hematite ore pellets because of the low reactivity of the primary hematite in the induration process. Therefore, anthracite was usually blended with the hematite to promote the induration process as the hematite can be easily reduced to magnetite even in a weak reducing atmosphere.12) Binder can modify the interfacial characteristics of the iron ore particles, provide adhesion to the fines and hold them together.13) The binder could not only improve the growth rate and strength of green pellets but also increase the strength of indurated pellets.14) Bentonite is a commonly used binder for iron ore pelletization, however, some factors have constrained its use in recent years. The increasing demand of bentonite clay in the crude oil exploration process lead to the price of bentonite increased.10) In addition, the gangue minerals in the bentonite may reduce the pellet quality when the bentonite was added.15) Hence, the development of a substitute for bentonite has been paid much attention.
The utilization of calcined lime or hydrated lime as fluxing agents and binder has been studied by some researchers. However, the quality of the fired pellets looked not better than that of fluxed pellets when bentonite and limestone was used as binder and flux, respectively,16) indicating the preparation process required further improvement. Some researchers have found that the green and dry pellets containing both hydrated lime and bentonite has poor strength, even easily broke as the bentonite will lose its activity when the calcium ions from hydrated lime will react with bentonite to replace its sodium ions.17) Therefore, the bentonite was totally replaced by hydrated lime in this study. Jagannath found that there was only a tiny difference between the metallurgical performances of limestone fluxed pellet and calcium lime fluxed pellet with basicity of 0.2.18) The hydrated lime may be more suitable for preparation of basic pellets as it can provide abundant CaO in the induration process and elevate the basicity of the fired pellets. The effect of basicity on the properties of hydrated lime fluxed pellets was studied in this research.
In present study, the preparation process of magnesium-containing fluxed pellets with basicity of 1.45 was studied for the blast furnace with fired pellets and lump ores as burden. Few researches on the manufacture of fluxed pellets with such a high basicity could be found in previous literature. The sinter can be totally replaced by the high basicity pellets, and only the fired pellets and lump ores constituting a binary burden for blast furnace for the purpose of energy conservation and emission reduction. If bentonite is used as the binder for the production of pellets with such a high basicity, much more CaO from limestone should be added to counteract the SiO2 from the bentonite and maintain the high basicity, leading to poor balling behaviors and lower iron grade of fired pellets. Therefore, the hydrated lime was used not only as the binder but also providing CaO for pelletizing while the dolomite was used as a source of MgO and CaO in the current study. A preheating process was adapted to adjust the decomposition rate of the fluxing agents in order to prevent the formation of crack in the pellet during the induration process. The production of fluxed pellets in which bentonite and limestone were used as the binder and flux, respectively, was also investigated for comparison. The differences in pelletization behaviors between the limestone fluxed pellets and hydrated lime fluxed pellets with basicity of 0.45–0.95 were investigated and the related mechanisms were clarified in this paper.
The raw materials which were used for preparing the green pellets are as follows: hematite concentrate, hydrated lime, limestone, dolomite, bentonite, quartz sand and anthracite.
The chemical compositions of hematite procured from the north of Brazil are shown in Table 1, it has good chemistry with high iron grade, low silica and trace sulfur and phosphorus. However, the content of Al2O3 is 1.63%, which is relatively high and may cause an adverse impact on the reduction degradation index of the fired pellets without properly handle.19) In the meantime, its specific surface area (SSA) is below 1500 cm2/g and need to be elevated before balling. The HPGR (High Pressure Grinding Roller) technology was adapted to process the hematite concentrate before balling in order to improve its ballability and decrease the binder dosage. As can be seen in Table 2, the SSA of the hematite concentrate increased from 1475 cm2/g to 1930 cm2/g after HPGR treatment. Figure 2 also indicated that the surface roughness of hematite particles was increased and more finer particles were generated after HPGR treatment.
| Item | Fetotal | FeO | SiO2 | CaO | MgO | Al2O3 | S | P | LOIa |
|---|---|---|---|---|---|---|---|---|---|
| Hematite concentrate | 65.94 | 0.14 | 1.31 | 0.01 | 0.01 | 1.63 | 0.01 | 0.07 | 1.53 |
| Limestone | 0.42 | / | 1.49 | 52.22 | 2.10 | 0.45 | <0.01 | 0.06 | 42.62 |
| Hydrated lime | 0.14 | / | 1.42 | 67.10 | 0.32 | 0.23 | 0.08 | 0.07 | 29.33 |
| Dolomite | 1.18 | / | 1.11 | 32.05 | 19.15 | 0.10 | 0.02 | 0.02 | 44.88 |
| quartz sand | / | / | 99.90 | / | / | / | / | / | / |
| Bentonite | 5.89 | / | 51.82 | 0.30 | 2.60 | 21.77 | 0.02 | 0.01 | 13.20 |
LOIa-Loss on ignition
| Item | −0.045 (mm) | 0.045–0.074 (mm) | +0.074 (mm) | SSAs (cm2/g) |
|---|---|---|---|---|
| Hematite concentrate (before HPGR) | 68.43 | 12.35 | 19.22 | 1475 |
| Hematite concentrate (after HPGR) | 75.80 | 10.40 | 13.8 | 1930 |
| Limestone | 80.00 | 8.37 | 11.63 | / |
| Dolomite | 83.93 | 9.10 | 6.97 | / |
| Quartz sand | 81.27 | 7.62 | 11.11 | / |
| Hydrated lime | 84.25 | 15.75 | 0 | / |
| Bentonite | 85.23 | 14.77 | 0 | / |
| Anthracite | 85.02 | 14.98 | 0 | / |
SSAs: specific surface areas, HPGR: High Pressure Grinding Roller
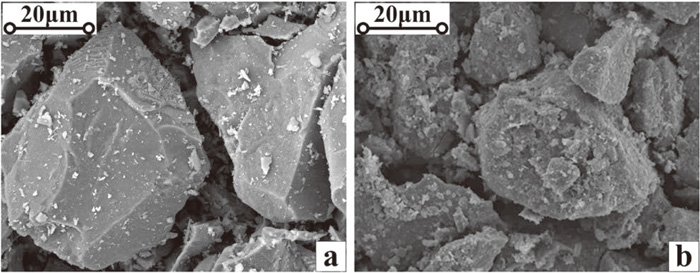
Morphology of hematite concentrate under SEM (a-before HPGR, b-after HPGR).
