Abstract
Aims: Small arteries can be visualized in the ocular fundus, and findings of retinopathy based on Scheie classification are often applied to evaluate the impact of hypertension and atherosclerosis. However, the relationship between damage in the large and small arteries has not been investigated sufficiently, especially in the early stages. The present study investigated possible associations between large artery atherosclerosis and small artery retinopathy in untreated middle-aged individuals.
Methods: Untreated middle-aged workers undergoing periodic health check-ups (n=7,730, 45±8 years) were enrolled in this study. The absence or presence and extent of retinopathy were characterized by ophthalmologists as hypertensive (H0-4) and atherosclerotic grades (S0-4) based on Scheie classification. Large artery atherosclerosis was examined based on functional assessment of the cardio-ankle vascular index (CAVI) and morphological assessment of the carotid intima-media thickness (IMT) by ultrasound.
Results: We found significant differences in CAVI and carotid IMT between individuals with and without hypertensive or atherosclerotic retinopathy. Multivariable regression analysis showed that the presence of hypertensive and atherosclerotic retinopathy was significantly associated with CAVI and carotid IMT. Logistic regression analysis with the endpoint of a hypertensive or atherosclerotic lesion revealed that CAVI and carotid IMT are independent determinants of retinopathy.
Conclusions: CAVI and carotid IMT were significantly associated with the presence of retinopathy based on Scheie classification in untreated middle-aged subjects, implying that atherosclerotic examination in large arteries could reveal early-stage small artery retinopathy.
See editorial vol. 29: 1-2
Introduction
The onset of cardiovascular disease is mainly based on the development and progression of atherosclerosis that provokes a decrease in blood flow in systemic or local circulation, resulting in ischemic changes that lead to primary organ damage
1-
5)
. Hence, preventing the progressive processes of atherosclerosis is one of the most critical strategies in public health. Hypertension, dyslipidemia, diabetes mellitus, and smoking promoted atherosclerosis are established cardiovascular risk factors related to unhealthy lifestyles; thus, individuals having such risk factors are at increased cardiovascular risk
6-
13)
. In addition to these factors, aging and the presence of chronic kidney disease (CKD) are also considered high-risk conditions of cardiovascular disease
14-
16)
.
Atherosclerosis is a chronic disease of the arterial wall characterized by functional and structural alterations in the vascular endothelium and smooth muscle layer
1-
5)
. In the clinical setting, the extent of subclinical atherosclerosis is mainly evaluated in large arteries by non-invasive examinations, including the cardio-ankle vascular index (CAVI) and carotid intima-media thickness (IMT)
17)
. CAVI is a functional index that indicates arterial stiffness, which is pathologically characterized by decreased arterial elasticity
17-
20)
, whereas carotid IMT is a morphological index of atherosclerotic vascular damage that reflects pathological thickening in the vascular wall
19-
23)
.
Small arteries can be visualized in the ocular fundus, and findings of retinopathy based on the Scheie classification are often applied to evaluate the impacts of hypertension and atherosclerosis
24)
. Notably, hypertensive changes in the fundus (i.e., hypertensive retinopathy) are the only way in which to visually capture vascular morphological changes due to high blood pressure (BP), making them important findings in the target organ damaged by hypertension
25,
26)
. The presence of small artery retinopathy in the fundus has been reported to be associated with increased risk of cardiovascular diseases and future cardiovascular events
27-
29)
. However, the relationship between arterial damage in the small and large arteries, including the muscular and elastic arteries, has not been investigated sufficiently, particularly in early-stage atherosclerosis. Here, we hypothesized that atherosclerosis in small arteries and large arteries progresses in relation to one another from an early stage, and that examination of large artery atherosclerosis could reveal small artery retinopathy.
Aim
In the present study, our aim was to investigate possible associations between retinopathy in small arteries based on the Scheie classification and examinations of large artery atherosclerosis, and to test whether the atherosclerotic examination reflects the presence of retinopathy in seemingly healthy untreated middle-aged individuals.
Methods
The present study enrolled individuals attending their periodic physical check-up. The study was performed in accordance with the principles of the Declaration of Helsinki, and the ethics committees of Toyota Memorial Hospital approved the protocol. All data used in the analysis were anonymized, and opt-out opportunities were provided to participants.
Subjects
This study screened 15,764 individuals who visited the Health Support Center WELPO in 2008–2009 for a periodic health check-up. The center provides health care for Toyota Motor Corporation (Toyota, Japan) employees and their spouses, and all employees receive annual medical examinations under the Industrial Safety and Health Law of Japan. All obtained data were supplied as medical examination records. Of the 15,764 people screened, 4,901 individuals were excluded due to blank of data on fundus examination, because they were non-workers (spouses), or due to an uncertain workstyle. An additional 3,133 individuals taking medication were excluded to eliminate the effects of medications because the detailed classifications of the medications were unknown. The data from the remaining 7,730 untreated workers were finally used for the analyses.
After overnight fasting, body height and weight were measured using an automated BF-220 instrument (Tanita, Tokyo, Japan), and systolic and diastolic BP were measured using a validated oscillometric technique in a seated position. Blood was sampled from the antecubital vein in the morning for laboratory measurements, and CAVI and carotid IMT were measured to assess subclinical atherosclerosis. CAVI was measured in a supine position to evaluate peripheral artery disease or arterial stiffness, followed by ultrasound examination to measure the carotid IMT. Individuals with a systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg were defined as having hypertension
25)
. Individuals with high-density lipoprotein cholesterol (HDL-C) levels <40 mg/dL, low-density lipoprotein cholesterol (LDL-C) levels ≥ 140 mg/dL, or triglycerides ≥ 150 mg/dL were defined as having dyslipidemia
17)
. Individuals with a fasting blood glucose (FBG) level ≥ 126 mg/dL were defined as having diabetes
30)
. The estimated glomerular filtration rate (eGFR) was calculated using a modified formula from the Modification of Diet in Renal Disease study for the Japanese population and eGFR <60 mL/min/1.73 m2 was defined as CKD
11,
31)
.
