2023 年 70 巻 1 号 p. 1-7
2023 年 70 巻 1 号 p. 1-7
Algal sulfated polysaccharides are known to be effective hyaluronidase inhibitors. We evaluated hyaluronidase inhibitory activity of sulfated polysaccharide (SP) from Caulerpa lentillifera. Results showed that SP with IC50 of 163 µg/mL appears to allosterically inhibit the hyaluronidase activity. Main sugar composition and sulfate content of SP was estimated to be Gal, Glc, Xyl, Man, uronic acids, and sulfate in the weight percent of 27.7: 28.9: 14.6: 22.5: 3.4: 21.7. We modified the SP by desulfation and partial hydrolysis with trifluoroacetic acid (TFA) to investigate the effect of sulfate content and molecular weight on inhibition. Hyaluronidase inhibitory activity of desulfated SP, 0.1 M TFA-hydrolyzed SP and 0.5 M TFA-hydrolyzed SP were significantly lower than that of native SP, revealing that sulfate content or molecular weight is important for hyaluronidase inhibition.
AIR, alcohol insoluble residue; CPC, cetylpyridinium chloride; CPC-P, cetylpyridinium chloride precipitation; CPC-S, cetylpyridinium chloride supernatant; SP, sulfated polysaccharide from C. lentillifera; DSP, desulfated SP; HWE, hot water extraction; SP0.1, partially hydrolyzed SP by 0.1 M TFA; SP0.5, partially hydrolyzed SP by 0.5 M TFA.
Among 100 Caulerpa spp., Caulerpa lentillifera and C. racemosa are the two most popular edible green algae in the Asia-Pacific Region. Caulerpa lentillifera is one of the main sea products cultivated on a large scale in Okinawa, Japan, where it is known as Umi-budou.1)2)3) Polysaccharide is the most abundant compound in Caulerpa spp., containing up to 83.2 % of algal dry weight.3) Rather than role of basic nutrition, algal polysaccharide have been studied for wide range of physiological and biological activities, so that they play key roles in the fields of pharmaceutical, nutraceutical, cosmeceutical and functional foods.4) In recent years, bioactivities of polysaccharides, extracted from C. lentillifera have been reported including antioxidant, anticoagulant and anticancer,5)6) immune-stimulatory,2)7) anti-inflammatory,8) anti-tumor, and therapeutic microbial effects.2) Hyaluronidase inhibitory activity is another important activity, reported for algal polysaccharides. Furthermore, inhibitory activity of some enzymes such as dipeptidyl peptidase-IV and α-glucosidase were observed in C. lentillifera extract previously.9) Therefore, in this study, we focused on the inhibitory activity of hyaluronidase with polysaccharide from C. lentillifera.
Hyaluronic acid is a principal component of the extracellular matrix in connective tissue, distributed through organs and body fluids in human body.10) Since hyaluronic acid is extremely hydrophilic, it plays a vital role in maintaining skin smoothness and moistness, and reducing wrinkles, while involves in many fundamental physiological and pathological processes.11) Hyaluronidase, an enzyme for hyaluronic acid hydrolysis involves in development of inflammatory diseases, tumor invasiveness, metastasis, invasive breast adenocarcinoma, metastatic human melanoma, colon carcinoma, and glioblastoma cell lines.12) Therefore, hyaluronidase inhibitors are vital to overcome phenomena accompanied by abnormal decomposition of hyaluronic acid such as above mentioned diseases, deregulation of skin homeostasis and wounds. Particularly, hyaluronidase inhibitors likely to become increasingly important as therapeutic agent in pharmaceutical industry and antiaging agents in the cosmetic industry. Despite reporting several types of hyaluronidase inhibitors, up to date, algal acidic polysaccharides have found to be a promising potential inhibitor, for example, sulfated polysaccharide from Cladosiphon okamuranus,13) Lessonia nigrescens, Laminaria angustata,14) Undaria pinnatifida,12) Padina pavonica,15) Fucus vesiculosus,16) Monostroma nitidum,17) and Porphyridium purpureum.18) However, little is known about hyaluronidase inhibiting polysaccharide from C. lentillifera, which has already been reported to contain sulfated polysaccharide.2)7)8)
Although many studies measure the hyaluronidase inhibitory activity from algal sulfated polysaccharide, the mechanism and influencing factors were not widely understood. Li et al. suggested that sulfate content and molecular weight of the bioactive polysaccharide significantly affect their biological activities.19) Sulfated polysaccharide from green algae Monostroma nitidum and brown algae Cladosiphon okamuranus explained a strong positive relationship between sulfate content and hyaluronidase inhibitory activity.13)17) Despite lack of previous data about the effect of molecular weight of algal sulfated polysaccharide on hyaluronidase inhibitory activity, some compound such as outer layer of green coffee bean and phlorotannin from Fucales showed a positive relationship between hyaluronidase inhibitory activity and molecular weight.20)21) In contrast, Asada et al. demonstrated that in despite of hyaluronidase inhibitory activity of alginate from Lessonia nigrescens increased with increasing molecular weight up to some extent, the highest molecular weight compound (388 kDa) had decreased inhibitory activity.14) Therefore, it is clear that sulfate content and molecular weight can affect diversely on hyaluronidase inhibitory activity. So far, the effect of both sulfate content and molecular weight of sulfated polysaccharides on hyaluronidase inhibitory activity were not investigated together. Here, we investigated the hyaluronidase inhibitory activity of sulfated polysaccharides from C. lentillifera and evaluate the impact of highly influential factors: sulfate contents and molecular weight of the polysaccharides on its activity.
