2021 Volume 68 Issue 3 Pages 63-67
2021 Volume 68 Issue 3 Pages 63-67
Erianthus arundinaceus (ER) is greatly appreciated among domestic energy crops in Japan for the production of fermentable sugars from lignocellulosic polysaccharides. In this study, we developed an efficient Ca(OH)2-based pretreatment of both stems and leaves of ER at ambient temperature with the addition of a washing step for enzymatic saccharification. The recoveries of glucans and xylans in the pretreated ER after four countercurrent washing cycles were 91 and 76 %, respectively, the former being considerably higher than that of rice straw (RS) (72 %). Their saccharification ratios in the washed sample under the pressure of 1 atm CO2 were 80 and 92.5 %, respectively. The application of this simple sugar production process from ER would further support the domestic bioprocess development. ER is also foreseen to provide the additional feedstock favorable for harvesting from winter to spring in Japan, preventing a risk for feedstock shortage generated by single harvesting such as RS.
ER, Erianthus arundinaceus; RS, rice straw; DM, dry matter; TG, total glucan; TX, total xylan.
Expectations for establishing a carbon-free society through the sophistication of resource recycling are rapidly increasing as a measure to mitigate intensifying climate changes. In particular, developing a technology for producing ethanol for fuel, biomass-derived plastics, biodegradable plastics, etc. from lignocellulosic resources, such as agricultural waste, that do not compete with food is considered important.1)2) Under the basis of such a global trend, the “Roadmap for Bioplastics Introduction” was formulated in 2021 by the Japanese government (https://www.env.go.jp/recycle/mat21030210_1.pdf). In this document, the development of processes for using domestic herbaceous resources, such as rice straw (RS), is mentioned as highly important, as well as resource crops, from the viewpoint of diversification of raw material processing because of growing demands. Considering the production of fermentable sugars in Japan, the combination of various biomass resources harvested through different seasons, such as RS and stem and leaves of Erianthus arundinaceus (ER), has been recommended to minimize the storage period.3) Ike et al.4) have proposed a method for the enzymatic saccharification of these two raw resources under pressurized conditions after a pretreatment with calcium hydroxide (Ca(OH)2) followed by a carbon dioxide neutralization. In this process, for the needs of year-round supply with raw materials, it is assumed that the harvested plant resources will be used either directly, without storing, or they will be stored under the dry storage conditions. However, in Japan, there are areas where drying RS is quite difficult (mainly in the coastal areas of the Sea of Japan) because of heavy autumn rainfalls. Therefore, to promote the usage of raw materials in such regions, developing a process that would be based on the wet storage technology is necessary. Under these prerequisites, we have investigated a method for obtaining saccharified products after wet storing coupled by a Ca(OH)2 pretreatment at an ambient temperature. In our previous investigations, we have evaluated the removal efficiency of alkali from the pretreated RS by a washing step and the saccharification efficiency of washed substrates either under atmospheric pressure or using pressurized CO2 gas.5) In the present report, based on the results of the abovementioned research, we evaluated both the washing process and the enzymatic saccharification efficiency of Ca(OH)2-pretreated ER, which represents another strategic raw plant material. Furthermore, we discussed the overall comparative performance of the two types of raw material conversion processes that we have developed so far.
