2023 年 70 巻 4 号 p. 81-97
2023 年 70 巻 4 号 p. 81-97
This review discusses sugar isomerization with organogermanium compounds. Organogermanium compounds markedly increase the aldose-ketose (glucose-fructose or lactose-lactulose) isomerization ratio, double the initial reaction rate, and significantly reduce the base-catalyzed degradation of sugars. 1H-nuclear magnetic resonance analysis reveals that the affinity of organogermanium compounds with a 3-(trihydroxygermyl)propanoic acid (THGP) structure toward ketoses is 20-40 times stronger than that toward aldoses; thus, such organogermanium compounds form complexes more readily with ketoses than with aldoses. Stable ketose complexes, which contain multiple cis-diol structures and high fractions of furanose structures, suppress the reverse ketose-aldose reaction, thereby shifting the equilibrium toward the ketose side. These complexes also protect sugar molecules from alkaline degradation owing to the repulsion between anionic charges. The increased rate of the initial reaction in the alkaline isomerization process results from stabilizing the transition state by forming a complex between THGP and a cis-enediol intermediate. The cyclic pentacoordinate or hexacoordinate THGP structures give rise to a conjugated system of germanium orbitals, which is extended through dπ-pπ interactions, thereby improving the stability of the complex. Based on these results, we have developed a bench-scale lactulose syrup manufacturing plant incorporating a system to separate, recover, and reuse organogermanium poly-trans-[(2-carboxyethyl)germasesquioxane]. This manufacturing plant can be used as a model of an alkaline isomerization accelerator for continuous industrial production.
THGP, 3-(trihydroxygermyl)propanoic acid; Ge-132, poly-trans-[(2-carboxyethyl)germasesquioxane]; HFS, high-fructose syrup; NMR, nuclear magnetic resonance; GPhe, β-(trihydroxygermyl)phenylalanine; N-Ac-GPhe, N-acetyl-β-(trihydroxygermyl)phenylalanine; GAla, β-(trihydroxygermyl)alanine; N-Ac-GAla, N-acetyl-β-(trihydroxygermyl)alanine; GSuc, 2-(trihydroxygermylmethyl)succinic acid; DM-THGP, 3,3-dimethyl-3-(trihydroxygermyl)propanoic acid; HPLC, high-performance liquid chromatography; Km, Michaelis constant; Vmax, maximum velocity; Ks, complex formation constant; Kd, apparent dissociation constant; ED, electrodialyzer; EDI, electrodeionizer; ED-BP, ED with a bipolar membrane.
The aldose-ketose isomerization process, which generally employs an isomerase or an alkaline catalyst, produces sugars such as high-fructose syrup (HFS) and lactulose syrup. HFS is a mixed liquid sugar obtained by converting D-glucose to the sweeter D-fructose through an enzyme-catalyzed reaction. The original glucose for this transformation is obtained from starch. In this process, the starch is initially liquified using α-amylase and subsequently saccharified with a combined action of glucoamylase and pullulanase, resulting in glucose production. Japanese researchers in the mid-to-late 1960s played an extremely important role in the initial investigations into the isomerization of glucose using glucose isomerase.1)2) The development of immobilized glucose isomerase progressed throughout the 1970s, and a viable filled-type bioreactor was eventually established to produce HFS from glucose continuously. The isomerization ratio of this enzymatic reaction reaches equilibrium at approximately 50 % (reaction temperature: 60 ºC); however, during sweetener production, isomerization is generally stopped after the conversion of approximately 42 % of the original substrate to prevent changes in color and degradation of the components. The fructose in the reaction solution is then separated from glucose using an ion-exchange resin. The fructose-rich fraction (approximately 90 % fructose) is combined with glucose to obtain a product with a fructose content of 55 %, the typical fructose content of commercially available sweeteners. The manufactured HFS is commonly used to replace sugar in soft drinks, bread, and liquid seasonings. In Japan, over one million tons of HFS are produced yearly,3) making it a major starch application. The base-catalyzed Lobry de Bruyn-Alberda van Ekenstein transformation, developed simultaneously with enzymatic isomerization, shows strong potential for practical application but has various limitations preventing its commercial use, including low yield. Recently, however, the reaction has been used as a method to produce sweeteners containing rare sugars4) (such as psicose) and chemical intermediates5) (such as biomass-derived 5-hydroxymethylfurfural and levulinic acid).
Lactose, also known as 4-O-β-D-galactopyranosyl-D-glucose, is a disaccharide comprising galactose and glucose. It is naturally found in cow's milk and whey, a byproduct of cheese production, at a concentration of roughly 5 %. Japan, for instance, produces nearly 19,000 t of whey annually, as per the data for the fiscal year 2022.6) Through alkaline isomerization, lactose is converted into lactulose, a keto-type disaccharide. This transformation essentially involves replacing glucose residues with fructose residues, resulting in the formation of 4-O-β-D-galactopyranosyl-D-fructose.7) Lactulose is used worldwide as a medicine and functional food to treat hepatic encephalopathy8) and constipation9) as well as a prebiotic to improve intestinal flora.10) However, the alkaline isomerization of lactose does not produce a lactulose yield greater than 25 %, and multiple degradation products are formed during this reaction, thereby reducing the production efficiency of the batch method. Moreover, an enzyme that converts lactose to lactulose sufficiently for practical application in the pharmaceutical and food industries has yet to be discovered. Hence, large-scale lactulose production remains challenging and expensive, and its practical applications are limited.
Chemical equilibrium is generally controlled by adjusting the reaction conditions, including the temperature, pressure, and solvent polarity, among other parameters. Shifting the reaction equilibrium toward the ketose side is necessary to increase the isomerization yield. In addition to optimizing the parameters above, removing reaction products (ketoses such as fructose and lactulose) from the system will also shift the equilibrium toward the products. Tewari and Goldberg11) established the relationship between the reaction temperature and equilibrium constant and found that the reaction temperature should be maintained at 87 ºC to ensure an isomerization ratio of 55 %. Similarly, Visuri and Klibanov12) achieved an isomerization ratio of 55.9 % in a solution containing 85 % ethanol. The enzyme glucose isomerase is relatively resistant to high temperatures and solvent concentrations; however, its reactivity is poor under these conditions.
Carbohydrates (especially ketoses) tend to form complexes with calcium and oxoanions such as boric acid, sodium aluminate,13)14)15)16) and germanium dioxide.17)18)19)20)21) In an alkaline solution, germanium dioxide produces a germanate ion, and the reverse ketose-aldose reaction is suppressed by stabilizing the ketose form of the sugar, which increases the isomerization ratio. However, germanium dioxide accumulates in the kidneys and causes serious renal failure when consumed continuously or in large amounts.22) Consequently, it is deemed unsafe to use in pharmaceuticals and food products. To address this issue, we directed our attention toward organogermanium compounds (discussed in Section 2) that have been extensively researched. These compounds feature germanium atoms covalently bonded with organic acids such as propanoic acid.
In 1991, we began studying the interactions of sugars with organogermanium compounds to promote the isomerization of sugars.23)24)25)26)27)28)29)30)31) The present work has identified the effect of organogermanium compounds on sugar isomerization, the affinity of these compounds toward sugars and, consequently, their ability to form organogermanium-sugar complexes as well as the mechanism by which the isomerization reaction can be promoted.32)33)34)35) The influence of organogermanium compounds on the enzymatic and alkaline isomerization reactions between aldoses and ketoses was investigated by considering their affinity for saccharides with the 3-(trihydroxygermyl)propanoic acid (THGP) structure. The complexation of ketoses with various cis-diol structures and organogermanium compounds with the THGP structure was also evaluated using 1H-nuclear magnetic resonance (NMR) spectroscopy. Several factors promoted the isomerization reaction of organogermanium compounds, including the formation of stable complexes with ketoses, protection against degradation under alkaline conditions, formation of complexes with cis-enediol intermediates, and stabilization of the transition state by the extension of the conjugated orbital system via cyclic pentacoordinate or hexacoordinate THGP structures. Finally, a bench-scale syrup manufacturing plant incorporating a reaction accelerator system that could separate, recover, and reuse organogermanium compounds was constructed and used to prepare a lactulose-containing syrup.
This review discusses the fundamental and applied research into promoting sugar isomerization reactions using organogermanium compounds.
This section summarizes the sugar isomerization reactions that occur in the presence of organogermanium compounds with the THGP structure (THGP and THGP derivatives).