Algae materials. Caulerpa lentillifera purchased from the local market of Ishigaki island, Okinawa, in 2007, was used in this study. Alcohol insoluble residue (AIR) from C. lentillifera was obtained in the same manner as previously reported and kept frozen at -30 °C until start of the experiment.22) Briefly, fresh seaweed was washed with tap water, lyophilized and powdered. The algal powder was sequentially treated with 80 % ethanol, chloroform/methanol (1:1, v/v) and acetone. The residue was collected as AIR after filtration through filter paper.
Extraction and purification of polysaccharides from C. lentillifera. Polysaccharides were extracted as described by Konishi et al.22) In brief, AIR was stirred in water, and heated at 80 °C for 1 h. Supernatants from two consecutive extractions were pooled after centrifugation and named hot water extraction (HWE). Purification of acidic polysaccharides from HWE was based on cetylpyridinium chloride (CPC) precipitation as described by Tako et al.23) In brief, HWE was kept at 37 °C for 16 h after adding 2 % of CPC solution and collected CPC supernatant and CPC precipitation by centrifugation. The CPC supernatant was precipitated by the addition of 2 volume of ethanol and the CPC precipitation was re-precipitated with a three-fold volume of ethanol after dissolving in 4 M CaCl2. Then, both ethanol precipitations were stirred overnight with distilled water and freeze dried after dialysis. The final resultants from CPC supernatant and CPC precipitation were named CPC-S and CPC-P respectively.
Analysis of chemical composition. Total sugar and uronic acid (UA) contents were determined by the phenol-sulfuric acid method24) using glucose (Glc) as the standard, and m-hydroxybiphenyl method25) using galacturonic acid as the standard, respectively. To estimate sulfate content and sugar composition, polysaccharides were hydrolyzed in 2 M trifluoroacetic acid (TFA) at 121 °C for 1 h. The hydrolysate was dried to remove TFA and dissolved in distilled water. The hydrolysate was subjected to high-performance liquid chromatography with an AS4A-SC column (4 mm × 250 mm, Dionex Co., Tokyo, Japan) to measure sulfate content. The column was eluted at 1 mL/min at room temperature with buffer containing 1.7 mM NaHCO3 and 1.8 mM Na2CO3.22) The weight of sulfate in the sample was calculated from a calibration curve using Na2SO4 as a standard based on the molecular weight of HSO3. The content of sulfate was calculated using the following equation.
Hyaluronidase inhibitory activity. Hyaluronidase inhibitory activity of the extracted polysaccharides (HWE, CPC-S and CPC-P) was estimated following procedures outlined in previous reports with a slight modification.12)14) Hyaluronic acid sodium salt from rooster comb was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) and molecular weight was estimated to be 2.38 × 106 by size-exclusion chromatography by using a column of TSKgel G5000 PWXL (7.8 mm × 300 mm, Tosoh Co., Kyoto, Japan) as the method described in this study (Data not shown). Hyaluronidase type I-S from bovine testis (451 U/mg) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and a enzyme unit (U) is defined as the amount of enzyme that liberates one micromole of N-acetylglucosamine from hyaluronic acid per minute at 37 °C and pH 4.0.26) First, the reaction mixture containing 100 µL of hyaluronidase (5 mg/1.5 mL) in 50 mM sodium acetate buffer (pH 4.5) and 200 µL of polysaccharide sample was incubated at 37 °C for 15 min. Then, 700 µL of hyaluronic acid (1 mg/mL) as a substrate, containing 100 µL 1.5 M NaCl and 18 µL of 1 M sodium acetate buffer (pH 4.0) was added and further incubated at 37 °C for 15 min. The reaction was stopped by boiling at 100 °C for 10 min. Quantitative analysis of N-acetyl-amino sugar (product) was determined by modified Morgan-Elson method.27) Percentage inhibition was calculated as follows:
In order to evaluate the dose-dependent manner of inhibitor, inhibition rate was measured at different concentrations of CPC-P (0-339 µg/mL), and also of fucoidan (0-358 µg/mL) extracted from Okinawa mozuku (Cladosiphon okamuranus) as described by Tako et al.23) to compare the mechanism of inhibition with known hyaluronidase inhibitory polysaccharide. Furthermore, the mechanism of action of hyaluronidase inhibition was characterized using two different strategies - desulfation and partial hydrolysis - to examine the effect of sulfate content and molecular weight.
Chemical desulfation of CPC-P was performed using the method of Shiroma et al.28) Sample (100 mg) was dissolved in distilled water (20 mL) and passed through DOWEX 50W × 8 (H+, 100 mesh) resin purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). After neutralization with pyridine, the solution was lyophilized. The lyophilized pyridinium salt was dissolved in DMSO:MeOH (9:1; v/v, 20 mL). The mixture was heated at 80 °C for 4 h and then desulfated product was dialyzed against distilled water and lyophilized to obtain desulfated CPC-P (DSP).