ER was sampled during 2012 by harvesting in a form of chips a few centimeters in diameter, which were subsequently dried and stored. Both Ca(OH)2 pretreatment at an ambient temperature and washing steps were performed using the method as previously reported for the W2C2 process (Fig. 1, Table S1; see J. Appl. Glycosci. Web site).5) Specifically, after the addition of Ca(OH)2 and distilled water to ER, the plant material was packed in an aluminum package and stored at room temperature for one month. The basic washing process involved washing the pretreated ER twice with water and then twice with water infused with CO2. Countercurrent washing was applied to economize the amount of wash water. The preliminary washing cycles were performed four times to equilibrate the washing water in the main countercurrent washing process in the model experiments #1 and #2. To evaluate washing efficiency, the washing water (WW2-P4, WW3*-P4, WW4-P4) was used for both model experiments #1 and #2. The calcium (Ca) residual ratio in the pretreated ER washed twice with water (W2R, 40 %) was lower than that of RS (48 %) reported in an earlier study5) (Fig. 2). Since the solubility of Ca(OH)2 in water was very low (1.7 g/L at 25 ℃), the most of calcium atom in washing water was supposed to be ionized with organic acids released from ER or RS with alkali treatment. Indeed, the concentration of acetic acid estimated by HPLC (Aminex HPX-87H, Bio-rad laboratories Inc., Tokyo, Japan) in washing solution WW1-P1 in Fig. 1 (28.3 mM) was higher than that of RS (16.7 mM) in the past experiment.5) The difference of lignin contents between ER and RS (25.2 % vs. 15.8 %) might affect the released acetic acid contents, and calcium washing efficiencies as a result.4) However, further washing steps with water were less effective, resulted in 30 % of Ca was remained after washing 4 times (data not shown). Therefore, water infused with CO2 was used to wash out the remainder Ca in the third and fourth washing step. The Ca residual rate of ER washed twice with water and twice with carbonated water (W2C2R) was 9 %, which was similar to that of rice straw (8 %) washed by the same method.5) The recovery efficiency of total glucans (TG), mainly composed of cellulose and starch, is considered an important parameter in washing step since glucans represent substrates for the forthcoming saccharification process. Total xylans (TX), which are mainly composed of xylose, must also be efficiently recovered if xylose is available for microorganisms in the fermentation process. After the washing step of ER, 91 % of TG and 76 % of TX were retained in the W2C2R fraction. The recovery of TX gradually decreased with the washing cycles, and its loss rate was higher than that of TG. The reduction of washing water or the use of the CaCCO (Ca capturing by carbonation) process that has no washing step might be effective to minimize both TG and TX loss.6)7)8) The loss of water-soluble carbohydrates mentioned above might be more severe in the situation that the lignocellulosic materials contain a considerable amount of sugars. We had previously reported that sweet sorgahm bagasse and wet sugarcane bagasse could be substrates for saccharification enzymes via CaCCO process.9)10) In the case that some amounts of sugar remained in the baggases after juice extracting process, these feedstocks may be unsuitable for washing process because a certain ammount of loss of sugars are inevitable.

ER, calcium hydroxide (Ca(OH)2)-pretreated ER; WmR, solid residue recovered at the washing step m; WWm-Pn, wash water recovered at the washing step m of a preliminary sequence n. After each washing step, the liquid fraction obtained after solid residue removal was centrifuged to recover a insoluble matters (IM) and a supernatant. Aeration was performed for each WW3 fraction, and each fraction was centrifuged to separate CaCO3, as a precipitate, and the WW3* fraction, as a supernatant for the next washing cycle. Two independent experiments (experiments #1 and #2) were performed to calculate the average data. WmR-n, solid residue recovered at the washing step m of the experiment n; IMm-n, IM recovered as insoluble matters after centrifugation at the washing step m of the experiment n; WWm-n, wash water recovered as supernatant after centrifugation at the washing step m of the experiment n. Both W2C2R-1 and W2C2R-2 fractions were used for enzymatic saccharification.
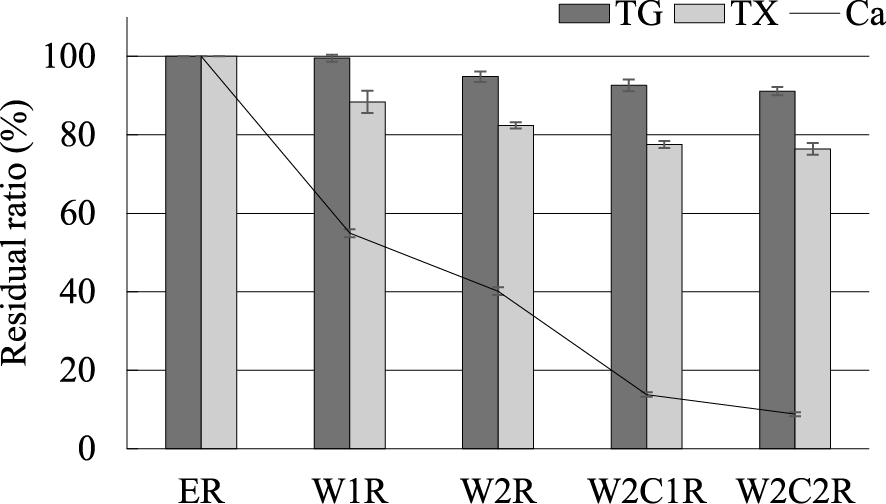
TG, dark gray bar; TX, light gray bar; Ca, line. The residual ratios were calculated as follows: weight of each substance in the pretreated ER (ER) or washed residues (g)/weight of each substance in the pretreated ER (ER) × 100 (%). The average values of the two experiments (experiments #1 and #2, Fig. 1) are shown.