2.1 Organogermanium compounds.
Germanium is a group 14 element with the symbol Ge, an atomic number of 32, and an atomic weight of 72.63 (IUPAC, 2023). The simple form of germanium is customarily called “metallic germanium”; however, it is considered a “semimetal” that exhibits intermediate properties between those of metals and nonmetals. Germanium, which has the properties of a semiconductor, is used in transistors and diodes for amplification and rectification, respectively, and has revolutionized the field of electronics. Germanium is currently used extensively in the fields of electronics and chemistry: metallic germanium is employed in solar cells and infrared lenses; germanium dioxide is used as a polymerization catalyst in producing polyethylene terephthalate bottles; and germanium tetrachloride is employed as an additive in optical fibers.
The interactions between germanium compounds and biological components have also attracted attention in the field of biochemistry.17)18)36)37) Still, the solubility of germanium compounds and concerns regarding their safety22)38) have limited progress in their research and development. Organogermanium compounds, which are water-soluble and highly reactive in solution, have been developed to overcome these limitations.39) Oikawa and Kakimoto successfully produced the water-soluble organogermanium compound poly-trans-[(2-carboxyethyl)germasesquioxane] (commonly known as Ge-132) in 1967.40)41)42) Ge-132 has been shown to be extremely safe and has a variety of pharmacological applications.43)44)45)
Ge-132, a water-soluble polymer with the chemical formula [(GeCH2CH2COOH)2O3]n, is stable under various conditions. Figure 1 shows the synthesis of Ge-132 from metallic germanium.46) Ge-132 can be obtained in high yield and purity by the dehydration condensation of trihydroxygermylpropanoic acid ((HO)3GeCH2CH2COOH) produced by the reaction of germanium, hydrogen chloride, and acrylic acid. X-ray crystallographic studies of Ge-13241) revealed its three-dimensional network structure with a 12-membered planar ring, in which three oxygen atoms are bonded to one germanium atom inside the core (Figs. 2A and B), and the carboxyethyl groups of the alkyl substituents are alternately positioned above and below this plane, forming a three-dimensional network with dimers connected by hydrogen bonds (Figs. 2C and D). According to its 17O-NMR data, Ge-132 adopts the THGP structure in aqueous solution.46) In addition to the 12-membered ring of Ge-132, the structure of polymeric THGP includes a ladder-type polymer with eight-membered rings which have the same chemical formula and a linear polymer with the Ge-OH structure (Fig. 3).46)47)48)
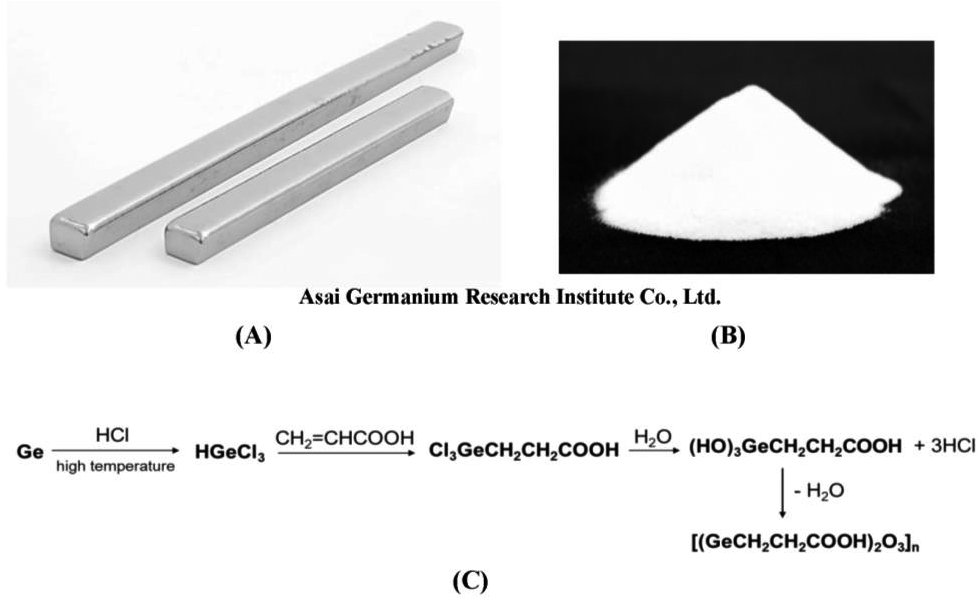
(A) High-purity polycrystalline germanium ingot; (B) Ge-132 powder; and (C) Synthesis of Ge-132 from high-purity polycrystalline germanium. High-purity polycrystalline germanium and hydrogen chloride are reacted at approximately 500 ºC to produce highly reactive trichlorogermane, which undergoes an addition reaction with acrylic acid to produce trichlorogermylpropanoic acid. The hydrolysis of trichlorogermylpropanoic acid forms trihydroxygermylpropanoic acid. These compounds can be dehydrated and condensed to obtain polymeric Ge-132 with high yield and purity.

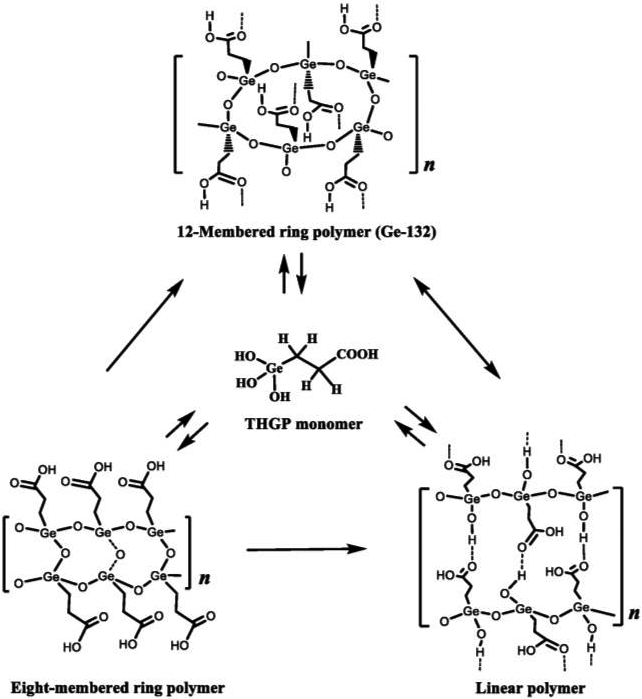
Polymers with three different structures can be produced by adjusting the recrystallization conditions. Each polymer exhibits a topological polymorphic relationship; thus, structural transformation is achieved by soaking the corresponding crystal in deionized water under the appropriate reaction conditions.
The development of pharmaceuticals containing Ge-132 began in the 1970s when its immunomodulatory effects49) were first studied extensively. Ge-132 was found to suppress osteoporosis50) and induce antioxidative,51) antinociceptive,52) anti-inflammatory,53) and anti-rheumatic effects.54) Numerous analyses, including acute toxicity,55) subacute toxicity,56) repeated dose toxicity,57)58) reproductive and developmental toxicity,59)60)61)62) antigenicity,63) and carcinogenicity,64) tests have confirmed the safety of Ge-132. Many new functions of Ge-132 were discovered during the development of its food and cosmetic applications.65)66)67)68)69)70) The simple-structured THGP has been suggested to interact with the biological components of living organisms to produce various effects.
The following sections will discuss THGP-based organogermanium compounds with various biospecific properties, including Ge-132, regarding their reactivity with sugars in aqueous solutions and isomerization-promoting properties.
2.2 Promotion of glucose-to-fructose isomerization with organogermanium compounds.
The organogermanium compounds with the THGP structure discussed in this section are shown in Fig. 4.
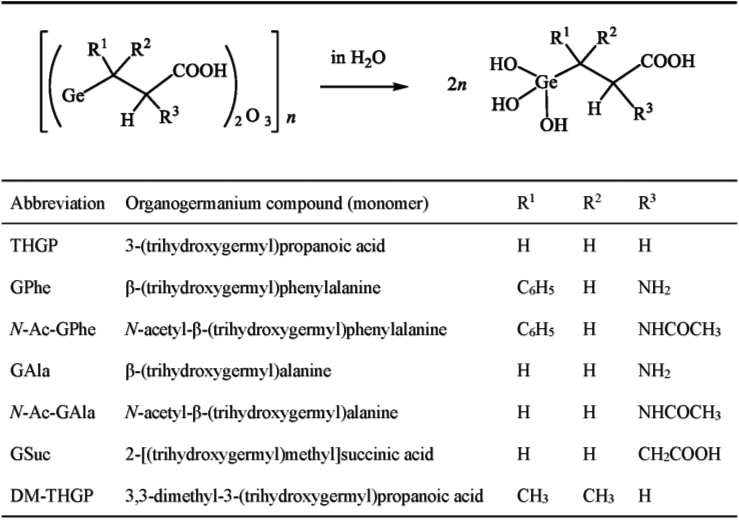
Poly-trans-[(2-carboxyethyl)germasesquioxane] (Ge-132) is hydrolyzed to THGP in aqueous solution. The structures of the organogermanium compounds are those adopted in aqueous solution. All organogermanium compounds were synthesized at Asai Germanium Research Institute Co., Ltd.