Biochemical Analysis
Biochemical tests, including the determination of total cholesterol, LDL-C, HDL-C, triglycerides, creatinine, and FBG, were performed using standard laboratory assays as described previously
32)
. Concentrations of glycated hemoglobin A1c (HbA1c) were measured by high-performance liquid chromatography and expressed according to the National Glycohemoglobin Standardization Program.
Assessment of Arterial Stiffness in a Muscular Artery
Arterial stiffness was assessed by CAVI using a Vasera VS-1000 automatic system (Fukuda Denshi, Tokyo, Japan), as described previously
17-
20)
, after resting in the supine position. Electrocardiogram electrodes and a microphone were placed on both wrists and the sternum to detect heart sounds. Cuffs were wrapped around both upper arms and both ankles. Cardio-ankle pulse wave velocity (PWV) was measured by dividing the distance from the aortic valve to the ankle artery by the sum of the difference between when the time the pulse waves were transmitted to the brachium and when they were transmitted to the ankle, combined with the time difference between the second heart sound on the phonocardiogram and that on the notch of the brachial pulse wave. CAVI is expressed as stiffness parameter β according to the following equation: CAVI=a [2ρ/ PP×[ln Ps/Pd] PWV2]+b, (where a and b are constants, ρ is blood density, PP is pulse pressure, Ps is systolic pressure, and Pd is diastolic pressure). Theoretically, BP does not affect the CAVI measurement, and the mean CAVI from the left and right parts of the body was used for analysis.
Assessment of Carotid IMT
The IMT of the carotid artery was assessed by ultrasound using the Aplio 500 device (Canon Medical Systems, Otawara, Japan), as described previously
32)
. All estimations of carotid IMT and plaque were performed by well-trained clinical laboratory technicians who were blinded to other clinical information. The common carotid artery (CCA) is one of the elastic arteries, and the CCA IMT was evaluated manually using a 7.5 MHz frequency probe, with all individuals in the supine position. CCA IMT was evaluated in the far wall ~20 mm from the carotid bifurcation using recorded images of the carotid artery, with the mean IMT from both sides used for analysis
21-
23)
. Carotid plaque was identified as an elevated lesion with a maximum thickness ≥ 1.1 mm and a point of inflection on the surface of the intima-media complex in the CCA, carotid bulb, and internal carotid artery. Carotid plaque was considered representative of subclinical atherosclerosis
21-
23)
.
Assessment of Retinopathy in the Fundus
Fundus photographs were taken using a non-mydriatic retinal camera (CR-DGi, Canon, Inc., Tokyo, Japan). All photographs were evaluated by ophthalmologists who were blinded to the participants’ backgrounds. Hypertensive and atherosclerotic changes graded the retinopathy according to the Scheie classification
24)
. Hypertensive retinopathy was graded as follows: H0, normal; H1, barely detectable arterial narrowing; H2, obvious arterial narrowing with focal irregularities plus light reflex changes; H3, grade H2 plus retinal hemorrhages and/or exudates; H4, grade H3 plus papilledema. Atherosclerotic retinopathy was graded as follows: S0, normal; S1, there is broadening of the light reflex from the arteriole with minimal or no arteriovenous compression; S2, light reflex changes and crossing changes are more prominent; S3, the arterioles have a copper wire appearance, and there is more arteriovenous compression; S4, the arterioles have a silver appearance, and arteriovenous crossing changes are most severe.
Statistical Analysis
Data were analyzed using SPSS Statistics 19 (IBM Corp., Chicago, IL, USA). Normally distributed data are expressed as mean±standard deviation. Dichotomous variables (gender, smoking status, complications, and retinopathy) were assigned values of 0 (female, non-smoker, without each complication, and absence of retinopathy) or 1 (male, smoker, with each complication, and presence of retinopathy). Differences in continuous variables among more than three groups were tested using analysis of variance (ANOVA) followed by Scheffe’s post-hoc analysis. Logistic regression analyses were performed to determine the independent variables. Multivariable regression analyses were performed as appropriate. Receiver operating characteristics (ROC) curve analysis was performed to determine the cut-off level, area under the curve (AUC) with 95% confidence interval (CI), sensitivity, and specificity. Two-tailed p<0.05 was considered significant.
Results
A total of 7,730 untreated workers, including 7,120 males and 610 females, aged 35 to 61 years, were enrolled in the present study (
Table 1)
. CAVI and carotid IMT showed nearly normal distributions with median values of 7.25 and 0.55 mm, respectively (
Supplementary Fig.1)
. Carotid plaque was found in 1,110 of the 7,730 enrolled individuals. Most of the enrolled individuals were classified as H0 grade (i.e., without hypertensive retinopathy;
Table 1
). Only a few individuals had grade 2 hypertensive or atherosclerotic findings, and grade 3 or 4 findings were not observed in this study population (
Table 1)
. The proportion of obesity, CKD, hypertension, dyslipidemia, or diabetes was significantly increased with hypertensive retinopathy, but that of current smoking decreased (
Supplementary Table 1)
. The proportion of CKD, hypertension, dyslipidemia, or diabetes was also increased with atherosclerotic retinopathy (
Supplementary Table 1)
.
Table 1.