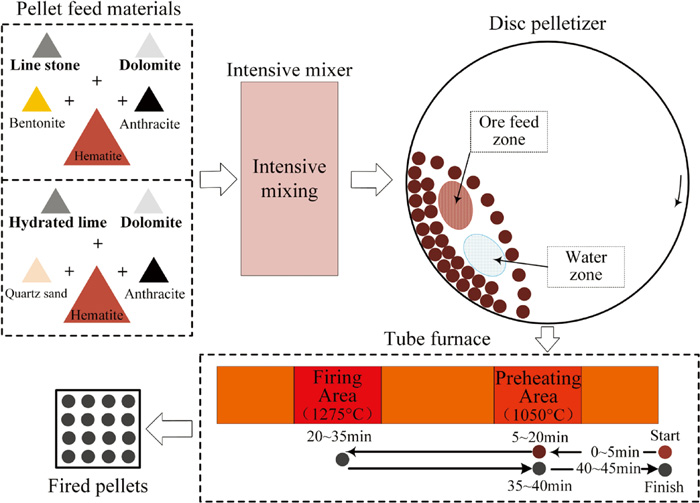
Flowsheet of fired pellets manufacture. (Online version in color.)
The limesone, dolomite, quartz sand and anthracite were finely ground separately in laboratory ball grinder to get suitable fineness for pelletizing and their size distributions are listed in Table 2. The dolomite mainly comprising of CaMg(CO3)2 was used to provide MgO and part of CaO for the pellets, and the MgO content in the fired pellets was designed at 1.3%. Limestone and quartz sand were used to adjust the basicity (CaO/SiO2) in the fired pellets. The pulverized anthracite was blended into pellet feed to improve the strength of preheated pellets and reduce the heat supplying from the combustion of gas fuel during preheating process, which is a popular practice for pellet plants where travelling grate process is used to produce fired pellets. The contents of particles below 0.074 mm fractions in these materials were both higher than 80%.
The physical properties of the bentonite are listed in Table 4. It can be seen that the bentonite contained 90.5 wt% montmorillonite and its water absorption rate in two hours could reach 598 wt%, indicating an excellent binder for pelletizing. The hydrated lime was used as the binder as well as a fluxing agent for pelletizing and its fineness was close to the bentonite (From Table 2).
| Proximate analysis/wt.% | Main chemical compositions of ash/wt.% | |||||||
|---|---|---|---|---|---|---|---|---|
| Mad | FCad | Vad | Aad | Fetotal | CaO | MgO | Al2O3 | SiO2 |
| 1.63 | 72.06 | 8.39 | 17.92 | 6.15 | 1.54 | 1.04 | 19.99 | 63.38 |
(Mad: Mositure, FCad: Fixed Carbon, Vad: Volatile Matter, Aad: Ash)
| Methylene blue adsorption /(g·(100 g)−1) | Montmorillonite /wt.% | Swelling capacity /(mL·g−1) | Colloid index /% | Water absorption for 2 h/wt.% |
|---|---|---|---|---|
| 40.0 | 90.5 | 57.5 | 100.0 | 598.0 |
In the preparation process of pellet feed materials, the hematite concentrate (after HPGR) was thoroughly mixed with the additives which included dolomite, limestone, quartz sand and anthracite in an intensive mixer to ensure proper blend without clogging of particles. The amount of ingredients for the preparation of fluxed pellets in which bentonite or hydrated lime were used as binder respectively are given in Table 5.