Determination of molecular weight . Extracted CPC-P was partially hydrolyzed using 0.1 M or 0.5 M TFA at 70 °C for 3 h to obtain low molecular weight CPC-P. After removing TFA by drying, the hydrolysates were dissolved in distilled water. Partially hydrolyzed CPC-P in 0.1 M and 0.5 M TFA were named SP0.1 and SP0.5, respectively. Molecular weights of CPC-P, DSP, SP0.1 and SP0.5 were determined by size-exclusion chromatography (LC-6A; Shimadzu Co., Kyoto, Japan) equipped with a column of TSKgel G5000 PWXL (7.8 mm × 300 mm, Tosoh Co., Kyoto, Japan) and a refractive index detector RID-10A at 35 °C.28) The column was eluted by water at a flow rate of 1 mL/min. Pullulan P-10 (molecular weight = 0.96 × 104), P-50 (4.71 × 104), P-200 (20.0 × 104) and P-800 (70.8 × 104) (Showa Denko Co., Tokyo, Japan) were used as the molecular weight standards.
Preparation and characterization of C. lentillifera polysaccharides.
The yield and chemical composition of polysaccharides extracted from C. lentillifera are shown in Table 1. Polysaccharides were initially extracted from 5 g of AIR of C. lentillifera by hot water treatment and HWE consisted of mainly galactose (Gal), Glc, xylose (Xyl), mannose (Man) together with UA, and a small amount of fucose (Fuc), rhamnose (Rha), arabinose (Ara) residues. After purifying the polysaccharide with CPC, yields of CPC-S and CPC-P were 275 mg and 463 mg, respectively. Sugar composition analysis showed that CPC-S contained 91.4 % Glc, but UA, Gal, Xyl, and Man represented less than 5 %. The CPC-P contained 21.7 % sulfate and 53 % total sugar, including mainly Gal, Glc, Xyl, and Man. The molar ratio of sugar in CPC-P was estimated to be Gal, Glc, Xyl, and Man in the ratio of 0.2: 0.2: 0.12: 0.16 (Table 2). These data indicate that CPC-P is SP.
| Sample | Yield (mg) | Total sugar in mg (%) | Monosaccharide (wt %) | SO3− (%) | |||||||
| Fuc | Rha | Ara | Gal | Glc | Xyl | Man | UA | ||||
| HWE | 1942 | 753 (39%) | 0.3 | 0.2 | 0.3 | 21.6 | 39.8 | 20.4 | 14.1 | 3.3 | 11.7 |
| CPC-S | 275 | 247 (90%) | 0.1 | n.d. | n.d. | 2.5 | 91.4 | 0.7 | 0.7 | 4.6 | 6.6 |
| CPC-P | 463 | 244 (53%) | 0.6 | 0.3 | 0.8 | 27.7 | 28.9 | 14.6 | 22.5 | 3.4 | 21.7 |
n.d.: less than 0.1. (in 5 g AIR)
| Algae | IC50 (µg/mL) | Molar ratio of monosaccharide | SO3− (%) | Molecular weight | References | ||||||||
| Fuc | Ara | Gal | Glc | Xyl | Man | Rha | Rib | UA | |||||
| Caulerpa lentillifera | 163.3 | n.d. | 0.02 | 0.20 | 0.20 | 0.12 | 0.16 | n.d. | − | 0.03 | 21.7 | 169 × 104 | This study |
| Undaria pinnatifida | 13.0 | 0.14 | − | 0.14 | − | − | − | − | − | n.d | 0.71* | 6 × 104 | Katsube et al., 200312) |
| Cladosiphon okamuranus | 25.6 | 0.75 | − | − | − | 0.04 | − | − | − | 0.21 | 13.2 | − | Tako and Minami, 200813) |
| Fucus vesiculosus | 2.9 | 0.49 | 0.02 | 0.03 | 0.08 | 0.04 | − | − | − | 0.34 | 27.0 | 735 kDa | Pozharitskaya et al., 202016) |
| Porphyridium purpureum | 210.0 | − | − | 0.31 | 0.14 | 0.43 | − | − | 0.03 | 0.09 | 4.5 | 500 kDa | Mase et al., 201318) |
| Monostroma nitidum | 145.0 | − | − | n.d. | 0.08 | 0.02 | − | 0.75 | − | 0.15 | 20.0 | 70 × 104 | Yamamoto et al., 201635) |
*Molar ratio, n.d.: less than 0.01, Rib: Ribose.
Hyaluronidase inhibitory activity of polysaccharides extracted from C. lentillifera.
The hyaluronidase inhibitory activities of HWE, CPC-S and CPC-P (SP) were 32.3, 24.7 and 90.4 %, respectively, at a polysaccharide concentration of 339 µg/mL (Fig. 1). It was clear that there was a low percentage of inhibition (0-2 %) at low SP concentration (0-40 µg/mL) but higher SP concentration (200-339 µg/mL) required to inhibit reaction at a higher rate (70-92 %) (Fig. 2a). Thus, the inhibition curve was distinctly sigmoid in shape and IC50 of SP was 163 µg/mL. We compared the mode of hyaluronidase inhibition of SP with known hyaluronidase inhibitors such as fucoidan and it showed a hyperbolic curve as shown in Fig. 2b.
