2017 Volume 66 Issue J-STAGE-1 Pages 9-17
2017 Volume 66 Issue J-STAGE-1 Pages 9-17
Among clinical laboratory tests, urine testing has a particularly long history, and it continues to serve as an important screening test for kidney, urinary tract, and systemic diseases. Urine testing can accurately detect five abnormalities of urine, i.e., (1) pyuria, (2) bacteriuria, (3) hematuria, (4) proteinuria, and (5) metabolically abnormal urine (e.g., crystalluria, glycosuria). Urinary sediment examination is a morphological examination that accurately identifies and roughly counts urine formed elements, i.e., epithelial cells, blood cells, casts, salts/crystals, and bacteria, to provide information for detecting any pathological condition accompanying urine abnormalities in combination with qualitative urinary test findings. For urinary sediment examination procedures in Japan, the Japanese Committee for Clinical Laboratory Standards (JCCLS) proposed guidelines on urinary sediment examination procedures, namely GP1-P3 (urinary sediment examination procedures 2000), in 2000, and the revised version, named GP1-P4 (urinary sediment examination procedures 2010), was released in 2010. In this part, we outline the urinary sediment examination procedures according to GP1-P4 (with some modifications).
Urine specimens are classified by time or type of collection (Table 1.1).
| Classification by time of collection | Classification by type of collection |
|---|---|
| 1) Morning urine: the first urine voided in the morning 2) Spot urine: any daytime urine specimen except the morning urine 3) Post-loading urine: (1) after exercise (2) after prostatic massage etc. 4) 24-h urine: in principle, 24-h storaged specimens should not be used for urinary sediment examination. |
1) Natural urine: urine voided naturally (1) Total urine: urine completely collected in one natural voiding (2) Partial urine: part of a single natural urine voiding: (i) first urine: the first part of voided urine (ii) midstream voided urine: collected during voiding but excluding the first and last parts 2) Catheter urine: urine collected with a urethral catheter 3) Bladder puncture urine: urine collected by aspirating urine from a distended bladder through the anterior abdominal wall 4) Double voided urine: urine from a single voiding but dividing the specimen in accordance with the testing purposes 5) Others: urine sampled after urinary diversion surgery, e.g., ileal conduit surgery |
It is desirable to note the following points in order to report accurate results of urinary sediment examination.
1) First morning and mid-stream voided specimens are suitable for urinary sediment examination; thus, the patients should be given necessary instructions.
2) Clearly document the type of specimen and collection method (e.g., natural urine, catheter urine).
3) It is desirable to clean the urethral meatus before collecting urine. In particular, in case of a female, guidance on urine collection, including the use of a cleaning procedure, should be given to prevent the specimen from being contaminated by components from the vulva (e.g., red blood cells, white blood cells, squamous epithelial cells, bacteria).
4) Add formalin in a ratio of 1.0 mL per 100 mL of urine as a preservative if preservation is particularly needed. Glutaraldehyde is a preferred choice for sediment fixation.
5) In principle, the time of urine collection should be noted.
6) Quickly examine the submitted urine specimens within no more than 4 h after collection. The duration of storage affects urine properties differently between specimens. After prolonged storage, red blood cells, white blood cells, epithelial cells, and casts tend to decrease in number, whereas the number of bacteria and yeast tend to increase.
7) Specimens collected from females during menstruation are not appropriate for testing. In cases of absolute necessity, the specimens should be labeled as such.
3. Urine collection containers1) Collection containers should be made of resin-precoated clean paper, polystyrene resin, plastic, or glass. The inside of the containers should not be coated.
2) If only an aliquot of the specimen is to be submitted for testing, the entire specimen must be mixed well before aliquoting. The lid must be placed on the container.
3) When using urine-collecting bags (particularly for newborns and infants), care should be taken to attach the bags properly such that urine does not leak.
It is important to first observe and record the appearance of urine when preparing specimens for urinary sediment examination. Specific points including color, turbidity, hematuria, and foreign matters (e.g., feces, paper) is desirable to be reported as much as possible.
1. MixingMix the urine well to achieve uniformity.
2. Centrifugation1) Centrifugation tube: Use a sharp, pointed (spitz-type) centrifugation tube accurately graduated at 10 and 0.2 mL. It is desirable that the tube is composed of transparent polyacrylic styrene.
2) Urine volume: A volume of 10 mL is recommended in principle. Even if the available urine volume is lower than recommended, perform the test whenever possible. The use of a short sample should be recorded.