| Pellet types | Basicity | Ingredients of the blends/wt.% | ||||||
|---|---|---|---|---|---|---|---|---|
| HC | Quartz sand | Hydrated lime | Bentonite | Limestone | Anthracite coal | Dolomite | ||
| Pellet1 | 1.45 | 90.93 | 0 | 0 | 0.50 | 0.95 | 1.50 | 6.12 |
| Pellet2 | 1.45 | 90.57 | 0 | 0 | 0.65 | 1.16 | 1.50 | 6.12 |
| Pellet3 | 1.45 | 90.21 | 0 | 0 | 0.80 | 1.37 | 1.50 | 6.12 |
| Pellet4 | 0.95 | 91.50 | 0.38 | 0 | 0.50 | 0 | 1.50 | 6.12 |
| Pellet5 | 0.45 | 89.18 | 2.70 | 0 | 0.50 | 0 | 1.50 | 6.12 |
| Pellet6 | 1.45 | 90.07 | 0.65 | 1.50 | 0 | 0 | 1.50 | 6.28 |
| Pellet7 | 1.45 | 89.34 | 0.88 | 2.00 | 0 | 0 | 1.50 | 6.28 |
| Pellet8 | 1.45 | 88.61 | 1.11 | 2.50 | 0 | 0 | 1.50 | 6.28 |
| Pellet9 | 1.45 | 87.89 | 1.34 | 3.00 | 0 | 0 | 1.50 | 6.27 |
| Pellet10 | 0.95 | 88.10 | 2.12 | 2.00 | 0 | 0 | 1.50 | 6.28 |
| Pellet11 | 0.45 | 84.12 | 6.10 | 2.00 | 0 | 0 | 1.50 | 6.28 |
(HC: hematite concentrate)
Figure 2 illustrates the flowsheet of pelletization process. Green balls were prepared in a laboratory disc pelletizer with 1.0 meter diameter and 0.25 meter rim depth. The balling disc with a tilt angle of 45° was rotated at 38 r/min when pelletizing. The green balls with a size range of 8–16 mm were obtained after balling for 12 minutes. The moisture, drop strength from 0.5 m and compressive strength of the green balls with 10–12.5 mm were measured to evaluated the ability of the pellets to remain intact during their later processes. Thermal shock temperature of the green balls was measured to evaluate the thermal stability of the pellets during drying and preheating processes. Fifty freshly made green balls were loaded into a basket, which was hung up in a vertical tube furnace. Hot air of different temperatures with 1.8 m/s of flow rate flowed through the pellets and the temperatures where 4% of green pellets cracked or fractured were defined as the thermal shock temperatures. Subsequently, the remaining green balls were dried at 110°C for 2.5 hours in a vacuum drying oven to prepare the dry pellets for further induration process.
The induration process of the dry pellets was performed in an electric tube furnace. Firstly, the dry pellets went through a 5 minutes non-isothermal heating process to arrive at the preheating area and then 15 minutes of residence time at the isothermal preheating condition. subsequently, the preheated pellets were directly pushed to the high temperature firing area and firing for 15 minutes. Afterwards, the fired pellets were sent back to the preheating area and went through a 5 minutes soaking stage. Finally, the fired pellets were stepwise pulled out of the furnace in 5 minutes. The thermal regulation of the induration process was set up according to the grate-rotary kiln process and the soaking stage was set to hinder the generation of glassy phases in the fired pellets in the cooling stage.
The chemical compositions, cold crush strength and mineralogical characteristics of the fired pellets were investigated. The metallurgical performances including reducibility index (RI/%), reduction swelling index (RSI/%) and reduction degradation index (RDI/%) of the fired pellets were evaluated according to the ISO standards (RI: ISO 7215, RSI: ISO 4698 and RDI: ISO 4696-2).
2.2.2. Characterization of MaterialsThe chemical compositions analysis of the samples was carried out through X-Ray Fluorescence (XRF). The size distributions and specific surface areas of the pellet feed materials were measured by the wet sieving method and Blaine method, respectively. The microstructure of samples was examined by an optical microscopy (Leica DMLP) and a scanning electron microscope (JEOL). The micro-area chemical compositions of the fired pellets were measured by an energy diffraction spectrometer (JSM-6490LV). The mineral compositions of the fired pellets were carried out based on solubility difference of various phases in solvent.
The thermal decomposition behaviors of limestone (CaCO3), hydrated lime (Ca(OH)2) and dolomite (CaMg(CO3)2) were investigated to better understand the effect of fluxing agents on properties of the fired pellets in induration process. The decomposition reactions of CaCO3, Ca(OH)2 and CaMg(CO3)2 are described by Eqs. (1), (2), (3). The correlations of standard Gibbs free energy with temperature for Eqs. (1), (2), (3) were calculated by the reaction model of Factsage 7.1 and the results are shown in Fig. 3.
| (1) |
| (2) |
| (2-1) |
| (2-2) |
| (3) |
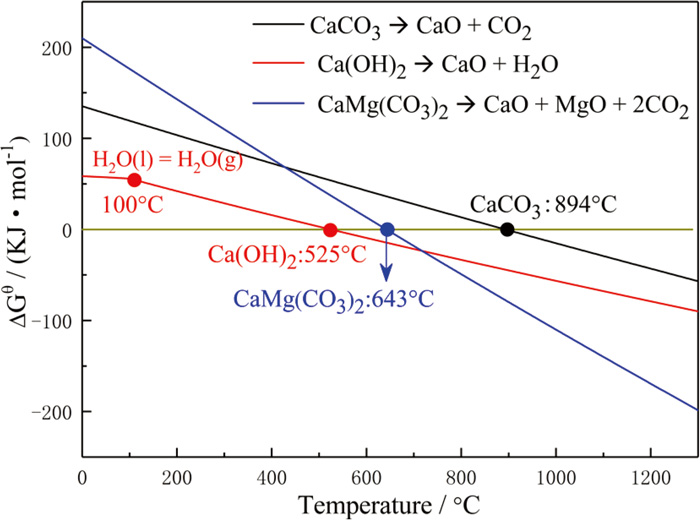
It can be observed that the decomposition equilibrium temperatures of CaCO3, Ca(OH)2 and CaMg(CO3)2 were 894°C, 525°C and 643°C, respectively. In addition, the previous study of decomposition kinetics of CaCO3 and Ca(OH)2 also indicated the higher temperature was required for the decomposition of limestone than hydrated lime, and the decomposition speed of limestone was lower than that of hydrated lime in the same thermal condition.20,21) The difference between decomposition characteristics of hydrated lime and lime stone would have an influence on the reactions between calcium oxide and other phases in the pellets during induration process. When the temperature is lower than the decomposition equilibrium temperatures of CaCO3, the CO2 generated from the oxidation of anthracite will react with Ca(OH)2 to form CaCO3, and CO2 can decrease decomposition efficiency of CaCO3.21,22) However, the carbonation of Ca(OH)2 can be avoided when the preheating temperature was higher than the decomposition equilibrium temperatures of CaCO3.