The time-course curves of the isomerization ratio obtained during the enzymatic isomerization of D-glucose to D-fructose using glucose isomerase in the presence of equimolar concentrations of organogermanium compounds are shown in Fig. 5A.32) Under normal conditions, i.e., in the absence of organogermanium compounds (control), the maximum observed isomerization ratio is 50 %; by contrast, in the presence of organogermanium compounds, the maximum observed isomerization ratios exceed 70 % (Table 1).32) Figures 5B and 5C show the conversion of D-glucose to D-fructose by heating under alkaline conditions (pH 12, 60 ºC) using a method based on the enzymatic isomerization reaction.32) The maximum yield of fructose reached 31 % under normal conditions (control); however, the yield increased by more than a factor of two with a maximum value of 61-75 % in the presence of organogermanium compounds with the THGP structure (Table 1).32)

(A) Enzymatic isomerization ratio (pH 8; 60 ºC), (B) D-Fructose yield and D-glucose residual of alkaline isomerization, and (C) Total saccharide (glucose + fructose) and pH of alkaline isomerization (initial pH: 12; 60 ºC). Each saccharide was quantified by high-performance liquid chromatography (HPLC).
Table 1. Maximum conversion of glucose-to-fructose in the presence of organogermanium compounds.32)
| Enzymatic isomerization (pH 8, 60 ºC) | Alkaline isomerization (pH 12, 60 ºC) | |||||
| Maximum isomerization ratio | Maximum fructose yield | Reaction time 60 min | ||||
| (%) | (min) | (%) | (min) | Total saccharide (%) | pH | |
| Control | 50 | 90-240 | 31 | 25 | 53 | 11.50 |
| THGP | 80 | 90-240 | 75 | 40 | 79 | 12.01 |
| GPhe | 77 | 120-240 | 61 | 50 | 79 | 11.99 |
| N-Ac-GPhe | 84 | 150-240 | 66 | 25 | 68 | 12.11 |
| GAla | 74 | 210-240 | 64 | 30 | 73 | 11.80 |
| N-Ac-GAla | 81 | 150-240 | 73 | 40 | 73 | 11.96 |
| GSuc | 85 | 90-240 | 71 | 30 | 69 | 12.02 |
Total saccharide (%) = fructose yield (%) + glucose residual (%).
In enzymatic isomerization, the carboxymethyl-substituted derivative and N-acetyl form, which possess structures favoring the cyclization of THGP derivatives (i.e., lactone-like ring formation of pentacoordinate germanium (Section 4.1)), displayed relatively higher isomerization ratios than the other derivatives. In alkaline isomerization, THGP displayed the highest fructose yield, and simpler structures with less steric congestion around the germanium atom showed higher yields than the more complex and sterically congested structures. In both isomerizations, the amino acid compound likely undergoes an aminocarbonyl reaction in which the amino group and the carbonyl group of the carbohydrate form a Schiff base, resulting in a slightly lower isomerization ratio. In addition, the isomerization ratio of an organogermanium compound with a butanoic acid side chain did not significantly differ from that of the control sample.25) Unlike THGP, the butanoic acid side chain cannot form a lactone-like ring; thus, its isomerization ratio does not change.
In alkaline isomerization (60 min), the lowest residual sugar ratio (glucose + fructose) observed in the control group was 53 %. In comparison, those observed in the presence of organogermanium compounds ranged from 68 to 79 % (Fig. 5C, Table 1). The reduction in the pH of the reaction solution was also suppressed in the presence of organogermanium compounds (Fig. 5C, Table 1). In addition, the rate of fructose formation during the initial stage of the reaction, where the reduction in pH and degradation of sugars were negligible, approximately doubled in the presence of THGP.35) These results indicate that organogermanium compounds with the THGP structure promote the alkaline glucose-fructose isomerization reaction, inhibit the degradation of sugars that occurs during this reaction, and suppress the resulting reduction in pH.71) These effects likely arise from the negative charge of the sugar-THGP complex.32) The mechanism of this process is described in Section 4.
A comparison of the Michaelis constant (Km) and maximum velocity (Vmax) values of the Michaelis-Menten plot of this enzyme reaction with those of the control group revealed no differences in Km (Control: 0.284, THGP (800 mM): 0.288) when glucose was used as the substrate; however, Vmax (Control: 1.057, THGP (800 mM): 1.358) was approximately 1.3 times greater owing to the formation of a THGP-fructose complex, which suppressed the reverse reaction.28) When fructose was used as the substrate, Km increased by approximately 5 % (Control: 0.261, THGP (800 mM): 0.276), and Vmax decreased by approximately 10 % (Control: 0.123, THGP (800 mM): 0.111).28) This phenomenon can be explained by the reduction in the concentration of free (i.e., non-complexed) fructose as a result of the formation of the fructose-THGP complex. Although this complex strongly affects the affinity of the substrate for the enzyme, it is not an isomerase substrate and is ultimately removed from the reaction system. The mechanism of the formation of this complex is described in Sections 4.1 and 4.2.
2.3 Promotion of lactose-to-lactulose alkaline isomerization with organogermanium compounds.
The alkaline isomerization of the disaccharide lactose was investigated. Figure 6 depicts the conversion of lactose to lactulose upon adding various molar ratios of THGP. The maximum yield of lactulose obtained from the control reached 24 %. This yield gradually increased with increasing THGP molar ratio, reaching a maximum value of 78-80 % at a molar ratio of 0.9-1.2 (Fig. 6A, Table 2).33) Therefore, the presence of THGP increases the lactulose yield by more than a factor of three. Adding more than equimolar amounts of THGP did not affect the maximum yield, indicating that the complexation of THGP with sugars is an equilibrium reaction.
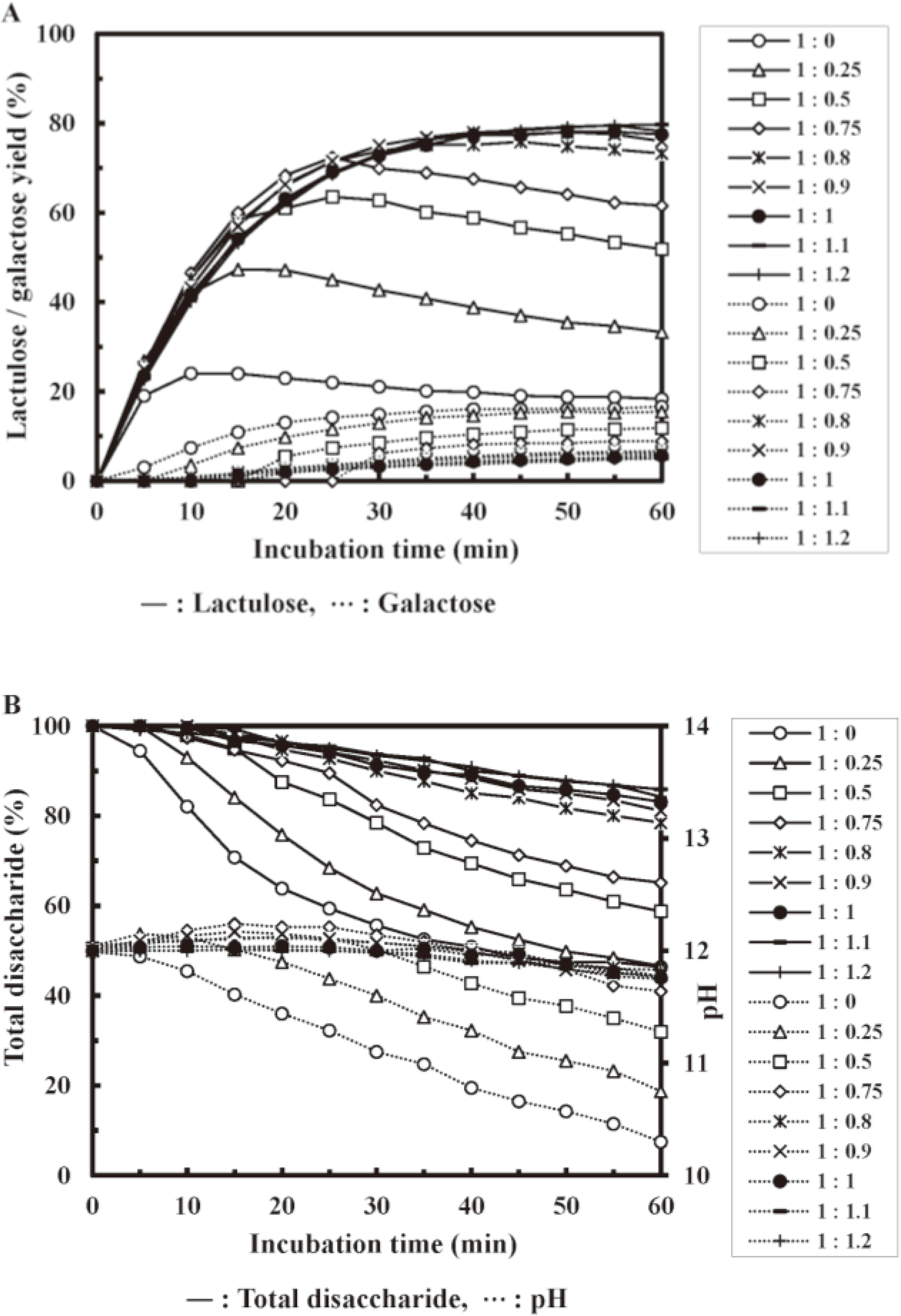
(A) Lactulose and galactose yields, (B) Total disaccharide (lactose + lactulose) and pH. Each saccharide was quantified by HPLC.