Characteristics of the study participants (
n = 7,730)
| Variable |
All participants |
| Age, years |
45.3±8.1 |
| Male gender |
7120 (92.1) |
| Body mass index, kg/m2
|
22.8±3.0 |
| Current smoker |
3023 (39.1) |
| Systolic BP, mmHg |
118.2±13.9 |
| Diastolic BP, mmHg |
75.0±9.4 |
| Creatinine, mg/dL |
0.80±0.13 |
| HDL-C, mg/dL |
60.4±15.6 |
| LDL-C, mg/dL |
118.8±27.9 |
| Triglyceride, mg/dL |
113.6±75.0 |
| FBG, mg/dL |
94.3±12.9 |
| HbA1c, % |
5.63±0.45 |
| Examination for subclinical atherosclerosis |
|
| CAVI |
7.29±0.78 |
| Carotid IMT, mm |
0.55±0.11 |
| Scheie classification in the retinal artery |
|
| Hypertensive grade (0-4) |
|
| H0 |
7513 (97.2) |
| H1 |
194 (2.5) |
| H2 |
23 (0.3) |
| Atherosclerotic grade (0-4) |
|
| S0 |
6671 (86.3) |
| S1 |
1050 (13.6) |
| S2 |
9 (0.1) |
Data are presented as the mean±standard deviation or n (%).
BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FBG, fasting blood glucose; CAVI, cardio-ankle vascular index; IMT, intima-media thickness.

Supplementaly Table 1.
Logistic regression analysis showing possible associations of each each cardiovascular risk factor and the presence of hypertensive or atherosclerotic retinopathy based on Scheie classification and atherosclerotic examination in all participants (
n = 7,730)
| Variable |
All subjects,
n = 7730
|
Absence or presence of hypertensive retinopathy |
Absence or presence of atherosclerotic retinopathy |
| H0, 7513 (97.2) |
H1 and H2, 217 (2.8) |
Unadjusted OR [95% CI] |
P value
|
S0, 6671 (86.3) |
S1 and S2, 1059 (13.6) |
Unadjusted OR [95% CI] |
P value
|
| Current smoking |
3023 (39.1) |
2958 (39.4) |
65 (30) |
0.66 [0.49 – 0.88] |
<0.01 |
2625 (39.3) |
398 (37.6) |
0.93 [0.81 – 1.06] |
0.274 |
| Obesity |
1484 (19.2) |
1424 (19.0) |
60 (27.6) |
1.63 [1.21 – 2.21] |
<0.01 |
1275 (19.1) |
209 (19.7) |
1.04 [0.88 – 1.23] |
0.633 |
| CKD |
3167 (41.0) |
3044 (40.5) |
123 (56.7) |
1.92 [1.46 – 2.52] |
<0.0001 |
2947 (44.2) |
670 (63.3) |
2.88 [2.52 – 3.29] |
<0.0001 |
| Hypertension |
651 (8.4) |
519 (6.9) |
132 (60.8) |
20.9 [15.7 – 27.9] |
<0.0001 |
449 (6.7) |
202 (19.1) |
3.27 [2.73 – 3.91] |
<0.0001 |
| Dyslipidemia |
2815 (36.4) |
2712 (36.1) |
103 (47.5) |
1.60 [1.22 – 2.10] |
<0.001 |
2379 (35.7) |
436 (41.2) |
1.26 [1.11 – 1.44] |
<0.001 |
| Diabetes |
230 (3.0) |
208 (2.8) |
22 (10.1) |
3.96 [2.50 – 6.29] |
<0.0001 |
123 (1.8) |
107 (10.1) |
5.98 [4.58 – 7.82] |
<0.0001 |
Data are presented as n (% in the grade) or unadjusted odds ratio (OR) [95% confidence interval (CI)]. CKD, chronic kidney disease (defined as estimated glomerular filtration rate <60 mL/min/1.73 m2).
Body mass index ≥ 25 kg/m2 was defined as obesity.
CAVI or IMT was significantly different between individuals with and without retinopathy, whereas we did not observe a step-wise increase in CAVI or IMT with increasing severity of retinopathy (grades 1 and 2;
Fig.1
). As the hypertensive and atherosclerotic grades did not exhibit a normal distribution in this population, linear regression analyses could not be applied to assess the relationship between the hypertensive and atherosclerotic grades and CAVI or IMT. Thus, we performed multivariable regression analyses in which atherosclerotic examination was considered a dependent variable, and the absence or presence of hypertensive and atherosclerotic retinopathy was included as an independent variable. The multivariable regression analyses revealed that the presence of hypertensive and atherosclerotic retinopathy was significantly associated with CAVI and carotid IMT after adjusting for possible confounders (
Table 2)
.
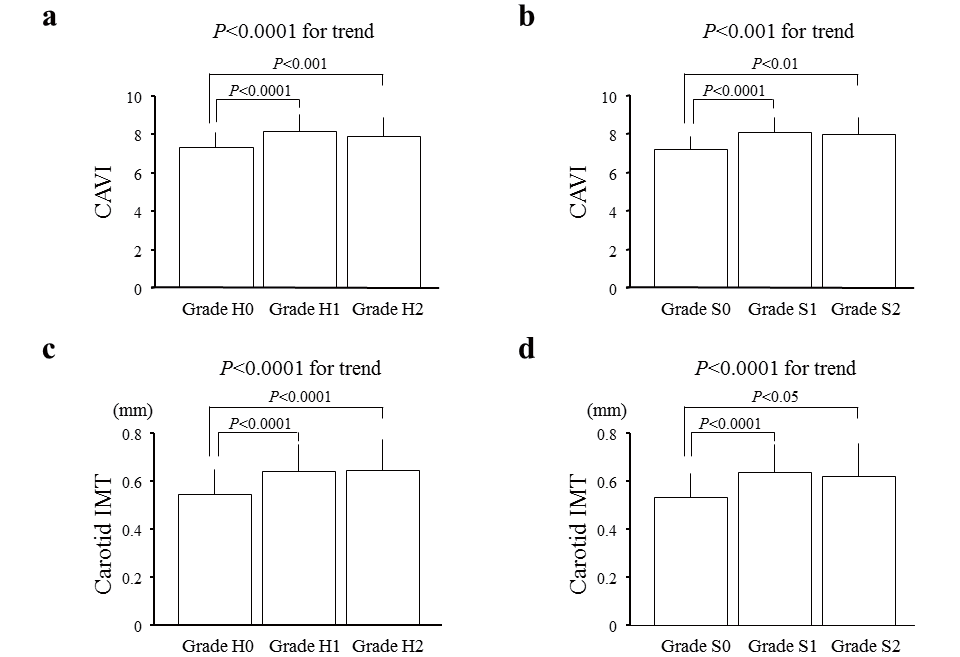
Table 2.