3.2. The Physical Properties of Green BallsIn the current study, bentonite was totally replaced by the hydrated lime which was sufficient to act as the binder and can eliminate the impact of SiO2 and Al2O3 from the bentonite on the basicity and quality of the fired pellets. The effects of binder types and dosage on the physical properties of green balls are displayed in Table 6. It can be seen that the increase of binder dosage could improve the strength and thermostability of the green balls. Bentonite and hydrated lime in the green balls imparted plasticity, held the particles together, and provided cohesion,23) which helped the green balls to sustain intact when it was under pressure or thermal shock. When the dosage of bentonite was 0.5%, the drop number from 0.5 m (DN) and compressive strength (GCS) of the green balls with basicity of 1.45 could reach 4.8 times and 19.7 Newton per pellet. The DN and GCS of the green balls could meet the requirement for most of the industrial production (DN > 4 times and GCS > 10 Newton per pellets).24) When the basicity of the pellets was 1.45, the strength of green balls made with 0.5% of the bentonite was close to the strength of pellet7 in which 2.0% of the hydrated lime was used as binder. More quartz sand was added into the pellet feed to counteract the CaO from the dolomite when the basicity decreased to 0.45. The hydrophobic quartz sand in the pellets has an adverse impact on the moisture transfer process and it can weaken the capillary force between the particle in the pellets.23) Therefore, the moisture and strength of the green balls declined while the thermal shock temperature (TS) increased with the decrease of basicity. Although the dosage of hydrated lime was higher than the bentonite, the green balls possessed the higher TS, which could shorten the drying time and improve the production efficiency for pelletizing plants. In addition, the hydrated lime was not only used as binder but also a fluxing agent, replacing limestone for producing basic pellets.
| Binder types | Pellets | Basicity | Dosage of binder/wt.% | Moisture/wt.% | DN/ times | GCS/ Newton·pellet−1 | TS/°C |
|---|---|---|---|---|---|---|---|
| Bentonite | Pellet1 | 1.45 | 0.50 | 8.2 | 4.8 | 19.7 | 280 |
| Pellet2 | 1.45 | 0.65 | 8.3 | 5.5 | 20.7 | 320 | |
| Pellet3 | 1.45 | 0.80 | 8.7 | 5.6 | 23.1 | 330 | |
| Pellet4 | 0.95 | 0.5 | 8.1 | 4.6 | 18.2 | 295 | |
| Pellet5 | 0.45 | 0.5 | 7.9 | 4.1 | 15.1 | 325 | |
| Hydrated lime | Pellet6 | 1.45 | 1.5 | 8.4 | 3.3 | 20.8 | 370 |
| Pellet7 | 1.45 | 2.0 | 8.7 | 5.0 | 24.2 | 385 | |
| Pellet8 | 1.45 | 2.5 | 8.7 | 5.9 | 23.3 | 390 | |
| Pellet9 | 1.45 | 3.0 | 8.6 | 6.0 | 23.8 | 430 | |
| Pellet10 | 0.95 | 2.0 | 8.4 | 4.8 | 21.5 | 395 | |
| Pellet11 | 0.45 | 2.0 | 7.8 | 4.0 | 15.2 | 425 |
(DN: Drop number from 0.5 m; GCS: Compressive strength of green balls; TS: Thermal shock temperature)
In the grate-rotary kiln process, the preheating process of the pellets was carried out in the grates, and then the preheated pellets were fed into the rotary kiln for further induration. The strength of preheated pellets could affect not only the operation in rotary kiln but also the strength of the fired pellets. Therefore, the effect of preheating temperature and basicity on cold crush strength (CCS) of preheated pellets was investigated and the results are illustrated in Fig. 4.
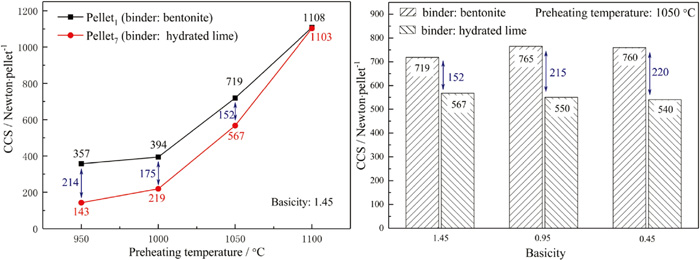
Effect of binder types, preheating temperature and basicity on CCS of preheated pellets (preheating for 15 min). (Online version in color.)
It can be seen that the CCS of the preheated pellets with basicity of 1.45 continuously increased when the preheating temperature raised from 950°C to 1100°C. The hardening mechanism of the pellets in the preheating process was mainly ascribed to the solid phase diffusion hardening. The higher preheating temperature was benefit for the diffusion and bonding of some particles in the pellets and thus elevated the strength of preheated pellets. When the preheating temperature increased to 1050°C, the CCS of two kinds of preheated pellets could both exceed 500 Newton per pellet and meet the requirement for rotary kiln operation (>400 Newton per pellet25)). The decrease of basicity could elevate the CCS of preheated pellets in which limestone was used as a fluxing agent, however, the influence of basicity on the CCS of preheated pellets in which hydrated lime was used as binder was inapparent.