Table 2. Maximum conversion of lactose-to-lactulose in the presence of organogermanium compounds (initial pH 12, 60 ºC).33)
| Lactose : THGP molar ratio | Maximum lactulose yield | Reaction time 60 min | |||
| (%) | (min) | Total disaccharide (%) | Galactose (%) | pH | |
| 1 : 0 | 24 | 10 | 46 | 17 | 10.30 |
| 1 : 0.25 | 47 | 15 | 47 | 16 | 10.75 |
| 1 : 0.5 | 64 | 25 | 59 | 12 | 11.28 |
| 1 : 0.75 | 72 | 25 | 65 | 9 | 11.64 |
| 1 : 0.8 | 76 | 45 | 79 | 7 | 11.77 |
| 1 : 0.9 | 78 | 50 | 81 | 6 | 11.78 |
| 1 : 1 | 78 | 50 | 83 | 6 | 11.76 |
| 1 : 1.1 | 80 | 55 | 86 | 5 | 11.75 |
| 1 : 1.2 | 80 | 55 | 84 | 5 | 11.83 |
Total disaccharide (%) = lactulose yield (%) + lactose residual (%).
During heating under alkaline conditions, there was a decrease in residual disaccharides (lactose + lactulose) once the reaction was completed. However, the presence of THGP significantly slowed down the rate of this decrease (Fig. 6B). Additionally, THGP reduced the amount of galactose produced by the degradation of lactose (Fig. 6A). The reduction in pH was significantly inhibited in the presence of THGP, as was the case when glucose was used as a substrate (Fig. 6B). In the initial reaction region, where the reduction in pH and degradation of sugar were negligible, the lactulose production rate was approximately doubled in the presence of THGP.35)
These results indicate that THGP promotes the alkaline isomerization of lactose to lactulose and inhibits sugar degradation, similar to glucose-fructose conversion.33) The corresponding mechanism is described in Sections 4.2 and 4.3.
Organogermanium compounds were likewise found to increase the yield of each ketose by 2-6 times in the arabinose-ribulose, ribose-ribulose, xylose-xylulose, and mannose-fructose alkaline isomerization reactions.29) Thus, organogermanium compounds can also be applied to promote the isomerization reactions of aldoses and ketoses.
To elucidate the mechanism by which organogermanium compounds promote the aldose-ketose isomerization reaction, we evaluated the affinity of these compounds toward sugar using 1H-NMR spectroscopy. The doublet signals at 5.78 and 4.16 ppm in the 1H-NMR spectrum of a 1:1 (0.28 M, neutral region) mixed solution of THGP and glucose were shown as evidence of complex formation. When fructose was added to the solution, the THGP-glucose complex signal disappeared, thus suggesting that THGP preferentially interacts with fructose even in the presence of glucose.27)
To quantify the affinity between THGP structures and sugars, the complex formation ratio and formation constant were determined using 3,3-dimethyl-3-(trihydroxygermyl)propanoic acid (DM-THGP) as a 1H-NMR reporter molecule.32)33) DM-THGP produces simple 1H NMR signals with no coupling, which allow the integral ratio of the original signals to the complex signals to be quantified.
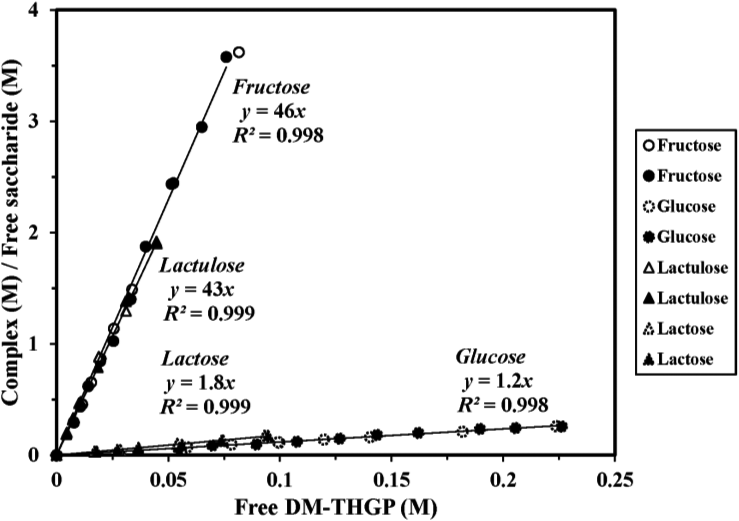
Figure 7 shows the 1H-NMR spectra of DM-THGP and DM-THGP/glucose, DM-THGP/fructose, DM-THGP/lactose, and DM-THGP/lactulose mixtures recorded in deuterated aqueous solutions.32)33) Two singlet signals corresponding to CH3 and CH2 groups were observed at 1.14 and 2.27 ppm, respectively, ascribed to free DM-THGP due to their lack of 1H coupling (A). Signals corresponding to the complexed DM-THGP were detected as a group of broadened signals shifted toward the signals of free DM-THGP (B-E). The complex formation ratio determined from the ratio of the integrals of the free and complexed DM-THGP signals was 19 % in the case of DM-THGP and glucose and 74 % in the case of DM-THGP and fructose.32) The complex formation ratios of DM-THGP and lactose and DM-THGP and lactulose were 14 and 59 %, respectively.33) As shown in Fig. 8, the complex formation constants for glucose, Ks(glucose), and fructose, Ks(fructose), were 1.2 and 46 M−1, respectively, indicating that the affinity of DM-THGP toward fructose is approximately 40 times stronger than that toward glucose.32) The complex formation constants for lactose, Ks(lactose), and lactulose, Ks(lactulose), were 1.8 and 43 M−1, respectively, indicating that the affinity of DM-THGP toward lactulose is approximately 24 times stronger than that toward lactose.33)

The enlarged spectra of the methyl signals in the 1.03-1.28 ppm region are also shown. (A) DM-THGP; (B) DM-THGP with D-glucose; and (C) DM-THGP with D-fructose-1:1 THGP and glucose ratio, 0.25 M; 20 ºC in D2O at neutral pH. (D) DM-THGP; (E) DM-THGP with lactose; (F) DM-THGP with lactulose-1:1 THGP and lactose ratio, 0.10 M; 20 ºC in D2O at neutral pH.