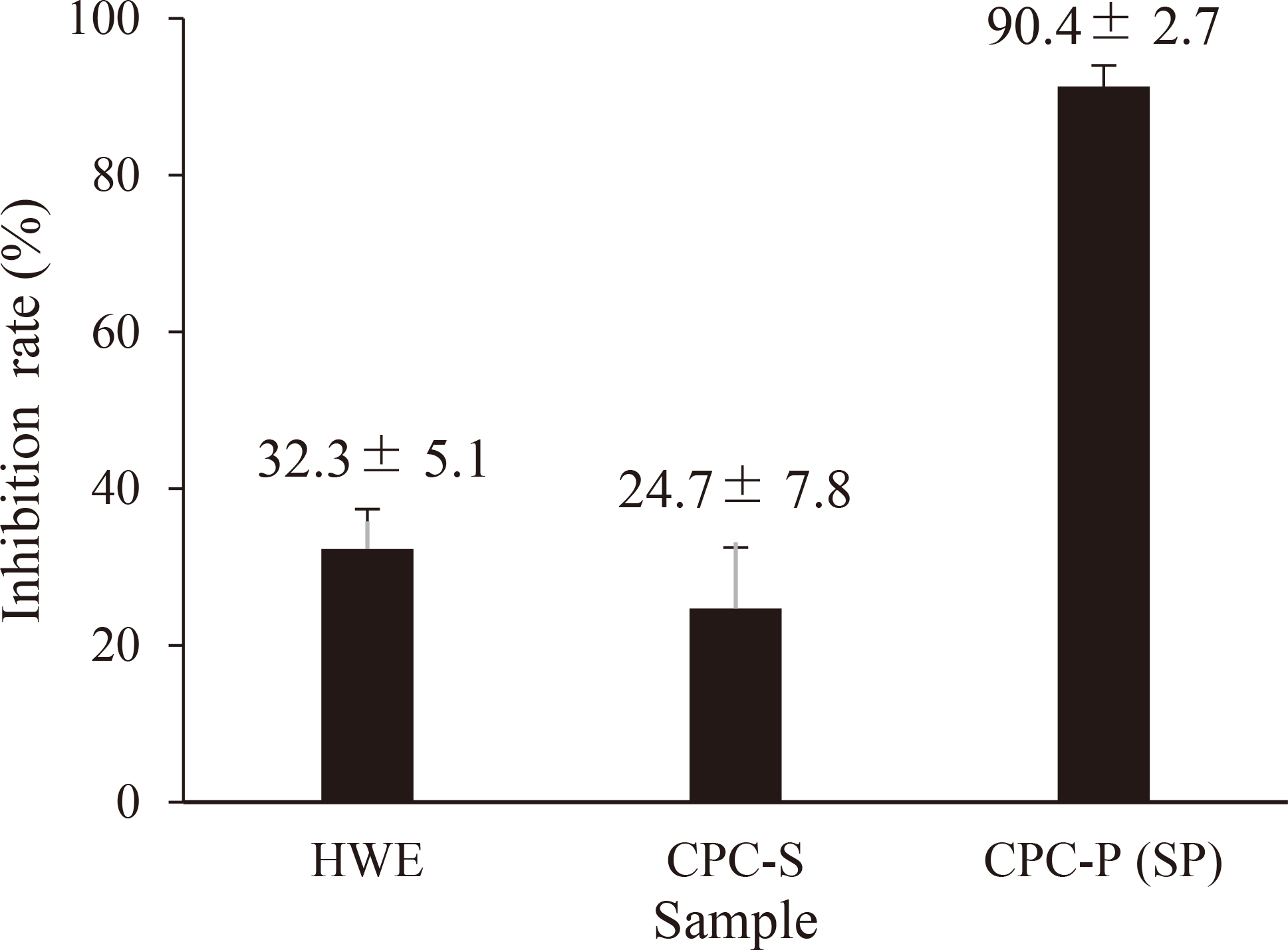
Data are shown as mean ± SD.