The CO2 and H2O could be generated and the pores could be formed with the decomposition and shrunk of fluxing agent particles (limestone, hydrated lime and dolomite) in the pellets. The diffusion of the gases could prevent the generation of hematite interlinkage necks among particles and decline the strength of preheated pellets.26,27) Section 3.1 indicated that the decomposition process of hydrated lime was easier to process than limestone. Therefore, the CCS of preheated pellets in which hydrated lime was used as a fluxing agent was lower than that of preheated pellets in which limestone was used a fluxing agent. Moreover, the difference of CCS between two kinds of preheated pellets was gradually vanished with the increase of preheating temperature and decomposition speed of limestone.21) When the basicity decreased from 1.45 to 0.45, the dosage of limestone declined while the additive amount of hydrated lime could not decrease due to the need of bind effectiveness. Therefore, the CCS of preheated pellets could slightly elevate with the decreased of basicity when limestone was used as a fluxing agent, while the effect of basicity on the CCS of preheated pellets in which hydrated lime was used as binder was inapparent.
3.3.2. Firing of Preheated PelletsThe CCS of fired pellets under various firing temperature and basicity were measured to evaluate their suitability for blast furnace operation. The increase of temperature could promote the particle-particle fusion and make stronger bonding in the pellets, and thus elevate the CCS of fired pellets.11) As can be seen from Fig. 5, the CCS of two kinds of fired pellets with basicity of 1.45 increased significantly when the firing temperature raised from 1200°C to 1250°C. The CCS of limestone fluxed pellets remained at around 2680 Newton per pellet while the CCS of hydrated lime fluxed pellets continued to increase when the firing temperature elevated from 1250°C to 1300°C. When the firing temperature reached 1275°C, the CCS of two kinds of fluxed pellets could both exceed 2600 Newton per pellet, which can meet the requirement for large blast furnace operation (>2500 Newton per pellet).28)
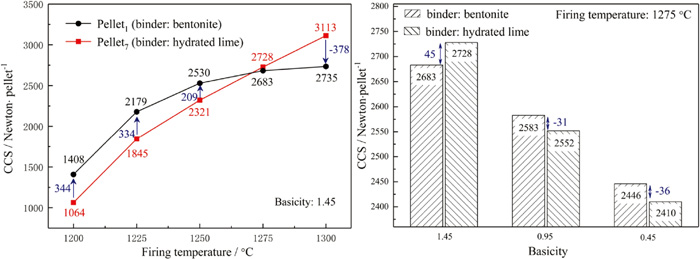
Effect of binder types, firing temperature and basicity on the CCS of fired pellets (preheating at 1050°C for 15 min, firing for 15 min). (Online version in color.)
The excessive firing temperature would increase the decomposition speed of fluxing agents, which would have an adverse impact on the strength of the firing pellets.21,26) The decomposition degree of hydrated lime in the preheating process was higher than that of limestone due to the different decomposition speed, and the limestone was hard to be decomposed completely in the preheating process according to some kinetics studies of decomposition of limestone.20,29,30) Therefore, the CCS of hydrated lime fluxed pellets exceeded the limestone fluxed pellets when the firing temperature was higher than 1275°C. The decrease of basicity was not benefit for the formation of bonding phases like CaO·Fe2O3,31) and would have an adverse impact on the CCS of the fired pellets. The decomposition of fluxing agents would decrease the CCS of fired pellets. When the basicity decreased from 1.45 to 0.45, most of CaO was provided by dolomite in the pellets in which bentonite was used as binder, while the additive amount of hydrated lime could not decrease due to the need of bind effectiveness. Therefore, the CCS of the hydrated lime fluxed pellets was lower than that of fired pellets in which bentonite was used as binder at lower basicity.
3.4. Properties of Fired PelletsThe preparation conditions of four kinds of fired pellets are showed in Table 7. The chemical and phase compositions, microstructure and metallurgical performances of the fired pellets were investigated to evaluate the quality of the fired pellets and better understand the effect of hydrated lime and limestone on the fired pellets.
| Pellet types | Basicity | Binder types | Preheating temperature/°C | Preheating time/min | Firing temperature/°C | Firing time/min |
|---|---|---|---|---|---|---|
| Pellet1 | 1.45 | 0.5% bentonite | 1050 | 15 | 1275 | 15 |
| Pellet5 | 0.45 | |||||
| Pellet7 | 1.45 | 2% hydrated lime | 1050 | 15 | 1275 | 15 |
| Pellet11 | 0.45 |
The chemical compositions of the four kinds of fired pellets are showed in Table 8. It can be observed that the content of MgO was close to 1.3% in these fired pellets. The binary basicity of pellet1 and pellet7 was found at 1.45, indicating the high basicity sinter could be completely replaced by the fired pellets in blast furnace burden. The FeO contents were found at 5.63% and 5.14% in pellet7 and pellet11, respectively, and they were obviously higher than that of Pellet1 and pellet5, indicating that a mass of Fe2O3 was reduced by reductant coal during the preheating process in hydrated lime fluxed pellets, and part of them being stabilized by MgO.