3) Centrifuge: This should be of the swing-type and not the angle-type.
4) Conditions of centrifugation: Centrifugation tubes must be placed in the centrifuge in a manner such that horizontal balance is maintained during operation. The centrifuge must be allowed to stop naturally before attempting to remove any centrifugation tubes.
(1) A relative centrifugal force (RCF) of 500 × g is recommended. Because the RCF at a given number of rotations varies with the size (radius) of the centrifuge, the number of rotations for each centrifuge should be calculated using the following formula:
RCF (g) = 11.18 × (rpm/1,000)2 × R
rpm: rotations per minute
R: radius, i.e., the distance (cm) from the center of the centrifuge spindle to the bottom of the centrifugation tube
(2) Five minutes is the recommended duration for centrifugation.
5) Sediment volume: The supernatant urine should be removed with an aspirator or pipette or by decantation to leave a sediment volume of 0.2 mL. This should be followed in principle because essential urine formed elements would be diluted if the sediment volume exceeds 0.2 mL.
3. Slide preparation for microscopy1) Loading urine on slide: Use 75 × 26-mm glass slides. The sediment should be mixed well using a pipette or another device to achieve uniformity while avoiding damage to urine formed elements. Place 15 μL of the sediment on a glass slide.
2) Applying the coverslip: Place an 18 × 18-mm coverslip over the sediment, ensuring that the sediment is uniformly distributed but not escaping from the edges of the coverslip.
A microscope with an eyepiece with a field number = 20 [×400 (objective lens 40×) field area is 0.196 mm2] is recommended. When using lenses with different conditions, a correction should be made according to the applicable information obtained from the manufacturers.
1. The order of microscopic examinationThe whole field (WF) of the preparation is examined under a low power field (LPF), and selected fields are then examined under a high power field (HPF).
1) LPF [×100 (objective lens 10×)] microscopic examination(1) Confirm that the urine formed elements are uniformly distributed.
(2) If the distribution is uneven, then it is advisable to make a new preparation. However, if this is not possible, then perform a microscopic examination such that the mean value for the WF can be obtained.
(3) Note that urine formed elements tend to gather along the sides of the coverslip.
(4) It is advisable to diaphragm an aperture stop under low-power magnification to not miss hyaline casts, cell aggregates, or other variables.
2) HPF [×400 (objective lens 40×)] microscopic examinationIt is desirable to examine 20–30 fields. The number of fields examined must be at least 10.
2. Specimen observation 1) Microscopic examination without stainingIn principle, urine sediments should be examined without staining.
2) Microscopic examination after stainingStaining methods are used for the confirmation and identification of urine sediment components if necessary. However, staining solutions should be carefully chosen because some have potent hemolytic activity.
3) Precautions(1) When a precipitate of urates, phosphates, carbonates, and other elements is formed in a large quantity in the sediment, it will severely hamper the microscopic examination of components. In such cases, dissolve the precipitate by heating the urine (at approximately 50°C with stirring) if it contains urates or by adding acetic acid if it contains phosphates and carbonates and then centrifuge the resulting sample to prepare a urinary sediment specimen.
(2) Care should be exercised during observation of large matters, such as cells and casts, because they tend to gather along the sides of the coverslip.
(3) The occurrence of urinary sediments is not necessarily in parallel with findings from qualitative or semiquantitative method such as urine protein test, urine occult blood reaction, and leukocyte esterase test. Even when the sample tested negative using multi-item test strips, urinary sediment examination can be useful for the differential diagnosis of kidney and urinary tract diseases as well as systemic diseases.
Urine formed elements are divided into components as shown in Table 1.2 according to JCCLS’s proposed guidelines on urinary sediment examination procedures GP1-P4 (2010).