To evaluate the enzymatic saccharification properties of the pretreated ER, both crude cellulase powder recovered from culture medium of Trichoderma reesei M2-1 strain11) and β-glucosidase solution (Novo 188, Novozymes Japan Ltd., Chiba, Japan) were used for the hydrolysis. The saccharification reaction followed the same procedure previously described.5) In particular, substrates of two types (pretreated ER or W2C2R) were added into airtight vials (six vials for each sample). Three vials of each substrate type were filled with nonpressurized CO2 to decrease pH since saccharification enzymes prefer slightly acidic conditions. As a control test, the pH values of another three vials were adjusted to pH 4.0–4.4 using HCl and 50 mM (final) sodium acetate buffer (pH 4.5). After a 48-h incubation with shaking at 50 ℃, the concentration of TG, glucose, TX, and xylose and pH value of each vial were measured, and recovery rates were subsequently calculated (Fig. 3). The pH values of both pretreated ER and W2C2R, neutralized with HCl and acetate buffer, were approximately 4.0–4.4, and no difference of the recovery rates in either TG or TX was observed between them. The pH value of the pretreated ER substrate filled with unpressurized CO2 was 6.4, indicating that pH conditioning was not fully successful. The pH value of W2C2R was lower (5.6) than that of the pretreated ER because its Ca ions have been removed by CO2-infused water during the washing steps. As a result, the recovery ratios of TG and TX in W2C2R were as high as that in the control vials, in contrast to their low values recorded for the pretreated ER. These results are in good agreement with the saccharification experiments on pretreated RS used as a substrate.5) A pH neutralization of the pretreated ER containing considerable Ca amounts would have needed significant amounts of acid. In addition, it would also result in the increment of the concentrations of salts in the solution and the decrement of xylose recovery rate by the inhibition of β-xylosidase, especially under high pH conditions. In the W2C2R solution, this issue was circumvented owing both low Ca content and low pH value even under unpressurized CO2 conditions. A higher salt content during the saccharification process has a high potential to become an obstacle in the forthcoming fermentation and purification steps. Therefore, the pH conditioning using nonpressurized CO2 was considered as downstream-fitted method. The obtained equivalents of glucose and xylose, which were recovered from 1 kg of ER (used as raw material before mixing with Ca(OH)2) and calculated from the recovery rates of W2C2R and ER and recovery rates during the saccharification step under nonpressurized CO2 conditions, were 263 and 151 g, respectively. In contrast, the equivalent of glucose and xylose recovered from 1 kg of RS are 217 and 99 g, respectively, as calculated from the parameters reported in the previous study.5) The estimation of ethanol yield from glucose and xylose contained in either ER or RS as substrates were 240 and 184 mL/kg respectively, on the assumption that ethanol conversion rates from glucose and xylose were 92 and 85 %.12) These values were lower than those (263 mL/kg for ER and 239 mL/kg for RS) estimated according to the parameters obtained from the aforementioned CaCCO process using high-CO2 