| Pellets | Constituents/wt.% | Basicity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TFe | FeO | SiO2 | CaO | Al2O3 | MgO | P | S | ||
| Pellet1 | 61.80 | 3.60 | 3.23 | 4.68 | 1.70 | 1.34 | 0.006 | 0.002 | 1.45 |
| Pellet5 | 62.46 | 3.27 | 4.62 | 2.09 | 1.69 | 1.28 | 0.005 | 0.003 | 0.45 |
| Pellet7 | 62.73 | 5.63 | 2.48 | 3.59 | 1.65 | 1.29 | 0.005 | 0.004 | 1.45 |
| Pellet11 | 60.18 | 5.14 | 7.94 | 3.57 | 1.52 | 1.30 | 0.004 | 0.003 | 0.45 |
Reduction degradation of fired iron ore pellets usually occurs at low temperatures (500°C–550°C) and has an adverse impact on the operation of blast furnace when a mass of fines is generated.32) The reduction degradation index (RDI) of the fired pellets are presented in Fig. 6. It was found that the RDI+3.15 and RDI+6.3 of hydrated lime fluxed pellets were lower than that of limestone fluxed pellets, meaning that the former has a lower reduction degradation. The decrease of basicity could increase the reduction degradation of the fired pellets, especially for the hydrated lime fluxed pellet.
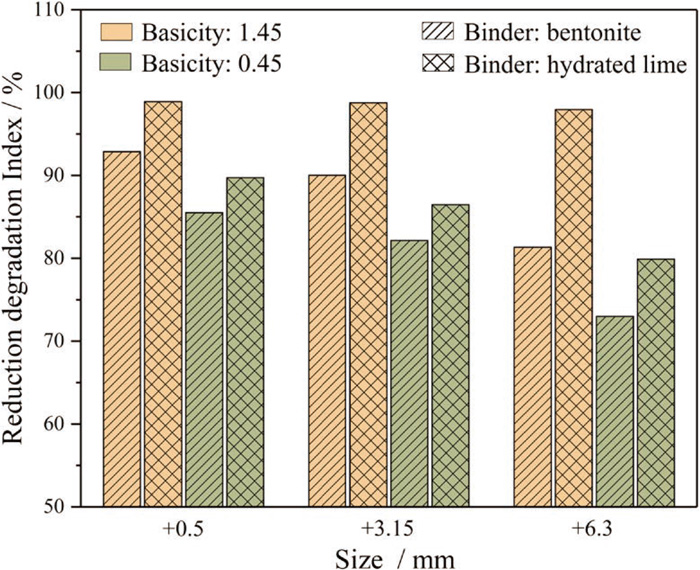
Reduction degradation index of the fired pellets. (Online version in color.)
The reducibility index (RI) can be used to evaluate the difficulty of iron oxides deoxidation in blast furnace operation. The improvement of the RI can decrease coke consumption and elevate productivity of blast furnace. It can be seen from Table 9, the hydrated lime fluxed pellet possessed higher reducibility than that of the limestone fluxed pellets when the basicity was 1.45. However, the reducibility of limestone fluxed pellets was close to that of hydrated lime fluxed pellet when the basicity was 0.45.
| Metallurgical performances | Pellet types | |||
|---|---|---|---|---|
| Pellet1 | Pellet5 | Pellet7 | Pellet11 | |
| Reducibility index/% | 68.55 | 68.14 | 70.58 | 67.81 |
| Reduction swelling index/% | 22.35 | 22.93 | 7.38 | 18.49 |
When the hematite is reduced to magnetite, the lattice transformation can lead to the volumetric expansion of the pellets, and the swelling of pellets in reduction may promote the generation of fines and decrease the gas permeability in the blast furnace.33) From Table 9, the reduction swelling index (RSI) of hydrated lime fluxed pellets with basicity of 1.45 was found at 7.38%, which was significantly lower than the RSI of limestone fluxed pellets. The decrease of basicity could significantly elevate the RSI of hydrated lime fluxed pellets but had little effect on the RSI of fired pellets in which bentonite was used as binder.
All of above results show that the hydrated lime fluxed pellets with high basicity possessed better metallurgical performances.