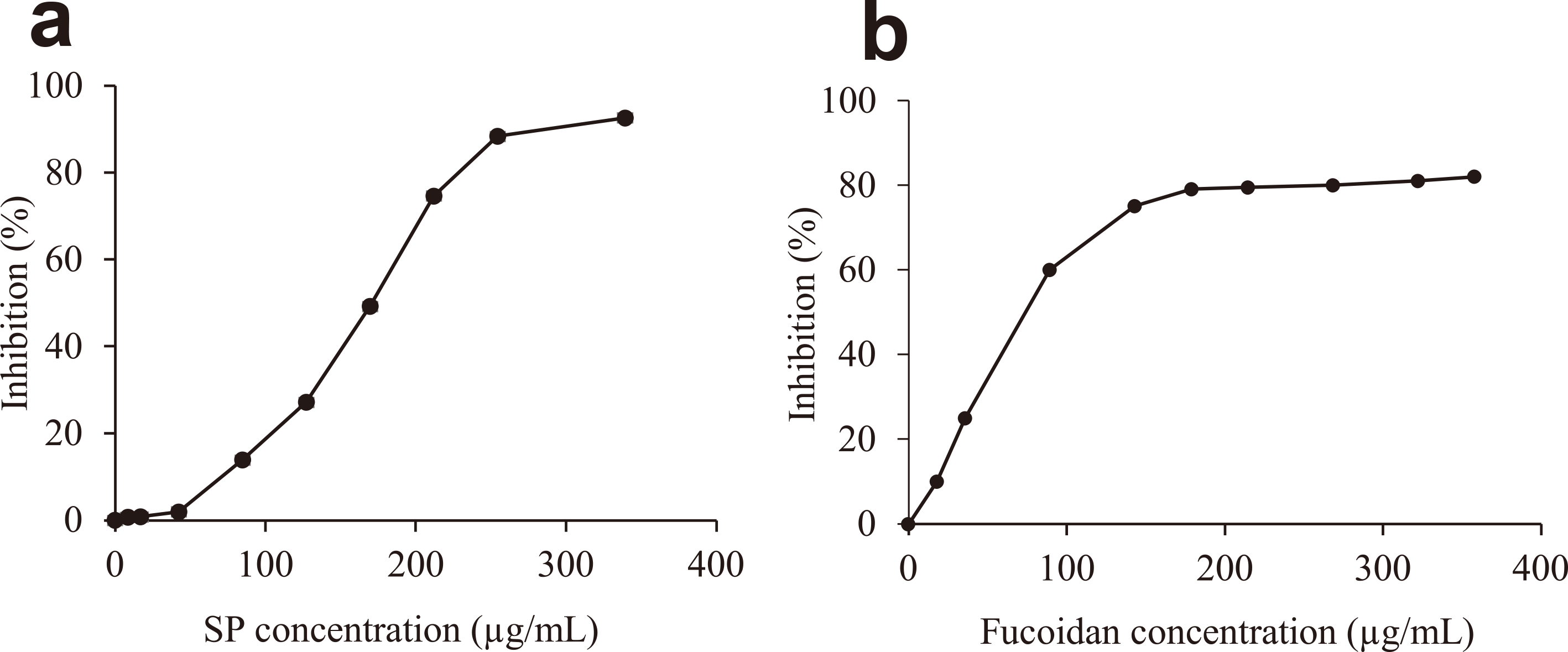
Effect of sulfate content of SP on hyaluronidase inhibitory activity.
We prepared DSP to investigate the effects of sulfate content in the polysaccharide on hyaluronidase inhibition. After desulfation, sulfate content of DSP decreased to 1.6 %. Results highlighted that desulfation of SP decreased to -0.9 % of inhibitory activity, which showed no inhibition of hyaluronidase (Fig. 3). Profiles of the molecular weight distribution of SP and DSP (Fig. 4a, d) indicated approximately similar molecular weight distribution