Results of multivariable regression analysis showing the association between atherosclerotic examinations and the presence of retinopathy based on Scheie classification in all participants (
n = 7,730)
| a) Cardio-ankle vascular index (CAVI) |
| Independent variable |
Dependent variable: CAVI |
| Model 1 |
Model 2 |
Model 3 |
| Coefficient (β) |
P value
|
Coefficient (β) |
P value
|
Coefficient (β) |
P value
|
| Hypertensive retinopathy |
0.081 |
<0.0001 |
0.081 |
<0.0001 |
0.050 |
<0.0001 |
| Atherosclerotic retinopathy |
0.371 |
<0.0001 |
0.080 |
<0.0001 |
0.071 |
<0.0001 |
| Model 1 included hypertensive and atherosclerotic retinopathy as covariates. Model 2 was adjusted for age (years), male gender (yes or no), body mass index (kg/m2), and smoking status (yes or no) in addition to the factors in Model 1. Model 3 was adjusted for mean blood pressure (mmHg), creatinine (mg/dL), high-density lipoprotein cholesterol (mg/dL), low-density lipoprotein cholesterol (mg/dL), triglyceride (mg/dL), and fasting blood glucose (mg/dL) in addition to the factors in Model 2. Mean blood pressure was calculated as sum values of diastolic blood pressure and (systolic blood pressure - diastolic blood pressure)/3.
|
| b) Carotid intima-media thickness (IMT) |
| Independent variable |
Dependent variable: carotid IMT |
| Model 1 |
Model 2 |
Model 3 |
| Coefficient (β) |
P value
|
Coefficient (β) |
P value
|
Coefficient (β) |
P value
|
| Hypertensive retinopathy |
0.072 |
<0.0001 |
0.043 |
<0.0001 |
0.030 |
<0.01 |
| Atherosclerotic retinopathy |
0.305 |
<0.0001 |
0.050 |
<0.001 |
0.046 |
<0.001 |
| Model 1 included hypertensive and atherosclerotic retinopathy as covariates. Model 2 was adjusted for age (years), male gender (yes or no), body mass index (kg/m 2), and smoking status (yes or no) in addition to the factors in Model 1. Model 3 was adjusted for mean blood pressure (mmHg), creatinine (mg/dL), high-density lipoprotein cholesterol (mg/dL), low-density lipoprotein cholesterol (mg/dL), triglyceride (mg/dL), and fasting blood glucose (mg/dL) in addition to the factors in Model 2. Mean blood pressure was calculated as sum values of diastolic blood pressure and (systolic blood pressure - diastolic blood pressure)/3.
|
To investigate the impact of atherosclerotic examinations on the presence of retinopathy and to identify the potential risk factors as categorical variables, logistic regression analyses were performed with the endpoint of the presence of retinopathy. The logistic regression analyses showed that CAVI was significantly associated with hypertensive retinopathy, whereas carotid IMT was significantly associated with hypertensive and atherosclerotic retinopathy (
Table 3)
. Among the 7,730 enrolled individuals, 1,115 had hypertensive or atherosclerotic retinopathy. Logistic regression analysis with the endpoint of the presence of hypertensive or atherosclerotic findings based on the Scheie classification revealed that CAVI and carotid IMT were independently associated with the retinopathy (
Table 4a, 4b)
. However, the model in which CAVI and carotid IMT were simultaneously included as independent variables showed that carotid IMT was still independently associated with the retinopathy while CAVI was not (
Table 4c)
.
Table 3.
Results of logistic regression analysis showing possible associations between the presence of hypertensive or atherosclerotic retinopathy based on Scheie classification, the presence of cardiovascular risk factors, and atherosclerotic examination in all participants (
n = 7,730)
| a) Cardio-ankle vascular index (CAVI) as the atherosclerotic examination of the large artery |
| Variable |
Hypertensive retinopathy |
Atherosclerotic retinopathy |
| Odds ratio |
95% confidence interval |
P value
|
Odds ratio |
95% confidence interval |
P value
|
| Age, per year |
1.157 |
1.124 – 1.192 |
<0.0001 |
1.988 |
1.890 – 2.092 |
<0.0001 |
| Gender male |
0.462 |
0.267 – 0.800 |
<0.01 |
1.346 |
0.803 – 2.257 |
0.259 |
| With current smoking |
0.773 |
0.551 – 1.084 |
0.136 |
0.795 |
0.623 – 1.014 |
0.065 |
| With obesity |
1.556 |
1.094 – 2.212 |
<0.05 |
1.250 |
0.922 – 1.695 |
0.152 |
| With chronic kidney disease |
1.056 |
0.749 – 1.489 |
0.756 |
0.891 |
0.688 – 1.153 |
0.380 |
| With hypertension |
11.561 |
8.478 – 15.764 |
<0.0001 |
1.191 |
0.860 – 1.648 |
0.292 |
| With dyslipidemia |
1.084 |
0.796 – 1.475 |
0.609 |
0.983 |
0.776 – 1.246 |
0.890 |
| With diabetes |
1.057 |
0.619 – 1.805 |
0.838 |
1.183 |
0.770 – 1.817 |
0.443 |
| CAVI, per 1.0 |
1.341 |
1.106 – 1.626 |
<0.01 |
1.077 |
0.925 – 1.253 |
0.338 |
|
Obesity: body mass index ≥ 25 kg/m2. Chronic kidney disease: estimated glomerular filtration rate <60 mL/min/1.73 m2.