3.4.3. Mineralogy of Fired PelletsFigure 7 and Table 10 illustrate the phase compositions and distribution of the fired pellets. In the preheating stage, the Fe2O3 was reduced by the CO which was produced by the gasification of anthracite, and then the Fe3O4 was generated in the hematite particle. When the anthracite was exhausted in the further induration process, parts of the Fe3O4 was transformed back to Fe2O3. Therefore, it could be found that a proportion of the magnetite was distributed in the hematite particles (Fig. 7, point 1 and 2). A great deal of melt mixture of Fe2O3–CaO–Al2O3–MgO–SiO2 was found in the limestone fluxed pellets (Fig. 7(P1 and P5), point 3) and hydrated lime fluxed pellets with low basicity (Fig. 7(P11), point 3). The eutectic mixture with low melting point was distributed between the particles and acted as the bonding phase in the pellets. The crystallization of CaO·FeO·SiO2 was hard to process in the cooling stage and the glassy phase could be found in the pellets (Fig. 7(P1 and P5), point 4). A certain amount of magnesium ferrite and large amount of calcium ferrite and silico-ferrite of calcium and aluminum (SFCA) could be found in the hydrated lime fluxed pellets with basicity of 1.45 (Fig. 7(P5), point 5, 6, 7). The calcium oxide was easier to be generated from hydrated lime than limestone,20,21) which would have a positive impact on the formation of calcium ferrite and SFCA in the hydrated lime fluxed pellets with high basicity during the induration process. The calcium ferrite and SFCA are good bonding phase which could connect the particles with each other and improve the strength of the fired pellets.34)
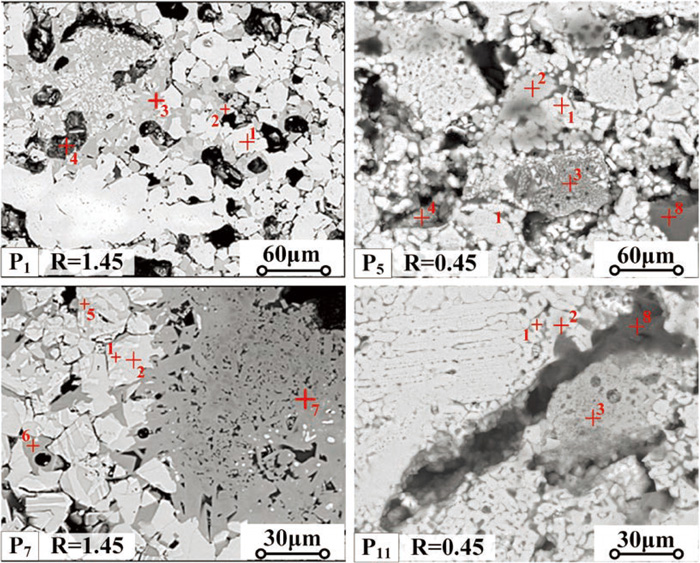
The electronic micrograph of the fired pellets (P1-Pellet1, P5-Pellet5, P7-Pellet7, P11-Pellet11). (Online version in color.)
| Point No. | Elements | Possible mineral phase | |||||
|---|---|---|---|---|---|---|---|
| Fe | Ca | Si | Mg | Al | O | ||
| 1 | 21.32 | 0 | 0 | 0 | 0 | 78.68 | Fe2O3 (hematite) |
| 2 | 43.54 | 0 | 0 | 0 | 0 | 56.46 | Fe3O4 (magnetite) |
| 3 | 29.68 | 8.57 | 5.22 | 1.81 | 2.64 | 52.08 | eutectic mixture |
| 4 | 23.24 | 11.58 | 10.61 | 0 | 0 | 54.57 | CaO·FeO·SiO2 (glassy phase) |
| 5 | 33.12 | 0 | 0 | 8.68 | 0 | 58.20 | MgFe2O4 (magnesium ferrite) |
| 6 | 30.38 | 12.40 | 0 | 0 | 0 | 57.22 | CaFe2O4 (calcium ferrite) |
| 7 | 22.03 | 6.73 | 3.88 | 0 | 10.70 | 56.66 | SFCA |
| 8 | 0 | 0 | 33.24 | 0 | 0 | 66.76 | SiO2 (quartz) |
The reflected light micrograph of the fired pellets is shown in Fig. 8. It can be observed that the diameter of the pores in the limestone fluxed pellets with basicity of 1.45 could reach 50 μm, which was obviously higher than that of pellet7, and this result was consistent with the analysis in section 3.3.2. The pores with relatively large size can decrease the CCS of fired pellets,35) therefore, the CCS of fired pellet7 was higher than of fired pellet1. The decrease of basicity was not benefit for the formation of bonding phases, therefore, the CCS of fired pellets with low basicity was lower than that of high basicity fired pellets.30) As can be seen from Figs. 7 and 8, the content of magnetite in the hydrated lime fluxed pellets was higher than the limestone fluxed pellets. In the preheating stage, the oxygen presented in the pellets was sufficient to kick off carbon combustion to produce CO, which then diffused to the surface of hematite particle and reacted with hematite to produce magnetite and CO2. The CO2 could further diffuse to carbon particles and reacted with carbon to form CO. These coupling reactions continued until the experiment was interrupted or one of the reactants was exhausted. Although part of the CO would diffuse to the outside of pellets and some oxygen would enter the pellet, part of the hematite could be reduced to magnetite in the CO diffusion process as an extremely low CO concentrate is required for the reduction of hematite.36) The reduction rate of hematite was higher in H2 atmosphere than in CO atmosphere,37) and the H2O generated from the decomposition reaction of hydrated lime could react with carbon to produce H2 while the CO2 generated from limestone reacted with carbon to form CO.38) As a result, the hematite was relatively easier to be reduced in the pellet7 and pellet11.
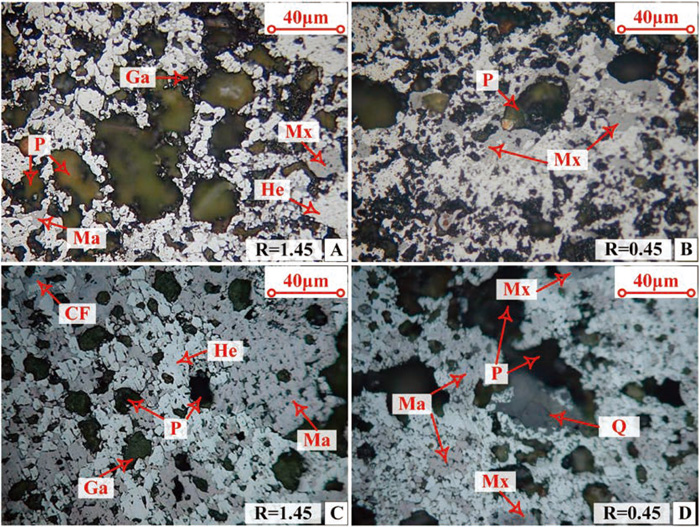
The optical micrograph of the fired pellets (A-Pellet1, B-Pellet5, C-Pellet7, D-Pellet11. He-hematite, Ma-magnetite, Ga-galss phase, Mx- melt mixture, CF-calcium ferrite, Q-quartz, P-pore). (Online version in color.)