In addition, the interactions of organogermanium complexes were quantitatively analyzed using ultraviolet difference absorption spectroscopy (Fig. S1; see J. Appl. Glycosci. website).25) The spectrum of GPhe exhibits an absorption band near 260 nm originating from a phenyl group. After adding sugar, new absorption peaks at 240, 266, and 272 nm were observed in the difference spectrum. The approximation formula in the figure was obtained by computer-simulating the experimental plots. The differential spectrum at 272 nm was used for calculating the apparent dissociation constants, Kd, of GPhe with glucose and fructose, since the peak at 272 nm was the most clear and reliable peak recorded. The Kd values of GPhe with glucose and fructose were calculated to be 575.8 and 28.5 mM, respectively, and its affinity for fructose was approximately 20 times stronger than that for glucose.25) Similarly, the calculated Kd values of GPhe with lactose and lactulose were 644.8 and 41.3 mM, respectively, and its affinity for lactulose was approximately 16 times stronger than that for lactose.26)
The results from both analytical methods indicated that organogermanium compounds with the THGP structure were significantly more likely to form a complex with ketose than with aldose (Table 3). These data strongly support the hypothesis that the isomerization-promoting effect of THGP primarily results from the inhibition of the reverse reaction from ketose to aldose owing to the observed difference in the affinities of organogermanium compounds toward the various saccharides, which affect their ability to form complexes.
Table 3. Complex formation ratio, Ks and Kd values of organogermanium with sugar.25)26)32)33)
| Organogermanium | Sugar | Complex formation ratio (%) | K s (M−1) | K d (mM) | Evaluation method |
| DM-THGP | Glucose | 19 | 1.2 | 1H-NMR32) | |
| Fructose | 74 | 46 | |||
| Lactose | 14 | 1.8 | 1H-NMR33) | ||
| Lactulose | 59 | 43 | |||
| GPhe | Glucose | 576 | UV25) | ||
| Fructose | 28.5 | ||||
| Lactose | 645 | UV26) | |||
| Lactulose | 41.3 |
UV: ultraviolet difference absorption spectroscopy.
4.1 Mechanism of isomerization promotion through the complexation of THGP and cis-diol structures.
In Section 3, we established that organogermanium compounds with the THGP structure form complexes with sugars and that the affinity of these compounds toward ketose is stronger than that toward aldose.32)33) However, the mechanism by which these complexes are formed has yet to be elucidated.
In an aqueous solution, glucose and fructose have five equilibrium states, as shown in Fig. 9.72)73) D-glucose is known to be present in aqueous solution in the form of α-D-glucopyranose (approximately 38 %) and α-D-glucofuranose (approximately 0.1 %), both of which have a cis-diol structure, along with β-D-glucopyranose (approximately 62 %) and β-D-glucofuranose (approximately 0.3 %), which do not have a cis-diol structure.72)73) D-fructose is present in aqueous solution in the form of β-D-fructofuranose (approximately 22 %), β-D-fructopyranose (approximately 68 %), and α-D-fructopyranose (approximately 3 %), which have a cis-diol structure, and α-D-fructofuranose (approximately 6 %), which does not have a cis-diol structure.74) Therefore, fructose has a significantly higher fraction of cis-diol structures in aqueous solution (approximately 93 %) relative to glucose (approximately 38 %).
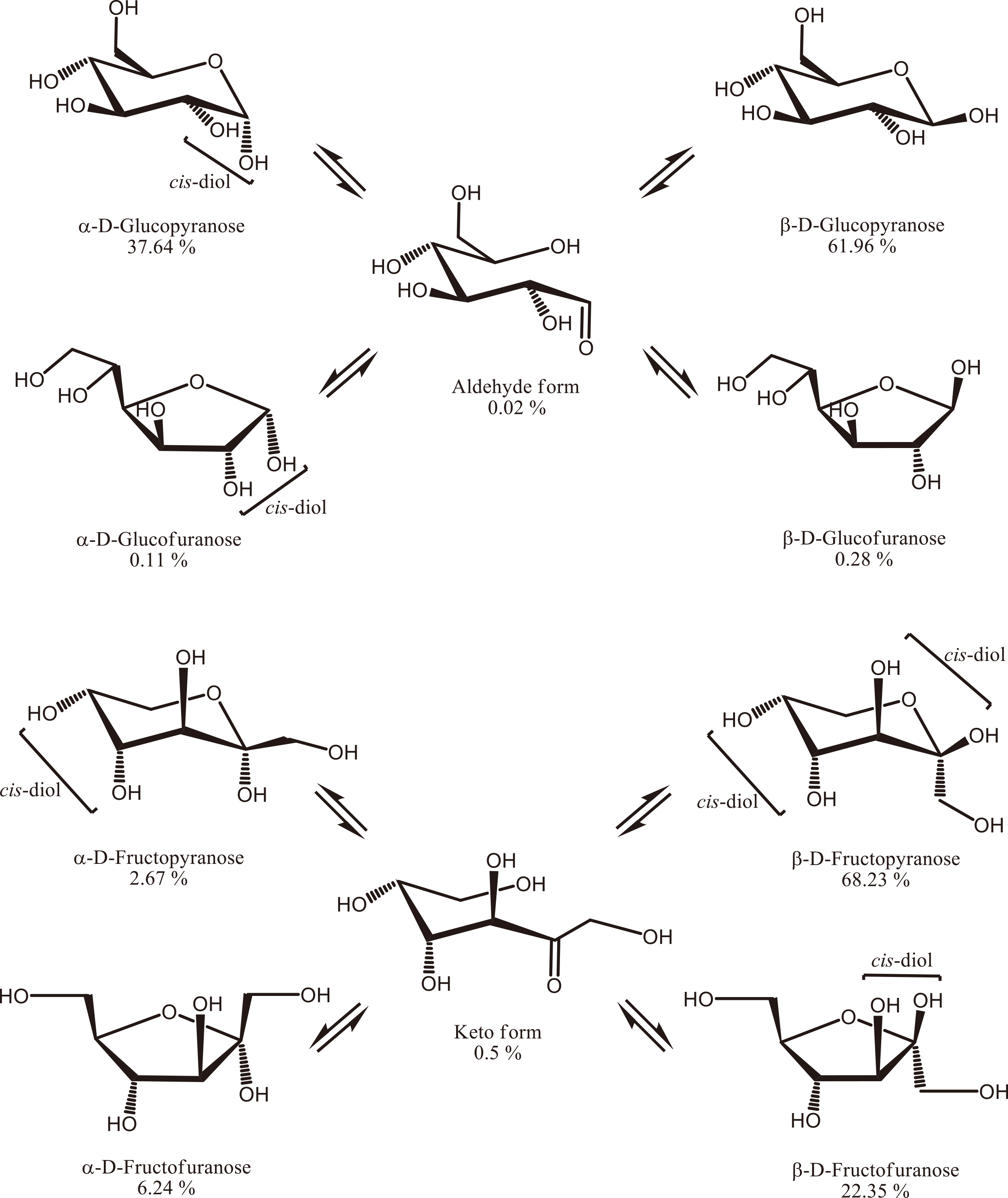
Figure 10 schematically illustrates the complex formation between the THGP structure and the cis-diol compound. The carboxylate anion of THGP initially undergoes intramolecular coordination to the vacant orbital of the germanium atom owing to the free rotation of the propanoic acid side chain (A), thereby forming a cyclic high-coordinate germanium structure (B and C). Germanium atoms typically have coordination numbers ranging from 4 to 6,75) which can be increased by accepting electrons into empty orbitals. THGP assumes a cyclic pentacoordinate structure (B) at pH values ranging from neutral to approximately 11, with a cyclic hexacoordinate structure (C) forming at a pH of approximately 12. The dehydration reaction of (B) or (C) with a cis-diol compound produces a stable pentacoordinate (D1 and D2) or hexacoordinate (D3) germanium complex, respectively. In this case, back-donation occurs from the π electrons of the bonding oxygen atoms to the vacant d-orbitals of the germanium atoms, resulting in dπ-pπ interactions that strengthen the Ge-O bond (E).76)77)78) In the pentacoordinate or hexacoordinate germanium structure, the Ge-O σ bond is strengthened by increasing the coordination number and electron density of the germanium atom. This dπ-pπ interaction favors trigonal bipyramidal, square pyramidal, and octahedral conformations with pentacoordinate or hexacoordinate germanium atoms over tetracoordinated germanium atoms, stabilizing the THGP-structured cis-diol complex (F1, F2, and F3). Furthermore, the complex is protected from hydrolysis by a steric hindrance from the cis-diol ligand and an inductive electron-donating effect (+I effect).