pressure saccharification reactor.4) Although comparing these two model experiments is difficult because of the differences in numerous factors (compositions of raw materials, substrate concentration, saccharification reaction conditions, etc.), the loss of substrate during the washing step may considerably affect the results. In addition, fixed costs should also be considered when choosing the most suitable system for considering various parameters such as raw material selection, final product expectation, and locational conditions. Therefore, the overall costs for installing a high-pressure saccharification reactor and a washing device are needed to be compared from case to case. The recovery of valuable chemical compounds, including ferulic acid and p-coumaric acid, in washing water might be appreciated as a tradeoff of the washing step.13)
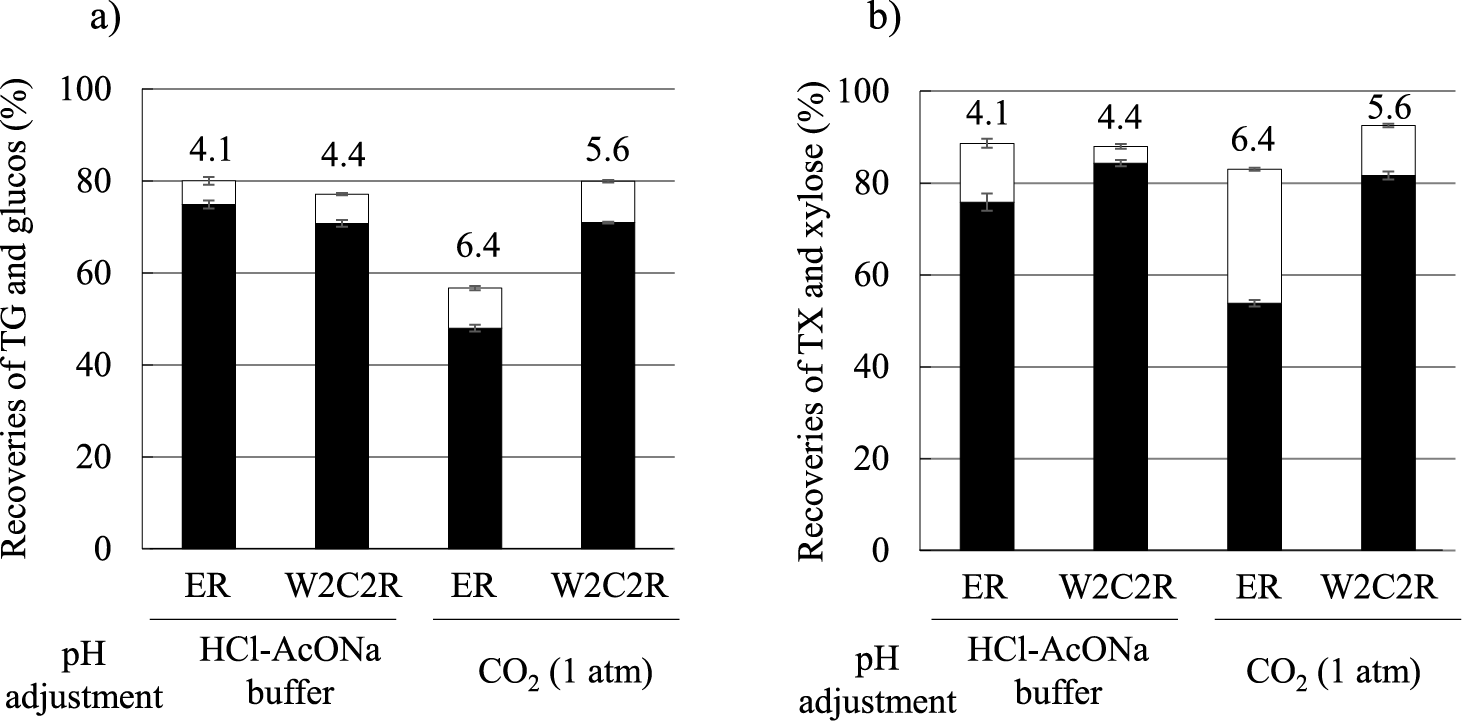
Panels (a) and (b) indicate the recovery rates of glucose and total glucans (TG) (a) and xylose or total xylans (TX) (b), respectively. Black bars indicate glucose (a) or xylose (b) recovery rates, respectively. White bars stacked on black bars indicate recovery rates of TG (a) or TX (b). The recovery rates of TG and TX were defined as follows: (weight of TG or TX released in the solution)/(weight of TG or TX in the substrate) × 100 (%). The recovery rates of glucose and xylose were defined as follows: (weight of released glucose × 0.9 or released xylose × 0.88 in the solution)/(weight of TG or TX in the substrate) × 100 (%). The numbers displayed on each bar indicated the pH after the saccharification reaction. The average values of the two experiments (experiments #1 and #2 from Fig. 1) with standard deviations are indicated.
Thus, to recover fermentable sugars from herbaceous lignocellulosics contained in ER, we evaluated a simple bioprocess composed of a Ca(OH)2 pretreatment at ambient temperature, a washing sequence using both water and carbonated water, and an enzymatic saccharification in CO2-saturated atmosphere at the pressure of 1 atm. To meet an increasing demand for domestic fermentable sugars for producing renewable fuels or plastics, a sustainable supply of feedstock from agriculture sources is foreseen as a promising option. ER could potentially offer several times more biomass over rice cultivated in the same area, and it may provide an alternative pattern of feedstock supply in terms of a harvesting season. Currently, it is expected for ER to arise less interest among farmers comparing to rice, which produces grains as the main commercial product, thus generating greater incomes. The present-day epilogue of all the facts mentioned above is reflected in smaller and more dispersed land areas used for the cultivation of ER. The information reported in this study, that is, fermentable sugars could be obtained from ER in the same manner as from RS, would facilitate a mixed use of both feedstocks for flexible sugar production in Japan.
The authors declare no conflict of interests.
We are grateful to Ms. K. Hiramoto and Ms. H. Nakayama for their excellent technical assistance. We would like to thank Enago (www.enago.jp) for the English language review. This work was supported by a grant from the Cabinet Office, Japan (Strategic Innovation Promotion Program (SIP) “Technologies for Smart Bioindustry and Agriculture”).