with a retention time at 4.5-5.5 min, suggesting that the desulfation reaction didn't affect any depolymerization or unfavorable chemical changes in the polysaccharide molecules. The peak molecular weight of SP and DSP were 169 × 104 and 155 × 104 respectively.
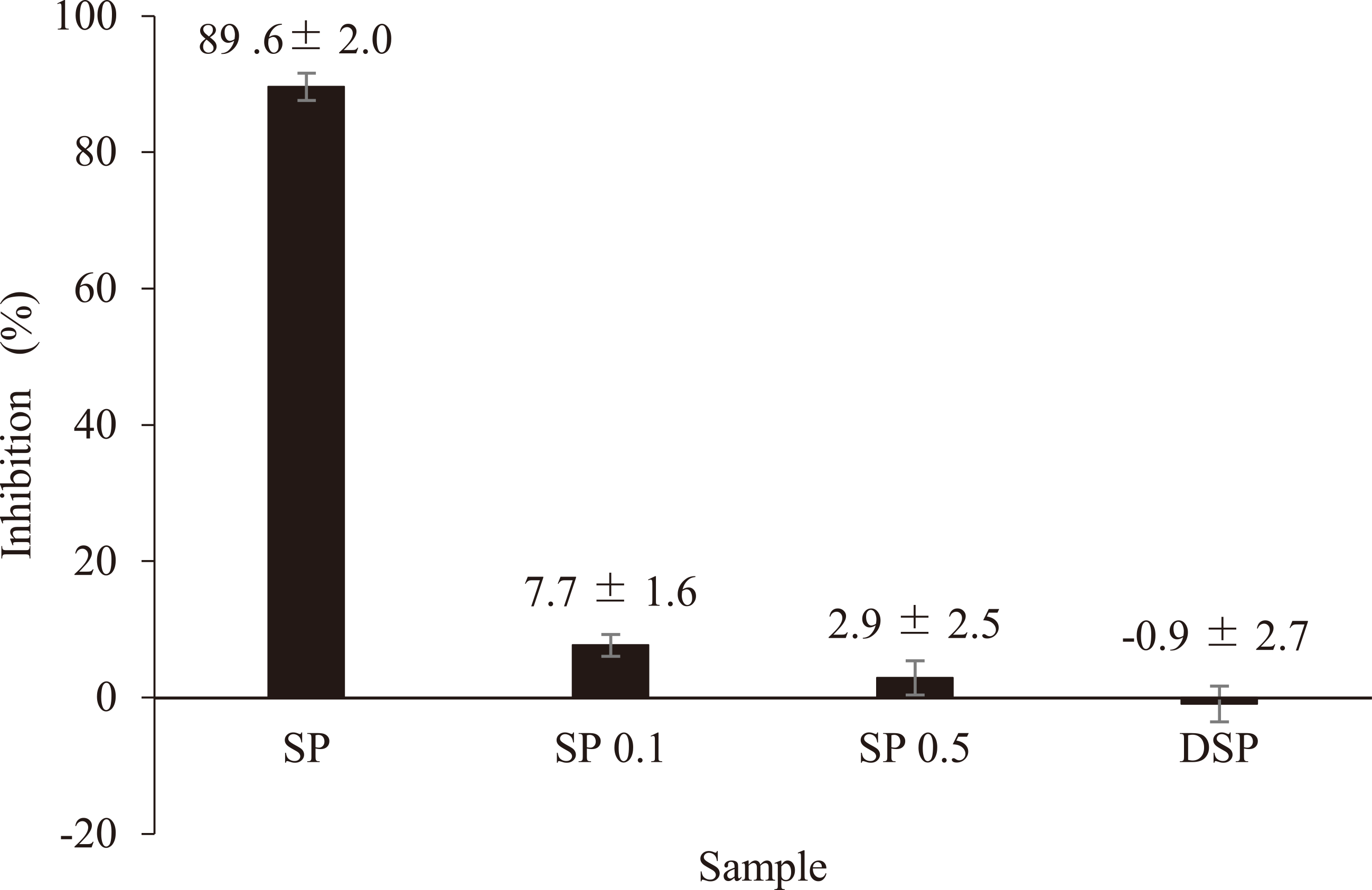
Data are shown as mean ± SD.
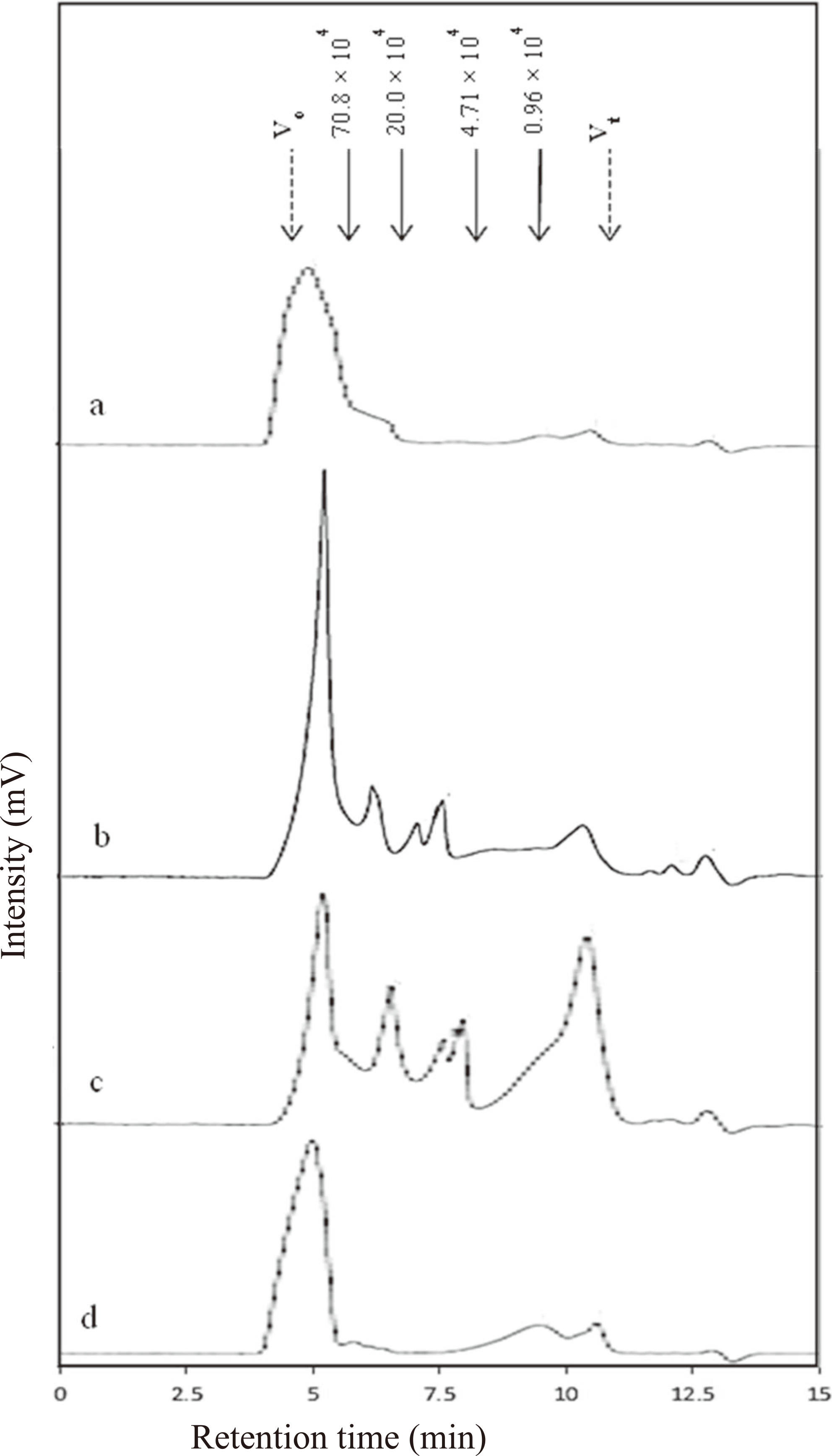
Solid arrows indicate the elution positions of size standards of pullulan with molecular weight of highest peak. Dashed arrows indicate the void volume (Vo) and total column volume (Vt).