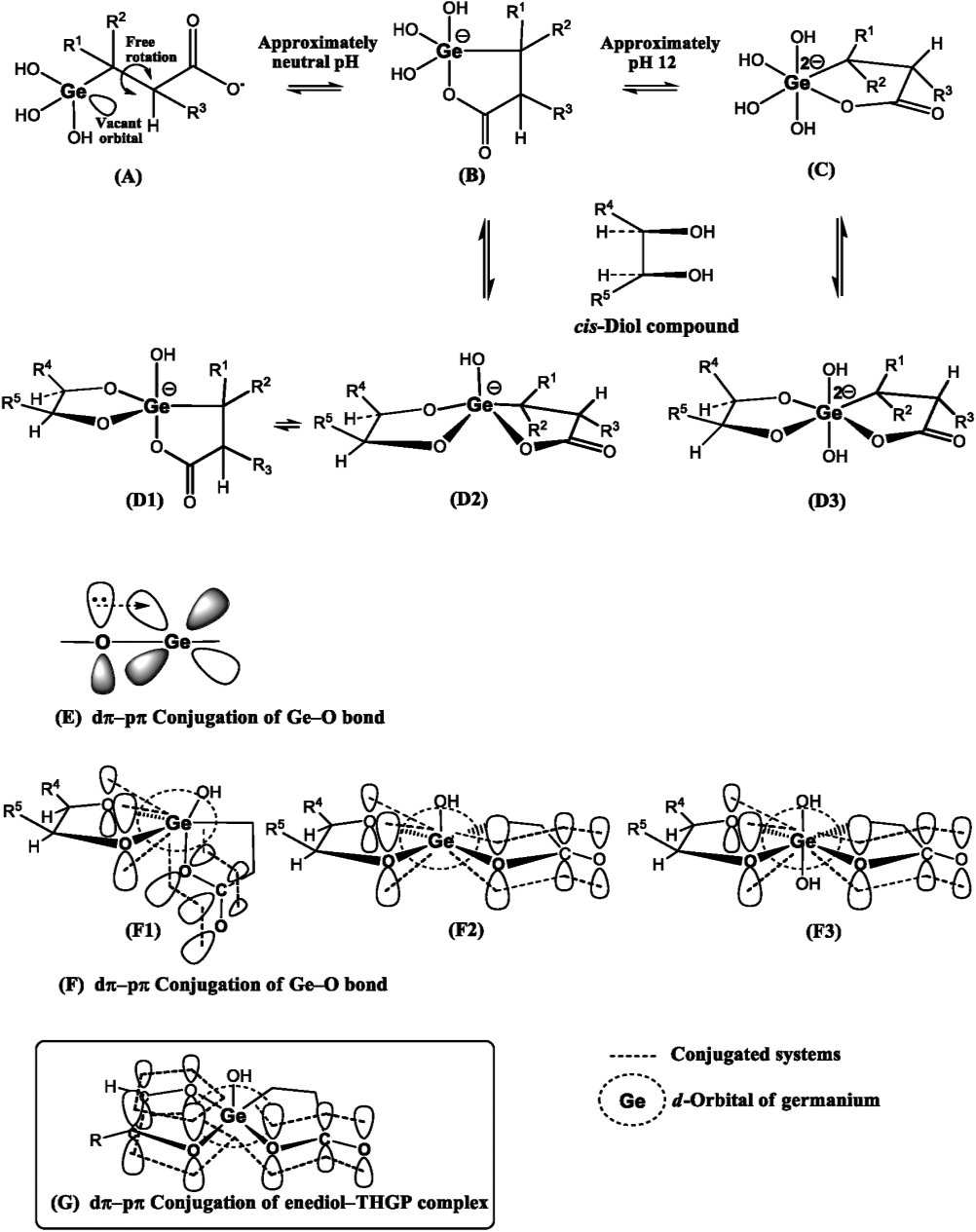
The carboxylate anion of THGP undergoes intramolecular coordination to the vacant orbital of the germanium atom due to the free rotation of the propanoic acid side chain (A). This coordination compound forms a cyclic pentacoordinate structure (B) at pH values ranging from neutral to approximately 11 and a cyclic hexacoordinate structure (C) at a pH of approximately 12. Pentacoordinate or hexacoordinate trihydroxygermanium forms a stable five-coordinate (D1, D2) or six-coordinate (D3) germanium complex, via the dehydration reaction with a 1,2-cis-diol compound. The dπ-pπ interaction (E) strengthens the Ge-O bond. The pentacoordinate germanium complex forms trigonal bipyramidal (D1, F1) and square pyramidal (D2, F2) structures, while the hexacoordinate germanium complex forms an octahedral (D3, F3) structure. The square pyramidal and octahedral structures represent relatively stable conjugated structures owing to the linear alignment of the p-orbital of the oxygen atom, empty orbital of the germanium atom, and p-orbital of the carbonyl group. In cases where the cis-diol ligand consists of an enediol, the conjugated orbital is further extended to form a more stable conjugated structure (G).
Our group previously verified the crystal structure of a 1:1 complex of adrenaline with a cis-diol structure and THGP by X-ray crystallography.79) This complex was formed via the dehydration condensation reaction between two of the three hydroxyl groups of THGP and a catechol hydroxyl group. Furthermore, THGP itself formed an intramolecular lactone-like ring with a pentacoordinate germanium atom. These results support the reaction mechanism presented in Fig. 10.
As shown in Fig. 9, the structural proportion of furanose-type glucose in aqueous solutions is less than 1 %, and NMR does not usually detect this molecule. Osawa et al. reported that THGP formed a complex with the 1,2-cis-diol of α-D-glucofuranose based on the 1H-NMR analysis of THGP and glucose (1:1) in a deuterated aqueous solution,80) indicating that THGP has high affinity for the cis-diol moiety of furanose. Ando et al. compared the 13C-NMR spectra of complexes of several furanose-type hemiacetals with that of THGP and confirmed that the signal attributed to the furanose form of fructose disappeared after complexation.81) These observations indicate that the furanose-type structures of both glucose and fructose readily form complexes with THGP. In addition, THGP produces stable complexes with diols having narrow dihedral angles (Section 4.2).
These findings demonstrate that THGP favors complexation with the furanose-type cis-diol structure over the pyranose-type structure and confirm that its high affinity for fructose arises from the significantly higher content of this structure in aqueous solutions than that of glucose. The formation of numerous complexes with fructose via the THGP structure inhibits the reverse reaction, thereby shifting the apparent reaction equilibrium toward fructose while promoting the isomerization reaction. We can reasonably assume that the fructose-THGP complex produced in the enzymatic isomerization reaction does not serve as a substrate for the enzyme but is expelled from the reaction system (Figs. 11A and B), thereby shifting the equilibrium and increasing both the isomerization ratio and fructose production rate.