| 1. Non-epithelial cells |
| 1) Blood cells |
| ① Red blood cells (RBCs) (non-glomerular-type RBCs, glomerular-type RBCs*1) |
| ② White blood cells*2 (neutrophils, eosinophils, lymphocytes, monocytes) |
| 2) Macrophages |
| 3) Others (e.g., endometrial stromal cells*3, mesothelial cells*4) |
| 2. Epithelial cells |
| 1) Basic epithelial cells |
| ① Renal tubular epithelial cells |
| ② Urothelial cells |
| ③ Columnar epithelial cells (e.g., urethral columnar epithelial cells, prostate epithelial cells, seminal vesicle epithelial cells, cervical epithelial cells, endometrial epithelial cells, intestinal epithelial cells) |
| ④ Squamous epithelial cells |
| 2) Degenerated/Virus-infected cells |
| ① Oval fat bodies*5 |
| ② Intracytoplasmic inclusion-bearing cells |
| ③ Intranuclear inclusion-bearing cells |
| ④ Other virus-infected cells (e.g., cells suspected to be infected by HPoV, cells suspected to be infected by HPV) |
| ⑤ Unclassifiable cells |
| 3. Atypical cells*6 |
| ① Epithelial malignant cells |
| ② Non-epithelial malignant cells |
| 4. Casts*7 |
| ① Hyaline casts |
| ② Epithelial casts |
| ③ Granular casts |
| ④ Waxy casts |
| ⑤ Fatty casts |
| ⑥ RBC casts |
| ⑦ WBC casts |
| ⑧ Vacuolar denatured casts |
| ⑨ Salt/Crystal casts |
| ⑩ Macrophage casts |
| ⑪ Others (e.g., fibrin casts, hemoglobin casts, myoglobin casts) |
| 5. Microorganisms/Parasites |
| ① Microorganisms (bacteria, fungi) |
| ② Parasites (protozoa, helminths) |
| 6. Salts/Crystals |
| ① Salts (e.g., amorphous phosphates, phosphates, amorphous urates, urates) |
| ② Normal crystals (e.g., calcium oxalate crystals, calcium phosphate crystals, urate crystals) |
| ③ Abnormal crystals (e.g., bilirubin crystals, cysteine crystals, cholesterol crystals, 2,8-dihydroxyadenine crystals) |
| ④ Drug crystals (e.g., sulfamethoxazole, sulfamethoxazole-trimethoprim) |
| 7. Others |
| ① Hemosiderin granules |
| ② Contaminants (Drugs/contrast agents, powder, feces, fibers, pollen, etc.) |
*1 Red blood cells in urine are divided into two major types, non-glomerular and glomerular types, according to the criteria for the morphological typing of urinary red blood cells (2010).
*2 White blood cells found in the urine are mostly neutrophils; however, counts of other types of cells, such as eosinophils, lymphocytes, and monocytes, are elevated in association with certain pathological conditions and thus are worth reporting.
*3 Often found with endometrial epithelial cells as contaminants in samples collected during a menstrual period.
*4 Can be found in specimens from patients with certain conditions such as abdominal wall/bladder fistula.
*5 These are cells containing fat granules that emerge in association with kidney diseases such as nephrotic syndrome. They can be divided into those derived from renal tubular epithelial cells and those derived from macrophages, but they are collectively referred to as the oval fat bodies. A different type of macrophage-derived, fat granule-containing cells found in cystitis, prostatitis, and other urologic diseases should be classified as macrophages rather than oval fat bodies. Cells containing fat granules shall be classified as cells of their origin if identifiable and otherwise classified as unclassifiable cells.
*6 The term “atypical cell” includes both malignant and benign cells in modern clinical cytology, but the former is preferentially indicated. Therefore, only malignant cells and those suspected to be malignant should be reported as atypical cells in routine microscopic examinations of urine sediments with accompanying comments on such cell information. When interpreting the test results, laboratory experts in urinary sediment examination, such as certified general clinical technologists, doctors in charge of cytology/pathology examinations, and attending physicians, should be consulted in principle. Cells difficult to classify should be reported as unclassifiable cells with a note on morphological information.
*7 Fibrin casts, hemoglobin casts, hemosiderin casts, myoglobin casts, Bence Jones protein casts, amyloid casts, platelet casts, and other casts should be documented if they can be identified on the basis of special staining along with other laboratory findings, clinical information, and other data.
Class should be identified using the following standards:
1) A hyaline cast containing three or more red blood cells, white blood cells, epithelial cells, and fat granules in the substrate should be classified as an RBC cast, WBC cast, epithelial cast, and fatty cast, respectively. Otherwise, the cast should be referred to as a hyaline cast. For example, a hyaline cast containing two red blood cells should be described as a hyaline cast. The same applies to casts containing white blood cells, renal tubular epithelial cells, or fat granules.
2) A cast is referred to as a granular cast if granules comprise at least one-third of cells in the substrate; otherwise, the cast should be documented as a hyaline cast.
3) A cast containing three or more each of multiple elements in the same substrate should be reported as follows:
① A granular cast containing three or more each of multiple cellular elements and/or other elements such as fat granules should be reported as a cast of each type as well as a granular cast.
② A waxy cast containing three or more cellular elements should be reported as both cellular and waxy casts.