Effect of molecular weight of SP on hyaluronidase inhibitory activity.
In order to examine the effect of molecular weight on the inhibitory activity, we partially hydrolyzed the SP using 0.1 or 0.5 M TFA to obtain a SP of lower molecular weight than native SP. The native SP showed molecular weight of about 169 × 104 with one main peak using size-exclusion chromatography (Fig. 4a). Approximately, 30 % of SP0.1 was composed of polymers of low molecular weight range of 42.2 to 0.4 × 104 (Fig. 4b) and 70 % of SP0.5 contained low molecular weight polymers of range 23.7 to 0.4 × 104 (Fig. 4c). Increasing the concentration of TFA in hydrolysis clearly increased production of low molecular weight compounds, and inhibitory activities of low molecular weight SP0.1 and SP0.5 were 7.7 and 2.9 %, respectively (Fig. 3).
The water-soluble polysaccharides derived from Caulerpa spp. mainly contain glucans and sulfated polysaccharide such as sulfated xyloarabinogalactans,29) and xylogalactomannans.30) Among these, previous studies revealed that sulfated polysaccharides were found to be a highly bioactive compound. This time we found hyaluronidase inhibitory activity with sulfated polysaccharide from C. lentillifera in terms of the effect of sulfate content and molecular weight. Our result confirmed that purified sulfated polysaccharide show significantly high activity of 90.4 % in 339 µg/mL of SP compared to other polysaccharide fractions. Chaiklahan et al. suggested that sulfated polysaccharide from Caulerpa spp. generally contained approximately 8-23 % sulfate.1) Presence of 21.7 % of sulfate in CPC-P confirmed that it was a sulfated polysaccharide, mainly composed of Gal, Glc, Xyl, Man, and a minor amount of UA (Table 3). In past years, several sulfated polysaccharides were extracted and purified from Caulerpa spp. as listed in Table 3. Comparison of monosaccharide composition with preceding studies found that Gal, Glc, Xyl and Man are generally present in sulfated polysaccharide in Caulerpa spp.2)30)31)32) In addition to Gal, Glc, Xyl, and Man a considerable amount of Ara contains in sulfated heteroglycan extracted from C. filiformis,28) and C. racemosa.32) Although sulfated polysaccharide derived from C. lentillifera consisted mainly of Gal, Glc, Xyl, and Man, their composition and molecular weight may differ due to different source material, habitat, environmental parameters, and processes of extraction and purification as shown in Table 3.33)
| Algae | Habitat | Purification method | Monosaccharide (wt%) | SO3− (%) | Molecular weight | References | |||||
| Gal | Glc | Xyl | Man | Ara | UA | ||||||
| C. lentillifera | Ishigaki, Okinawa | CPC precipitation | 27.7 | 28.9 | 14.6 | 22.5 | 0.8 | 3.4 | 21.7 | 1.69 × 106 | This study |
| C. lentillifera | Onna village, Okinawa | Superdex column | 44.2* | 2.2* | 49.3* | − | − | 4.3* | 21.5 | >100 kDa | Maeda et al., 20122) |
| C. lentillifera | Changai island, China | DEAE column | 43.2 | − | − | 38.7 | − | 2.3 | 21.3 | 3878 kDa | Sun et al., 201830) |
| C. filliformis | Cape town, South Africa | Remove glucan by α-amylase | 5† | n.d | 2† | 2† | 1† | − | 17.6 | − | Macki and Percival, 196031) |
| C. rasemosa | Gujarat, India | DEAE column | 43.0 | 2.0 | 27.0 | n.d | 28.0 | − | 12.0 | 80 kDa | Chattopadhyay et al., 200732) |
n.d.: less than 0.1, *Molar %, †Molar ratio.
It was reported that IC50 value of hyaluronidase inhibitors derived from sulfated polysaccharide of seaweeds were ranging from 2.9 to 210 µg/mL as shown in Table 2. We also examined the substantial hyaluronidase inhibition of SP with IC50 of 163 µg/mL. Yamamoto et al. revealed that 100 µg/mL of natural sulfated polysaccharides such as porphyrin, rhamnan sulfate, κ-carrageenan, fucoidan and λ-carrageenan showed 25, 40, 35, 70, and 80 % of hyaluronidase inhibitory activity, respectively17), while our study showed 20 % of activity at the same SP concentration (Fig. 2a). Furthermore, mode of inhibition of SP was predicted to be allosteric due to the sigmoid shape in dose-dependent curve, but it was hyperbolic shape in fucoidan. Different shapes of the hyaluronidase inhibitory curves such as sigmoid and hyperbolic were also recorded in phlorotannin from brown seaweeds Cystoseira nodicaulis and Fucus spiralis respectively.21) Furthermore, sigmoid dose-dependent curve was reported for pectic acid and further clarified its hyaluronidase inhibitory mechanism as non-competitive.34) Moreover, mode of hyaluronidase inhibition of SP derived from Monostroma nitidum17) was found to be competitive but inhibition was of mixed uncompetitive-noncompetitive type in Undaria pinnatifida.12) Taken all together, it became possible to state that different shapes of the dose-dependent inhibition curve might be due to various inhibition patterns of the inhibitors. However, further enzyme kinetic analysis is required to elucidate the exact mechanism of SP in hyaluronidase inhibition.