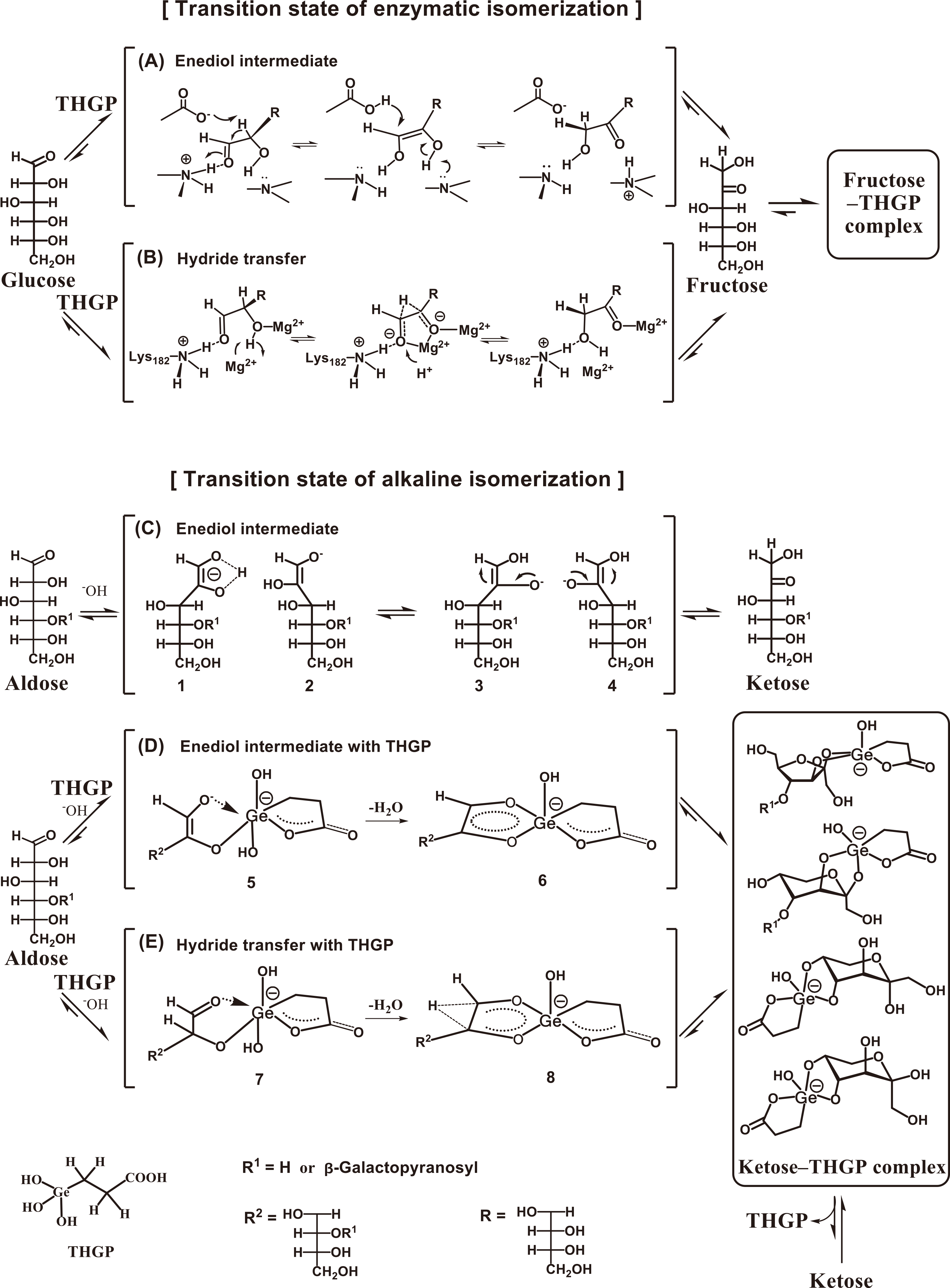
(A) Enzymatic isomerization via an enediol intermediate83)84)86) in the presence of THGP.32) Fructose forms a stable complex with THGP and is expelled from the equilibrium reaction system. (B) Enzymatic isomerization via hydride transfer85)86) in the presence of THGP.32) Fructose forms a stable complex with THGP and is expelled from the equilibrium reaction system. (C) Alkaline isomerization via an enediol intermediate,81) exists in the forms of cis-enediol (1 and 3) and trans-enediol (2 and 4) intermediates. (D) Alkaline isomerization via an enediol intermediate exists in the cis-enediol form (5) in the presence of THGP.32)35) The dehydration reaction proceeds via a cis-enediol intermediate (6), stabilizing the transition state. (E) Alkaline isomerization via hydride transfer performed in the presence of THGP,35) which produces the cis conformation (7). The dehydration process stabilizes the hydride transfer in the transition state (8).
4.2 Mechanism of isomerization promotion through transition state stabilization in the presence of THGP.
The aldose-ketose transformation in aqueous solutions was first studied under alkaline conditions. This transformation, also known as the Lobry de Bruyn-Alberda van Ekenstein transformation, involves the formation of an enediol intermediate via the polarization of the carbonyl group at the aldose C-1 position and abstraction of the proton from the aldose C-2 position by a base.82)
Schray and Rose reported that the C-2 hydrogen was transferred to the C-1 position during the enzymatic isomerization reaction using 3H-labeled xylose, and observed that enzymatic isomerization followed a similar mechanism.83)84) Meanwhile, Collyer et al. studied the enzyme-substrate complex using X-ray crystallography, which showed that the coordination of Mg2+ ions used as an activator caused a hydride transfer from the C-2 position to the C-1 position.85) This mechanism describing the enzymatic isomerization reaction involving the formation of glucose isomerase is now widely accepted;86) however, some details require further clarification.
The base-catalyzed interconversion of aldoses to ketoses is considered to proceed via an enediol intermediate in which electron pairs are transferred between the C-2 and C-1 positions; however, in an alkaline medium, the reaction profiles strongly depend on the type of sugar, type and concentration of the alkali, and presence or absence of an oxidizing agent. Nagorski and Richard used 1H-NMR spectroscopy to demonstrate that the isomerization of glyceraldehyde in weakly alkaline aqueous solutions is caused by the intramolecular migration of hydride ions.87) The isomerization reaction induced by hydride transfer was strongly enhanced by the addition of Zn2+ ions, while proton transfer via the enediol intermediate was catalyzed by the Brønsted base and promoted by the combined action of the Brønsted base and Zn2+ ions.88)
Figure 11 illustrates the mechanisms of enzymatic isomerization with glucose isomerase conducted in the presence of THGP (A and B), alkaline isomerization of aldose to ketose (C), and alkaline isomerization performed in the presence of THGP (D and E). THGP has high affinity for the cis-diol structure; therefore, the reaction is promoted by forming a stable complex with fructose (ketose). The isomerization reaction proceeds through two major pathways: via a 1,2-enediol intermediate (A, C, and D) and hydride transfer from C-2 to C-1 (B and E). We assume that THGP stabilizes the transition state of the alkaline isomerization reaction by forming a complex with the enediol intermediate (D).32)35) The enediol structure forms more stable complexes because the conjugated orbitals are extended by dπ-pπ interactions (Fig. 10G).
The complexation ratio of THGP with catecholamines (adrenaline and noradrenaline) is reportedly higher than that of THGP with adenosine or adenosine triphosphate.79) The diol groups of catechol are located in the plane of the benzene ring, and their complexation ability is strongly affected by their dihedral angles. Notably, THGP forms more stable complexes with diols having narrower dihedral angles. The cis-enediol and catechol diol arranged in the same plane have the same stereochemical structure; thus, the isomerization reaction may be promoted by a mechanism involving the stabilization of the transition state by the complexation of THGP with the enediol intermediate.
In the alkaline isomerization reaction pathway (E) involving hydride transfer from the C-2 position to the C-1 position, the electron-accepting property of the germanium atomic orbital enables dπ-pπ interactions, which stabilize the reaction transition state by forming a THGP complex with a cyclic structure.
In any case, THGP is presumably capable of lowering the activation energy of the alkaline isomerization reaction by forming a complex with the intermediate via either the enediol intermediate or hydride transfer route, and the conjugated pentacoordinate (or hexacoordinate) cyclic THGP structure can effectively stabilize the transition state. We believe that this function of THGP, which is similar to that of an artificial enzyme ligand, is the main factor that increases the initial rate of the alkaline isomerization reaction.
4.3 Degradation suppression mechanism of aldose -ketose alkaline isomerization.
In the alkaline isomerization reaction, sugar degradation by alkalis proceeds via the well-known Nef-Isbell mechanism.89)90) In Sections 2.2 and 2.3, we clarified that the presence of THGP suppressed the reduction in pH during the reaction and inhibited the production of galactose via the degradation of sugar in the lactose-lactulose isomerization process (Tables 1 and 2).
A schematic of the alkaline aldose-ketose isomerization process, including the Nef-Isbell mechanism, is shown in Fig. 12. In base catalysis, a water or galactose molecule is released during the β-elimination of an alkoxycarbonyl moiety from the enediol structure (C). This reaction produces an unsaturated ketone (F) and organic acid such as D-glucoisosaccharic acid (G), thereby reducing the system pH and inhibiting the isomerization reaction. However, organogermanium compounds with the THGP structure exhibit high affinity toward ketose, while the ketose-THGP complex acquires the anionic charge of germanium and, thus becomes negatively charged. This negative charge repels alkaline anions and protects ketose molecules from attack by alkaline reagents. Accordingly, the alkaline degradation process that occurs simultaneously with the alkaline isomerization reaction is inhibited, the reduction in pH is suppressed, and the alkaline isomerization reaction is promoted.