③ A cast that is in a transitional form from granular cast to waxy cast or in a mixed form thereof should be reported as both granular and waxy casts.
4) Tapered cylinder-like matters, so-called cylindroids, should be included in hyaline casts.
5) If a cast has a width of approximately 60 μm or greater, then it should be reported as a broad cast in addition to an applicable type of cast. It is advised to compare a cast of interest with other elements (e.g., red blood cells, white blood cells) found in the same sediment to help estimate its size.
The reporting format described below is for reference. Different patient groups have differing requirements for urine sediment reports. Where possible, the report should be tailored to the individual needs in consultation with attending physicians.
1. Reporting non-epithelial cells/epithelial cellsResults from HPF (40×) microscopic examination should be described.
Less than 1 cell/HPF 20–29 cells/HPF
1–4 cells/HPF 30–49 cells/HPF
5–9 cells/HPF 50–99 cells/HPF
10–19 cells/HPF 100 or more cells/HPF
(Note) The 50–99 and 100 or more cells/HPF groups may be integrated into the 50 or more cells/HPF group.
2. Reporting castsResults from the LPF (10×) microscopic examination should be described on the basis of approximate counts in the whole field (WF) or individual fields (LPF), or in a qualitative manner according to the criteria described in Table 1.3.
| − | 0/WF | 0/100 LPF | 0/100 LPF |
| 1+ | 1–4/WF | 1–4/100 LPF | 1/WF to < 1/10 LPF |
| 5–9/WF | 5–9/100 LPF | ||
| 2+ | 10–19/WF | 10–19/100 LPF | 1–2/10 LPF |
| 20–29/WF | 20–29/100 LPF | ||
| 3+ | 30–49/WF | 30–49/100 LPF | 3–9/10 LPF |
| 50–99/WF | 50–99/100 LPF | ||
| 4+ | 100–999/WF | 100–999/100 LPF | 1–9/LPF |
| 5+ | ≥ 1,000/WF | ≥ 1,000/100 LPF | ≥ 10/LPF |
Results from the HPF (40×) microscopic examination should be described in a qualitative manner according to the criteria described in Table 1.4.
| − | 0 or scattered in several fields |
| 1+ | Observed in each field |
| 2+ | Many or scattered in clusters |
| 3+ | Numerous |
Results from the HPF (40×) microscopic examination should be described in a qualitative manner according to the criteria described in Table 1.5.
| − | 0 |
| 1+ | 1/WF to 4/HPF |
| 2+ | 5–9/HPF |
| 3+ | ≥ 10/HPF |
Results from the HPF (40×) microscopic examination should be described in a qualitative manner according to the criteria described in Table 1.6. Abnormal crystals should be noted if any were found in the WF.
| Crystals | Salts | |
|---|---|---|
| − | 0 | 0 |
| 1+ | 1–4/HPF | Small quantity |
| 2+ | 5–9/HPF | Moderate quantity |
| 3+ | ≥ 10/HPF | Large quantity |
When using automated instruments such as flow cytometers, it is desirable to fully understand the instrument’s characteristics to best obtain information on urine formed elements. The use of urine specimens without centrifugation makes the test more rapid and compatible with quantitative reporting (counts/μL), which is becoming a global trend. This is being done to establish its position as a screening test in the future.
In the appendix, the criteria for the morphological typing of urinary red blood cells published by the Japanese Committee for Clinical Laboratory Standards (JCCLS) (2010) are outlined.
[Citation: Japanese Clinical Practice Guidelines for Hematuria Diagnosis 2013. Life Science Publishing]
Morphology information on red blood cells can be used to identify the plausible origin of hematuria. The guidelines provide terms and classification criteria for different shapes of red blood cells. For reporting, it is important to capture both individual shapes and the overall pattern of urinary sediments, and one should recognize that classification may not be possible for all cases of hematuria. Moreover, red blood cell morphology information should be documented in consultation with clinical staff.
Terms for red blood cell morphologyNon-glomerular-type red blood cell (isomorphic RBC) and glomerular-type red blood cell (dysmorphic RBC) are terms to describe red blood cell morphology.
Classification of red blood cells in urineTo clearly describe the morphological features of red blood cells in urine, non-glomerular-type and glomerular-type red blood cells are divided into four and three major subclasses, respectively. However, non-glomerular-type and glomerular-type red blood cells do not have to be divided into subclasses in routine examinations.