The endpoint of the analysis was the presence of hypertensive or atherosclerotic retinopathy based on Scheie classification. Listed variables were simultaneously evaluated in the multivariable analysis.
|
| b) Carotid intima-media thickness (IMT) as the atherosclerotic examination of the large artery |
| Variable |
Hypertensive retinopathy |
Atherosclerotic retinopathy |
| Odds ratio |
95% confidence interval |
P value
|
Odds ratio |
95% confidence interval |
P value
|
| Age, per year |
1.164 |
1.132 – 1.197 |
<0.0001 |
1.986 |
1.889 – 2.089 |
<0.0001 |
| Gender male |
0.503 |
0.292 – 0.866 |
<0.05 |
1.346 |
0.804 – 2.255 |
0.258 |
| With current smoking |
0.775 |
0.553 – 1.087 |
0.140 |
0.782 |
0.612 – 0.997 |
<0.05 |
| With obesity |
1.359 |
0.954 – 1.936 |
0.089 |
1.193 |
0.879 – 1.620 |
0.258 |
| With chronic kidney disease |
1.009 |
0.716 – 1.423 |
0.958 |
0.884 |
0.683 – 1.145 |
0.351 |
| With hypertension |
12.210 |
8.955 – 16.59 |
<0.0001 |
1.196 |
0.867 – 1.650 |
0.276 |
| With dyslipidemia |
1.063 |
0.781 – 1.447 |
0.698 |
0.977 |
0.771 – 1.237 |
0.844 |
| With diabetes |
1.130 |
0.666 – 1.916 |
0.650 |
1.177 |
0.769 – 1.802 |
0.452 |
| Carotid IMT, per 0.1 mm |
1.230 |
1.078 – 1.402 |
<0.01 |
1.120 |
1.012 – 1.240 |
<0.05 |
|
Obesity: body mass index ≥ 25 kg/m2. Chronic kidney disease: estimated glomerular filtration rate <60 mL/min/1.73 m2.
The endpoint of the analysis was the presence of hypertensive or atherosclerotic retinopathy based on Scheie classification. Listed variables were simultaneously evaluated in the multivariable analysis.
|
| c) Both cardio-ankle vascular index (CAVI) and carotid intima-media thickness (IMT) were simultaneously included as the atherosclerotic examination of the large artery |
| Variable |
Hypertensive retinopathy |
Atherosclerotic retinopathy |
| Odds ratio |
95% confidence interval |
P value
|
Odds ratio |
95% confidence interval |
P value
|
| Age, per year |
1.145 |
1.112 – 1.180 |
<0.0001 |
1.978 |
1.880 – 2.082 |
<0.0001 |
| Gender male |
0.456 |
0.263 – 0.790 |
<0.01 |
1.316 |
0.783 – 2.210 |
0.300 |
| With current smoking |
0.741 |
0.527 – 1.042 |
0.085 |
0.775 |
0.606 – 0.990 |
<0.05 |
| With obesity |
1.447 |
1.013 – 2.068 |
<0.05 |
1.206 |
0.887 – 1.640 |
0.232 |
| With chronic kidney disease |
1.031 |
0.730 – 1.456 |
0.861 |
0.887 |
0.685 – 1.148 |
0.362 |
| With hypertension |
11.279 |
8.263 – 15.396 |
<0.0001 |
1.171 |
0.845 – 1.622 |
0.344 |
| With dyslipidemia |
1.068 |
0.784 – 1.465 |
0.677 |
0.976 |
0.770 – 1.237 |
0.841 |
| With diabetes |
1.045 |
0.613 – 1.781 |
0.872 |
1.157 |
0.754 – 1.776 |
0.505 |
| CAVI, per 1.0 |
1.313 |
1.080 – 1.597 |
<0.01 |
1.064 |
0.914 – 1.238 |
0.422 |
| Carotid IMT, per 0.1 mm |
1.212 |
1.061 – 1.383 |
<0.01 |
1.117 |
1.009 – 1.237 |
<0.05 |
|
Obesity: body mass index ≥ 25 kg/m2. Chronic kidney disease: estimated glomerular filtration rate <60 mL/min/1.73 m2.
The endpoint of the analysis was the presence of hypertensive or atherosclerotic retinopathy based on Scheie classification. Listed variables were simultaneously evaluated in the multivariable analysis.
|
Table 4.
Results of logistic regression analysis showing the association between hypertensive and atherosclerotic retinopathy based on Scheie classification and possible factors including cardio-ankle vascular index (CAVI) and carotid intima-media thickness (IMT) in all participants (
n = 7,730)
| a) CAVI |
| Variable |
Odds ratio |
95% confidence interval |
P value
|
| Age, per year |
1.633 |
1.580 – 1.689 |
<0.0001 |
| Gender male |
1.376 |
0.882 – 2.148 |
0.160 |
| With current smoking |
0.841 |
0.678 – 1.042 |
0.114 |
| With obesity |
1.323 |
1.012 – 1.732 |
<0.05 |
| With chronic kidney disease |
0.823 |
0.655 – 1.034 |
0.094 |
| With hypertension |
2.262 |
1.669 – 3.066 |
<0.0001 |
| With dyslipidemia |
0.966 |
0.784 – 1.191 |
0.747 |
| With diabetes |
1.149 |
0.775 – 1.703 |
0.489 |
| CAVI, per 1.0 |
1.152 |
1.005 – 1.321 |
<0.05 |
|
Obesity: body mass index ≥ 25 kg/m2. Chronic kidney disease: estimated glomerular filtration rate <60 mL/min/1.73 m2.