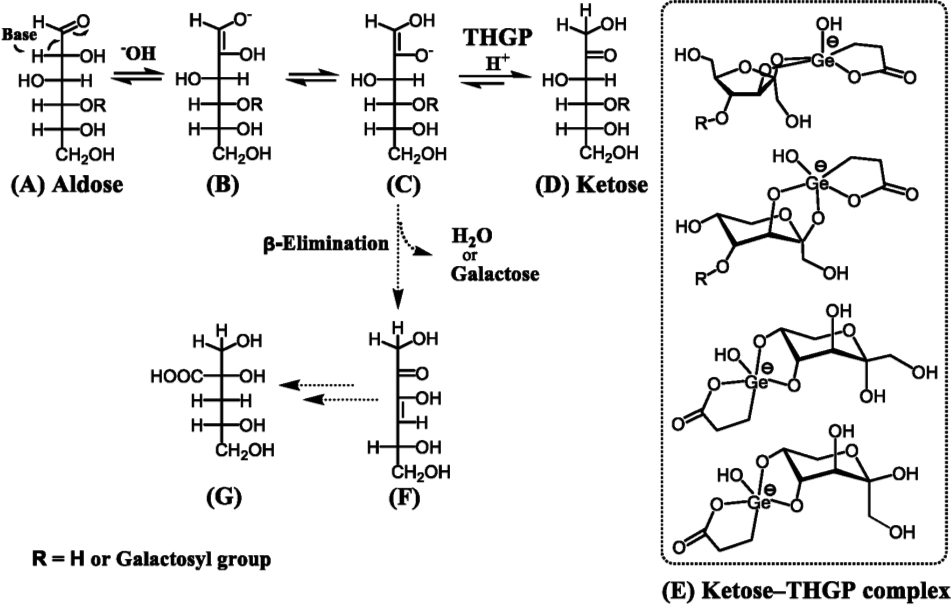
Aldose (A), enediol anion (B and C), and ketose (D): Alkaline aldose-ketose isomerization pathways. Enediol anion (C), unsaturated ketone (F), and organic acid (deoxyaldonic acid (G)): Partial Nef-Isbell mechanism of the alkaline degradation of aldose and ketose. Ketose-THGP complex (E): Stabilization of ketose through complexation with THGP. The negative charge acquired by the ketose-THGP complex protects the ketose from alkaline degradation.
We have thus far demonstrated that organogermanium compounds are effective catalysts for converting aldoses to ketoses owing to their high affinity for ketoses. In this section, the practical applications of our findings are discussed.
Based on the findings of this review, we first developed a system for producing lactulose via the alkaline isomerization of lactose using Ge-132 (THGP). A batch-type isomerization reaction was considered impractical owing to its low efficiency; therefore, a bench-scale plant was constructed as a novel and more efficient continuous manufacturing method. This plant consisted of a 1) continuous alkaline isomerization process, 2) continuous two-step electrodialysis process to separate the sugar and organogermanium compound, 3) continuous process to purify the sugar solution, 4) continuous process to concentrate the purified sugar, 5) process to separate the organogermanium compound and sodium hydroxide, and 6) process to purify the organogermanium compound (Fig. 13).34)
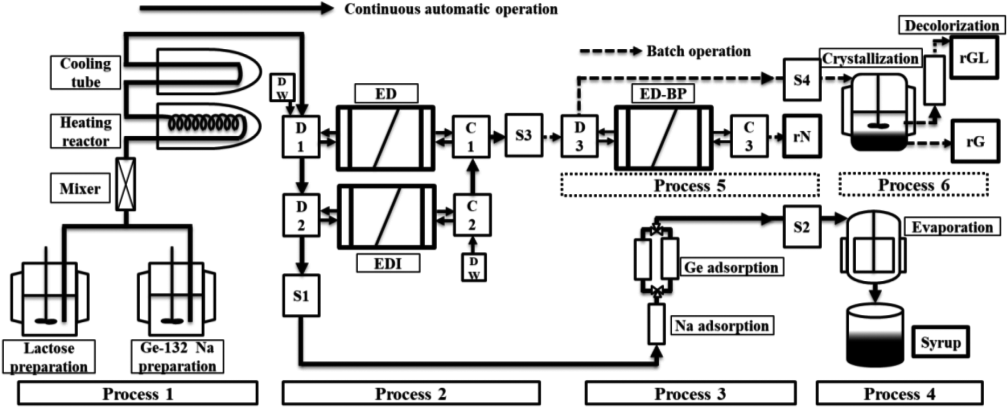
The bench-scale plant consists of four main processes and two reusable Ge-132 processes. Process 1) is a continuous alkaline isomerization process involving the dissolution, mixing, and reaction of the reagents. Process 2) is a continuous two-step electrodialysis process to separate sugar and Ge-132 (NaOH) based on the combination of ED and EDI. Process 3) is a continuous process to purify the sugar solution using an ion-exchange resin. Process 4) is a continuous process of concentrating the purified sugar solution using a thin-film evaporator. Process 5) is a process to separate Ge-132 and NaOH using an ED-BP. Process 6) is a process to purify Ge-132 via cooling crystallization and decolorization by an adsorbent. Deionization tank (D1, D2, D3); concentration tank (C1, C2, C3); storage tank (S1, S2, S3, S4); distilled water (DW); recovered NaOH (rN); recovered Ge-132 crystals (rG); recovered solution containing Ge-132 and lactulose (rGL).
Reusing the organogermanium compound was required to ensure the practicality of the plant. Thus, a two-step electrodialysis device was designed to separate Ge-132 from sugar. Ge-132 was separated at an efficiency of approximately 90 % using an electrodialyzer (ED).30) To further increase the separation efficiency of the system, we used an electrodeionizer (EDI)91)92) with a separation performance equivalent to that of an ultrapure water system to separate approximately 98 % of Ge-132. The circulating ED-EDI system separated more than 99.6 % of Ge-132. Most Ge-132 and sodium hydroxide species were successfully recovered using the ED with a bipolar membrane (ED-BP).
The manufactured bench-scale plant was operated continuously, and syrups containing lactulose (350 g/L), lactose (92 g/L), and galactose (31 g/L) were produced with an output of 37.7 mL/h. The final syrup contained only a tiny residual Ge-132 (2 mg/L). Because Ge-132 has long been used as a dietary supplement, it likely does not need to be removed entirely from the finished products (unlike other accelerators). The manufactured plant can be an effective system for developing food ingredients with new functionalities.
The effective continuous system producing lactulose-containing syrups using organogermanium compounds described in this chapter was first developed in this study. Optimization of the separation process to reduce sugar loss and further upgrade the purification process of Ge-132 from a batch system to a continuous one are necessary to improve the efficiency and simplicity of the system and enable its application in an actual production plant. In anticipation of a growth in the demand for lactulose, we will modify this system to improve its efficiency and cost-effectiveness in future studies.
Aldose-ketose isomerization is a reversible equilibrium process; thus, the corresponding isomerization ratio of the enzymatic and alkaline reactions typically do not exceed 50 and 30 %, respectively. However, the addition of organogermanium compounds to the reaction system increased the aldose-ketose isomerization ratio by 2-3 times to 60-85 %. The affinity of organogermanium compounds with the THGP structure toward sugar in aqueous solution was 40 times stronger for fructose than for glucose and 24 times stronger for lactulose than for lactose, as determined by 1H-NMR spectroscopy. Ultraviolet absorption spectroscopy produced similar results, indicating that the affinity of organogermanium compounds toward ketose was significantly higher than that toward aldose. The organogermanium compounds discussed herein therefore promoted the isomerization reaction by forming a stable complex with a ketose having a high proportion of furanose containing multiple cis-diol structures and inhibited the reverse reaction, thereby shifting the aldose-ketose equilibrium toward the ketose side. We also considered that the stable complex formed between the ketose and organogermanium compounds acquires the anionic charge of germanium and becomes negatively charged, thus protecting the ketose molecule from alkaline degradation. The observed increase in the rate of ketose formation during the initial stage of the reaction likely occurred because THGP lowered the activation energy of the reaction by stabilizing the cis-enediol intermediate of the alkaline isomerization reaction via complexation. Hence, THGP represents an enzyme-like molecule with a pentacoordinate or hexacoordinate structure that promotes conjugation and thus stabilizes the transition state.
Although THGP has a relatively simple structure, it demonstrates excellent reactivity in the sugar isomerization reaction, which was manifested by the germanium valence change and lactone-like ring formation in response to pH variations, stabilization of cis-diol complexes via dπ-pπ interactions, and strengthening of the Ge-O bond by the increased electron density of germanium atoms.
Finally, we developed a sugar-manufacturing process utilizing organogermanium (Ge-132) as a reusable catalyst. We established a method to efficiently convert aldose to ketose by taking advantage of the complexation of Ge-132 with the cis-diol structure in aqueous solution. Numerous products can potentially be produced by using the described production system under optimum conditions, thus facilitating the development of high value-added sugar products.
Takae Nagasawa is an employee of Asai Germanium Research Institute Co., Ltd., a company that specializes in the development, manufacture, and sales of Ge-132. Katsuyuki Sato was an employee of Asai Germanium Research Institute Co., Ltd. until May 2021.
The authors thank the late Dr. Norihiro Kakimoto (the former president of Asai Germanium Research Institute Co., Ltd.) and the late Dr. Keiji Umeda (the former director of the National Food Research Institute) for their helpful advice regarding the research design. We also thank the researchers and engineers who supported this study over the years.