• Non-glomerular-type red blood cell
[Discocyte]
Typical discocyte

Swollen discocyte
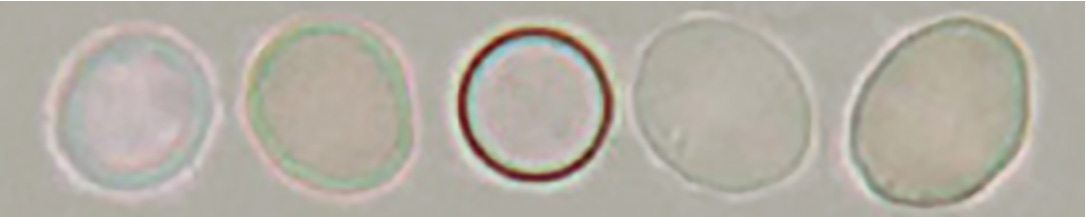
Some swollen discocytes have a thick margin and exhibit a doughnut-like shape. However, unlike doughnut-like dysmorphic red blood cells of the red blood cells of glomerular type, their margin is isomorphic.
Atrophic discocyte
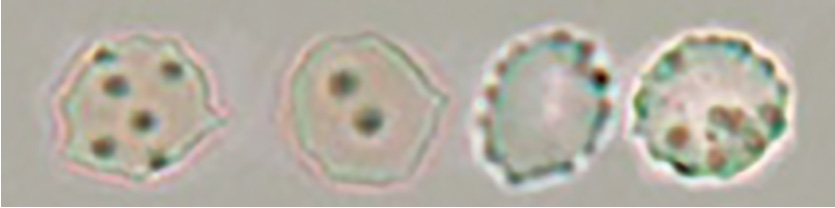
The term atrophic in this context does not describe a reduction in size. It denotes a shape with a laciniate margin formed by red blood cells undergoing low osmolality-driven expansion to a disc-like shape followed by high osmolality-driven shrinkage.
The atrophic type includes red blood cells with laciniate margins, which were previously referred to as confetti-like cells.
[Spherocyte]
Spherocyte
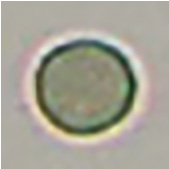
Atrophic spherocyte

Humped spherocyte
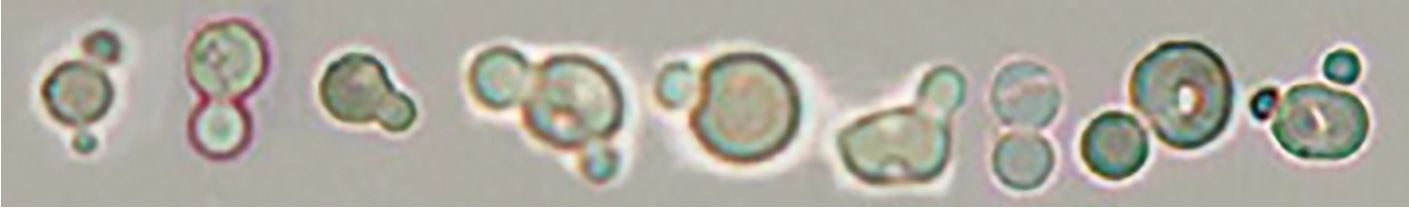
When humped spherocytes are detected, isolated hump segments of red blood cells are commonly found in the background. These red blood cell segments should not be counted as red blood cells.
[Discocyte/spherocyte transitional red blood cell]
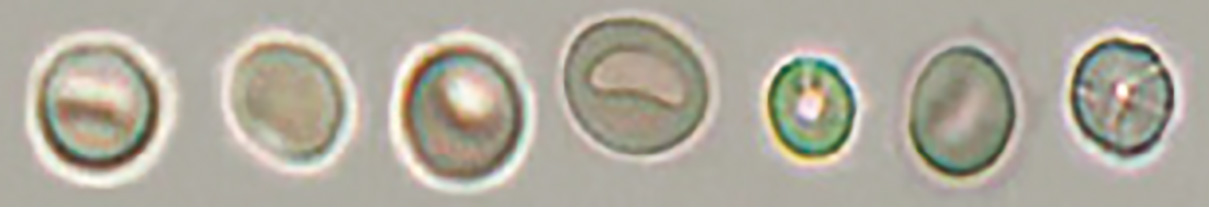
[Dehemoglobinized red blood cells with granular components aggregated in the membrane area]

In urine specimens collected after a prostate biopsy or from individuals with polycystic kidney disease, aggregated granules are found in the membrane part of dehemoglobinized red blood cells, unlike the typical morphology of dehemoglobinized red blood cells, as a result of influences from the prostatic fluid or cyst fluid.