The endpoint of the analysis was the presence of hypertensive or atherosclerotic retinopathy based on Scheie classification. Listed variables were simultaneously evaluated in the multivariable analysis.
|
| b) Carotid IMT |
| Variable |
Odds ratio |
95% confidence interval |
P value
|
| Age, per year |
1.634 |
1.582 – 1.688 |
<0.0001 |
| Gender male |
1.389 |
0.890 – 2.168 |
0.147 |
| With current smoking |
0.825 |
0.665 – 1.024 |
0.081 |
| With obesity |
1.227 |
0.937 – 1.606 |
0.137 |
| With chronic kidney disease |
0.812 |
0.646 – 1.020 |
0.073 |
| With hypertension |
2.310 |
1.708 – 3.124 |
<0.0001 |
| With dyslipidemia |
0.958 |
0.777 – 1.181 |
0.686 |
| With diabetes |
1.144 |
0.775 – 1.688 |
0.500 |
| Carotid IMT, per 0.1 mm |
1.183 |
1.080 – 1.296 |
<0.001 |
|
Obesity: body mass index ≥ 25 kg/m2. Chronic kidney disease: estimated glomerular filtration rate <60 mL/min/1.73 m2.
The endpoint of the analysis was the presence of hypertensive or atherosclerotic retinopathy based on Scheie classification. Listed variables were simultaneously evaluated in the multivariable analysis.
|
| c) Both CAVI and carotid IMT |
| Variable |
Odds ratio |
95% confidence interval |
P value
|
| Age, per year |
1.622 |
1.569 – 1.677 |
<0.0001 |
| Gender male |
1.331 |
0.851 – 2.082 |
0.210 |
| With current smoking |
0.811 |
0.653 – 1.007 |
0.058 |
| With obesity |
1.256 |
0.958 – 1.647 |
0.100 |
| With chronic kidney disease |
0.816 |
0.649 – 1.025 |
0.081 |
| With hypertension |
2.220 |
1.636 – 3.013 |
<0.0001 |
| With dyslipidemia |
0.955 |
0.775 – 1.178 |
0.670 |
| With diabetes |
1.105 |
0.746 – 1.637 |
0.617 |
| CAVI, per 1.0 |
1.133 |
0.988 – 1.299 |
0.074 |
| Carotid IMT, per 0.1 mm |
1.176 |
1.074 – 1.289 |
<0.001 |
|
Obesity: body mass index ≥ 25 kg/m2. Chronic kidney disease: estimated glomerular filtration rate <60 mL/min/1.73 m2.
The endpoint of the analysis was the presence of hypertensive or atherosclerotic retinopathy based on Scheie classification. Listed variables were simultaneously evaluated in the multivariable analysis.
|
ROC curve analysis discriminating individuals with hypertensive or atherosclerotic retinopathy revealed that the cut-off levels and AUC values for CAVI and carotid IMT were 7.55 and 0.80 (95% CI 0.79–0.81, p<0.0001) and 0.55 mm and 0.75 (95% CI 0.74–0.76, p<0.0001), respectively (
Fig.2)
. Subsequently, the effects of coincident retinopathy and carotid plaque were investigated; 409 of the 7,730 enrolled individuals were identified as having both retinopathy and carotid plaque. ROC curve analysis discriminating individuals with the complication of both retinopathy and carotid plaque using CAVI or carotid IMT revealed that the cut-off levels and AUC values for CAVI and carotid IMT were 7.55 and 0.79 (95% CI 0.77–0.82, p<0.0001) and 0.60 mm and 0.78 (95% CI 0.75–0.80, p<0.0001), respectively (
Fig.3)
.
Discussion
The main finding of the present study was significant differences in the CAVI and carotid IMT between patients with and without retinopathy, but not between those with grade 1 and 2 retinopathy. In addition, the presence of hypertensive and atherosclerotic retinopathy was significantly associated with CAVI and carotid IMT, which were independent determinants of the presence of retinopathy based on Scheie classification. Thus, a close association between atherosclerotic examination in large arteries and small artery retinopathy based on Scheie classification was confirmed in untreated middle-aged individuals, supporting the finding that vascular damage progresses in association with small and large arteries from an early stage.
Atherosclerosis examinations are widely performed, and CAVI and carotid IMT are representatives of functional and morphological assessments in large muscular and elastic arteries
17-
23)
. The usefulness of atherosclerotic examinations has been established not only in individuals with progressive atherosclerosis in clinical settings, but also in the asymptomatic general population at health check-ups
19,
20,
23,
32-
35)
. The ability to detect subclinical atherosclerosis in asymptomatic individuals is not so great with a single examination, though it could be improved by combining the examinations. In the present study, we investigated subclinical atherosclerosis in large arteries using CAVI for functional assessment and carotid IMT for morphological assessment and confirmed significant associations between these examinations and the presence of small artery retinopathy. Although an association between the small artery retinopathy and aortic stiffness measured by PWV was reported previously, the previous study included a small number of hypertensive individuals
36-
39)
. The present study enrolled a larger number of individuals with relatively low risk and evaluated both functional and morphological examinations, supporting the concept that the interaction between impairments in the small and large arteries was similarly consistent in the early stages.
Interactions between microvascular and macrovascular impairments were conceptualized previously, and vascular endothelial dysfunction and increased arterial stiffness are considered the fundamental mechanisms
40,
41)
. Oxidative stress and systemic inflammation have also been raised as common factors of vascular endothelial dysfunction
1-
5)
. Although, in the present study, we examined neither vascular endothelial function nor parameters regarding oxidative stress or inflammation, we previously reported that oxidative stress and inflammation are significantly associated with vascular endothelial dysfunction and increased arterial stiffness
10,
11,
35,
42)
. Decreased elasticity in the arterial walls has been reported as a mechanism underlying increased arterial stiffness, and physiological alterations with aging and inflammatory modifications being possible factors leading to decreased elasticity in the presence of increased arterial stiffness
19,
20)
. Thus, vascular impairment in the small and large arteries may simultaneously initiate and progress through increasing oxidative stress and inflammation.