Regarding influencing factors of hyaluronidase activity, Li et al. suggested that sulfate content and molecular weight of the polysaccharide significantly affect their biological activities.19) Therefore, we modified SP by desulfation to determine the effect of sulfate content and used TFA hydrolysis to assess the impact of molecular weight on the inhibition. It was found that existence and spatial positioning of sulfate groups on polysaccharides play key roles in their biological activity profile. Sun et al found that sulfate groups can substituted at C-3 positions of xylose and mannose, and C-6 positions of galactose of xylogalactomannan from C. lentillifera.30) In our study, inhibitory activity of native SP was significantly decreased -0.9 % after desulfation as shown in Fig. 3, suggested that the sulfate group in the inhibitor remarkably influenced hyaluronidase inhibitory activity. The present finding agreed with the finding of Yamamoto et al. of a strong positive relationship between sulfate content and hyaluronidase inhibitory activity in rhamnan sulfate from Monostroma nitidum with different sulfate contents (0.5, 7, 20, and 41 %) in different polysaccharide concentrations (0.1, 0.12, 0.16, 0.18, and 0.2 mg/mL).17)35) Moreover, Toida et al. found that chemically over-sulfated glycosaminoglycans (chondroitin sulfate, dermatan sulfate, heparan sulfate, heparin and hyaluronan) showed stronger hyaluronidase inhibitory activity (IC50: 1.35, 1.33, 0.78, 1.28, and 1.14 µg/mL, respectively) than the original glycosaminoglycans.36) Collectively, the present results provide support for the well-known fact that hyaluronidase inhibitory activity greatly depends on presence of the sulfate group.
Several reports have demonstrated a correlation between the molecular weight of inhibitor and hyaluronidase inhibitory activity.37)38)39) Although significantly stronger inhibitory activity was reported by high molecular weight native SP in our study, it was significantly low in SP0.1 and SP0.5 (Fig. 3), suggesting that decreasing of molecular weight may affect reduction of inhibitory activity. This reduction may also be due to the partial removal of the sulfate groups from the polysaccharide during hydrolysis. However, previous studies evident that occurrence of desulfation is negligible during the partial hydrolysis of sulfated polysaccharide with diluted TFA (0.1-0.75 M) at slightly elevated temperatures (60-100 °C) for several hours (1-2 h).40)41)42) Therefore, decreasing of molecular weight seems to cause reduction of inhibitory activity in our study. Similar results were reported by Asada et al. who demonstrated that hyaluronidase inhibitory activity of alginate from Lessonia nigrescens increased with increasing of the inhibitor molecular weight within the range of 150-370 kDa.14) In contrast, Asada et al. found that polysaccharides with the highest molecular weight (388 kDa), generally had decreased inhibitory activity.14) Furusawa et al. also discovered that higher molecular weight acidic polysaccharide from outer layer of green coffee bean contributed most to the hyaluronidase inhibition.20) All these results suggest that long chains of polysaccharide with high molecular weight are required for hyaluronidase inhibitory activity.
Nevertheless, the sulfate content of SP appeared to contribute more than molecular weight did to the inhibition, because DSP showed a significantly lower inhibitory activity despite having a high molecular weight similar to SP (Fig. 4a, d). Consistently, Furusawa et al. revealed that UA content of an acidic polysaccharide contributed more to the hyaluronidase inhibitory activity than its molecular weight.20) The present results indicate that hyaluronidase inhibitory activity of acidic polysaccharides mainly depended on action of the acidic group in the inhibitor compared to other factors. Beside sulfate content and molecular weight, hyaluronidase inhibitory activity and the mechanisms of action of sulfated polysaccharide are influenced by a number of factors, including type of polysaccharide backbones and glycosidic linkages present in the polysaccharide.39) This hypothesis could be further confirmed by comparison of inhibitory activity with sugar composition, sulfate content and molecular weight of SP and other sulfated polysaccharides reported in many studies (Table 2). Based on the result and references, it can be suggested that hyaluronidase inhibitory activity might be affected by more than single feature of the inhibitor molecule.
In the present study, SP, mainly consisted of Gal, Glc, Xyl, and Man found to effectively inhibit hyaluronidase activity. This activity correlated with sulfate content and molecular weight of SP. However, molecular weight alone was not likely sufficient, and the sulfate group was essential to inhibit hyaluronidase activity. It can be suggested that hyaluronidase inhibitory activity by SP might be affected by more than single feature of the inhibitor molecule.
The authors declare no conflicts of interest