• Glomerular-type red blood cell
[Doughnut-like dysmorphic red blood cell]
Doughnut-like dysmorphic red blood cell

Codocyte/doughnut-like dysmorphic red blood cell
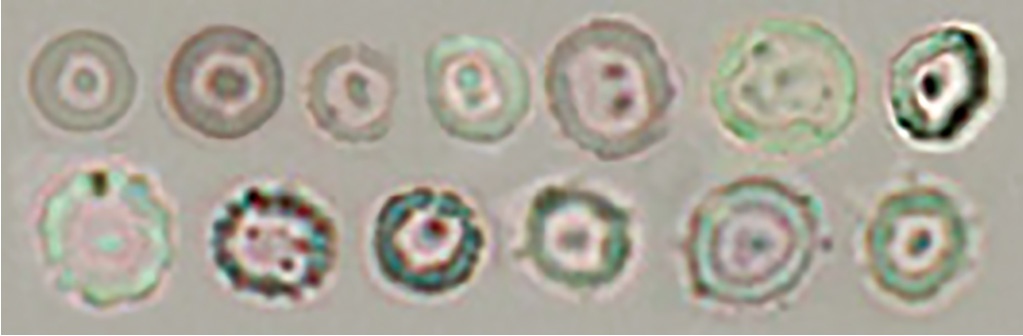
Humped/doughnut-like dysmorphic red blood cell

When humped/doughnut-like dysmorphic red blood cells are detected, isolated hump segments of red blood cells can be found concurrently in the background, as is the case with humped/spherocytes. These red blood cell segments should not be counted as red blood cells.
[Acanthocyte dysmorphic red blood cell]

[Doughnut/acanthocyte mixed-type dysmorphic red blood cell]

<Notes>
(1) The terms “swollen” and “atrophic” used for classification do not refer to size. Rather, they denote the final state of red blood cells; specifically, swollen and atrophic indicate expanded and shrunken states, respectively.
(2) The most probable cause of small spherical shapes among glomerular-type red blood cells is fragmentation of red blood cells occurring while passing through glomeruli/tubules.
Criteria for the morphological typing of urinary red blood cellsIt is determined on the basis of the shape of red blood cells observed under an optical microscope without staining. Classification as glomerular-type red blood cells requires 5–9 or more red blood cells identifiable as glomerular-type red blood cells to be observed in a ×400 (objective lens 40×) field.
The judgement involves classification into one of the following three stages: “glomerular-type red blood cells/dominant,” “glomerular-type red blood cells/moderate mixed,” and “glomerular-type red blood cells/minor mixed.” The classification criteria are based on the rank of the number of glomerular-type red blood cells relative to the total number of red blood cells.
| Total red blood cell count | |||||||
|---|---|---|---|---|---|---|---|
| 5–9/HPF | 10–19/HPF | 20–29/HPF | 30–49/HPF | 50–99/HPF | 100–/HPF | ||
| Glomerular-type red blood cell count |
5–9/HPF | Dominant | Moderate | Moderate | Minor | Minor | Minor |
| 10–19/HPF | Dominant | Moderate | Moderate | Minor | Minor | ||
| 20–29/HPF | Dominant | Moderate | Moderate | Minor | |||
| 30–49/HPF | Dominant | Moderate | Moderate | ||||
| 50–99/HPF | Dominant | Moderate | |||||
| 100–/HPF | Dominant | ||||||
HPF: high power field [×400 (objective lens 40×)]
<Notes>
(1) The pattern of glomerular-type red blood cell occurrence can lack variety, with the majority of cells being 2–4 μm in diameter and exhibiting a small spherical shape. In such cases, these cells should be counted as red blood cells regardless of the small size.
(2) When closely observed, these cells exhibit some characteristics of glomerular-type red blood cells. Humped/doughnut-like dysmorphic red blood cells can also be confirmed, albeit in a small number.
(3) The criteria for the morphological typing of red blood cells should be implemented at each institution after consultation with clinicians.
Source: Japanese Committee for Clinical Laboratory Standards (JCCLS) Urinary Sediment Examination Standardization Committee: “Urinary Sediment Examination Procedures GP1-P4,” 7–9, Examination of Urinary Sediment 2010, Japanese Association of Medical Technologists, 2011.