The prevalence of retinopathy was different based on the background of the enrolled individuals
36-
39)
. Although the proportion of individuals with hypertensive retinopathy (2.8%) was lower than the proportion with atherosclerotic retinopathy (13.7%), it may be partly due to the exclusion of currently medicated individuals from the present study. Among the cardiovascular risk factors, hypertension and obesity were independent determinants of hypertensive retinopathy although the BP levels were around the normal range and the untreated individuals with hypertension comprised only 8.4% of the total participants. This result reinforces that hypertensive retinopathy was strongly influenced by high BP
24)
. In contrast, the presence of atherosclerotic retinopathy was dependent primarily on age. Although current smoking status had a seemingly inverse influence on the presence of retinopathy, this may be because current smokers with retinopathy had been excluded from the study due to current medication use.
When the presence of retinopathy was analyzed based on the presence of hypertensive or atherosclerotic findings based on the Scheie classification, both CAVI and carotid IMT were independent determinants of retinopathy in models where these indices were separately included. However, only carotid IMT was independently associated with the retinopathy in the model where CAVI and carotid IMT were simultaneously included as independent variables. The detailed mechanism is not clear, but it may be partly attributable to the different features of the two indices. CAVI is a functional index of arterial stiffness, while carotid IMT is a morphological index of atherosclerotic vascular damage. In general, the elastic property of the large arteries buffers pulsation to deliver the flow with less pulsatile pressure to the peripheral microvascular; however, the coincidence of aging or cardiovascular risk factors promotes arteriosclerosis, limiting this buffering, and increases pulsatile pressure in the microvascular systems
40,
41)
. Hypertensive retinopathy might be directly influenced by high BP and reflecting the clinical severity of hypertension, while atherosclerotic retinopathy presents structural alteration in retinal small arteries due to continuous vascular damage
24,
40,
41,
43,
44)
. Thus, atherosclerotic retinopathy would manifest morphological property, while hypertensive retinopathy might contain functional property. In addition, in the present study, the number of individuals having hypertensive retinopathy was smaller than that of individuals having atherosclerotic retinopathy. Hence, when CAVI and carotid IMT were included in the same model, the association between CAVI and the retinopathy might have been relatively attenuated as compared to that between carotid IMT and the retinopathy.
The ROC curves analysis discriminating the presence of retinopathy revealed that the cut-off levels for the CAVI and carotid IMT for the presence of either retinopathy or the complication of both retinopathy and carotid plaque were equivalent or slightly higher than the mean. These findings imply that some middle-aged individuals may already have subclinical atherosclerosis in the large and small arteries, and also support the concept that vascular damage progresses in both the small and large arteries from an early stage. Notably, the cut-off for the CAVI for discriminating the presence of retinopathy was identical for detecting the combination of retinopathy and carotid plaque, which are representative of small artery and large artery diseases.
The present study has several limitations, and the findings should be interpreted with caution. Firstly, this study was a cross-sectional study of individuals with a heterogeneous background. Secondly, a large proportion of the enrolled individuals were male workers, and the proportion of female individuals was small. Thirdly, the causal relationship underlying the associations between large artery atherosclerosis and small artery retinopathy was not investigated in this study. Finally, vascular endothelial function was not evaluated. Therefore, further investigations with a longitudinal design and including a broad number of patients are necessary to obtain definitive conclusions.
Conclusions
The findings revealed a close association between retinopathy based on the Scheie classification and examinations of large artery atherosclerosis, rather than large artery stiffness, in seemingly healthy, untreated middle-aged workers. These findings imply that vascular damage progresses in both the small and large arteries from an early stage, and that atherosclerotic findings in large arteries indicate small artery retinopathy and vice versa.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1) Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation, 2001; 104: 191-196
- 2) Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation, 2004; 109: III27-III32
- 3) Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation, 2002; 106: 653-658
- 4) Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med, 1999; 340: 115-126
- 5) Libby P. Inflammation in atherosclerosis. Nature, 2002; 420: 868-874
- 6) Ikeda N, Saito E, Kondo N, Inoue M, Ikeda S, Satoh T, Wada K, Stickley A, Katanoda K, Mizoue T, Noda M, Iso H, Fujino Y, Sobue T, Tsugane S, Naghavi M, Ezzati M, Shibuya K. What has made the population of Japan healthy? Lancet, 2011; 378: 1094-1105
- 7) Nakamura Y, Yamamoto T, Okamura T, Kadowaki T, Hayakawa T, Kita Y, Saitoh S, Okayama A, Ueshima H; NIPPON DATA 80 Research Group. Combined cardiovascular risk factors and outcome: NIPPON DATA80, 1980-1994. Circ J, 2006; 70: 960-964
- 8) Iso H. Lifestyle and cardiovascular disease in Japan. J Atheroscler Thromb, 2011; 18: 83-88
- 9) Mozaffarian D, Wilson PW, Kannel WB. Beyond established and novel risk factors: lifestyle risk factors for cardiovascular disease. Circulation, 2008; 117: 3031-3038