The main mineral compositions of the fired pellets are displayed in Table 11. The content of magnetite was found as 15.74% in hydrated lime fluxed pellets with basicity of 1.45, which was much higher than that of in the limestone fluxed pellets. The CaO and MgO contributed from the limestone and dolomite mainly entered and stabilized the spinel structure of Fe2O3 by forming calcium ferrite, magnesium ferrite and SFCA in the pellet7. However, the CaO and MgO tended to combine with Fe2O3, Al2O3 and SiO2 by forming the melt mixing in the limestone fluxed pellets due to the difference between the decomposition characteristics of limestone and hydrated lime. In the fired pellet11 with basicity of 0.45, the CaO tended to react with SiO2 to form eutectic mixture due to the high ratio of SiO2 to CaO, therefore, pellet11 contained more eutectic mixture and less SFCA and calcium ferrite compared to pellet7. The data in Table 10 was consistent with the results of optical microscope and SEM-EDS analysis.
| Minerals | Hematite | Magnetite | Quartz | Melt mixture | Glassy phase | Calcium Ferrite and SFCA | Magnesium ferrite |
|---|---|---|---|---|---|---|---|
| Pellet1 | 77.10 | 9.20 | 0.17 | 9.90 | 2.30 | 0.13 | 0.30 |
| Pellet5 | 77.78 | 8.32 | 1.06 | 9.04 | 3.11 | 0.04 | 0.34 |
| Pellet7 | 71.24 | 15.74 | 1.55 | 1.31 | 2.13 | 4.25 | 2.38 |
| Pellet11 | 69.54 | 13.15 | 3.17 | 8.14 | 3.07 | 0.56 | 1.57 |
Table 9 shows that the RI of pellet1 was slightly lower than pellet7. On the one hand, the high-melting phases like magnesium ferrite in pellet7 (From Table 11) would not soften in reduction process and keep the pores open for the diffusion of reducing gas, while the pores in pellet1 were easy to be blocked by the low-melting eutectic mixture thereby hindered the diffusion of reducing gas and decreased RI of the pellets. On the other hand, the better reducibility phase such as SFCA in pellet7 could improve its RI.35) When the basicity decreased to 0.45, the contents of low-melting eutectic mixture significantly increased while the contents of high-melting phases like magnesium ferrite in hydrated lime fluxed pellet decreased, leading to an obviously decrease of RI. However, the contents of eutectic mixture and magnesium ferrite in the limestone fluxed pellets were little changed with the decrease of basicity. As a result, the difference of RI between the two kinds of pellets was negligible when the basicity was 0.45.
When the hematite was reduced to magnetite, the deformation of crystal structure will lead to the volume expansion of the fired pellets, stimulate the formation of crack and result in the degradation of the pellets.18) From Table 11, The content of magnetite in hydrated lime fluxed pellets was much higher than that of in the limestone fluxed pellets, hence the hydrated lime fluxed pellets possessed the lower RSI and RDI. Furthermore, the hematite and low-melting eutectic mixture bonds in the pellet1, pellet5 and pellet11 were not stable during reduction. Whereas, a mass of magnesium ferrite, calcium ferrite and SFCA in the hydrated lime fluxed pellets with basicity of 1.45 possessed the higher melting points and they were acted as the stable and strong bonding phase and counteracted the RDI during reduction process.39) When the hematite particles in the hydrated lime fluxed pellets are swelling, the stable magnesium ferrite, calcium ferrite and SFCA around the hematite particles will be compressed and prevent the swelling process and decrease the RSI of pellet7 effectively.27)
In current study, a novel approach of utilization of hydrated lime as effective binder and fluxing agent for the magnesium containing fluxed pellets production was investigated and the conclusions are drawn as follows:
(1) The bentonite binder could be totally replaced by hydrated lime to make fluxed pellets from hematite concentrate. When the hydrated lime was used as the binder, the green pellets with drop number of 4.8 times from 0.5 m, compressive strength of 19.7 Newton per pellet and thermal shock temperature of 385°C could be manufactured under optimum conditions, eliminating the adverse impact of silica and aluminum from the bentonite on the properties of fired pellets.
(2) The hydrated lime fluxed pellets with 1.45 basicity and 1.3% MgO possessed excellent properties and metallurgical performances (CCS of 2728 Newton per pellet, RDI (+3.15 mm) of 98.79%, RI of 70.58% and RSI of 7.38%) due to the generation of good reducibility phase such as SFCA, high-melting mineral such as magnesium ferrite and strong bonding phase like calcium ferrite in the induration process.
(3) The basicity and the decomposition characteristics of fluxing agents would affect the induration process, and the metallurgical performances of hydrated lime fluxed pellets with basicity of 1.45 were significantly better than limestone fluxed pellets and the hydrated lime fluxed pellets with lower basicity due to the different mineral composition.
We will thank Youth natural science foundation of Hunan province, China (NO. 51904347) to support our works.