- 10) Dohi Y, Takase H, Sato K, Ueda R. Association among C-reactive protein, oxidative stress, and traditional risk factors in healthy Japanese subjects. Int J Cardiol, 2007; 115: 63-66
- 11) Sugiura T, Dohi Y, Takase H, Yamashita S, Tanaka S, Kimura G. Increased reactive oxygen metabolites is associated with cardiovascular risk factors and vascular endothelial damage in middle-aged Japanese subjects. Vasc Health Risk Manag, 2011; 7: 475-482
- 12) Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation, 1998; 97: 1837-1847
- 13) Nishimura K, Okamura T, Watanabe M, Nakai M, Takegami M, Higashiyama A, Kokubo Y, Okayama A, Miyamoto Y. Predicting coronary heart disease using risk factor categories for a Japanese urban population, and comparison with the framingham risk score: the suita study. J Atheroscler Thromb, 2014; 21: 784-798
- 14) Lee M, Saver JL, Chang KH, Liao HW, Chang SC, Ovbiagele B. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ, 2010; 341: c4249
- 15) Vashistha V, Lee M, Wu YL, Kaur S, Ovbiagele B. Low glomerular filtration rate and risk of myocardial infarction: A systematic review and meta-analysis. Int J Cardiol, 2016; 223: 401-409
- 16) Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, Ura N, Kiyohara Y, Moriyama T, Ando Y, Fujimoto S, Konta T, Yokoyama H, Makino H, Hishida A, Matsuo S. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol, 2009; 13: 621-630
- 17) Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S; Committee for Epidemiology and Clinical Management of Atherosclerosis. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984
- 18) Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb, 2006; 13: 101-107
- 19) Qureshi G, Brown R, Salciccioli L, Qureshi M, Rizvi S, Farhan S, Lazar J. Relationship between aortic atherosclerosis and non-invasive measures of arterial stiffness. Atherosclerosis, 2007; 195:e 190-194
- 20) Sugiura T, Dohi Y, Takagi Y, Yokochi T, Yoshikane N, Suzuki K, Tomiishi T, Nagami T, Iwase M, Takase H, Seo Y, Ohte N. Close Association between Subclinical Atherosclerosis and Pulmonary Function in Middle-Aged Male Smokers. J Atheroscler Thromb, 2020; 27: 1230-1242
- 21) Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS; American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr, 2008; 21: 93-111
- 22) Terminology and Diagnostic Criteria Committee, Japan Society of Ultrasonics in Medicine. Standard method for ultrasound evaluation of carotid artery lesions. J Med Ultrason (2001), 2009; 36: 219-226
- 23) Nezu T, Hosomi N, Aoki S, Matsumoto M. Carotid Intima-Media Thickness for Atherosclerosis. J Atheroscler Thromb, 2016; 23: 18-31
- 24) SCHEIE HG. Evaluation of ophthalmoscopic changes of hypertension and arteriolar sclerosis. AMA Arch Ophthalmol, 1953; 49: 117-138
- 25) Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, Horio T, Hoshide S, Ikeda S, Ishimitsu T, Ito M, Ito S, Iwashima Y, Kai H, Kamide K, Kanno Y, Kashihara N, Kawano Y, Kikuchi T, Kitamura K, Kitazono T, Kohara K, Kudo M, Kumagai H, Matsumura K, Matsuura H, Miura K, Mukoyama M, Nakamura S, Ohkubo T, Ohya Y, Okura T, Rakugi H, Saitoh S, Shibata H, Shimosawa T, Suzuki H, Takahashi S, Tamura K, Tomiyama H, Tsuchihashi T, Ueda S, Uehara Y, Urata H, Hirawa N. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res, 2019; 42: 1235-1481
- 26) Smith W, Wang JJ, Wong TY, Rochtchina E, Klein R, Leeder SR, Mitchell P. Retinal arteriolar narrowing is associated with 5-year incident severe hypertension: the Blue Mountains Eye Study. Hypertension, 2004; 44: 442-447
- 27) Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA, 2002; 287: 1153-1159
- 28) Wong TY, Kamineni A, Klein R, Sharrett AR, Klein BE, Siscovick DS, Cushman M, Duncan BB. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the cardiovascular health study. Arch Intern Med, 2006; 166: 2388-2294
- 29) Yatsuya H, Folsom AR, Wong TY, Klein R, Klein BE, Sharrett AR; ARIC Study Investigators. Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis Risk in Communities Study. Stroke, 2010; 41: 1349-1355
- 30) Haneda M, Noda M, Origasa H, Noto H, Yabe D, Fujita Y, Goto A, Kondo T, Araki E. Japanese Clinical Practice Guideline for Diabetes 2016. Diabetol Int, 2018; 9: 1-45 (Erratum in: Diabetol Int, 2018; 10: 83, Erratum in: Diabetol Int, 2019; 11: 163)
- 31) Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis, 2009; 53: 982-992
- 32) Sugiura T, Dohi Y, Takagi Y, Yoshikane N, Ito M, Suzuki K, Nagami T, Iwase M, Seo Y, Ohte N. Relationships of Obesity-Related Indices and Metabolic Syndrome with Subclinical Atherosclerosis in Middle-Aged Untreated Japanese Workers. J Atheroscler Thromb, 2020; 27: 342-352
- 33) Saiki A, Sato Y, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T, Ban N, Kawana H, Nagumo A, Nagayama D, Ohira M, Endo K, Tatsuno I. The Role of a Novel Arterial Stiffness Parameter, Cardio-Ankle Vascular Index (CAVI), as a Surrogate Marker for Cardiovascular Diseases. J Atheroscler Thromb, 2016; 23: 155-168
- 34) Takase H, Sugiura T, Murai S, Yamashita S, Ohte N, Dohi Y. Carotid intima-media thickness is a novel predictor of new onset of hypertension in normotensive subjects. Medicine (Baltimore), 2017; 96: e7710
- 35) Sugiura T, Dohi Y, Takase H, Yamashita S, Fujii S, Ohte N. Oxidative Stress is Closely Associated with Increased Arterial Stiffness, Especially in Aged Male Smokers without Previous Cardiovascular Events: A Cross-Sectional Study. J Atheroscler Thromb, 2017; 24: 1186-1198
- 36) Masugata H, Senda S, Hoshikawa J, Okuyama H, Inukai M, Himoto T, Imai M, Goda F. Differences between hypertensive and atherosclerotic lesions in retinal arteries assessed by Scheie’s classification in hypertensive patients following stroke. Clin Exp Hypertens, 2010; 32: 335-354
- 37) Katsi V, Vlachopoulos C, Souretis G, Baou K, Dagalaki I, Alexopoulos N, Tousoulis D, Hatziyianni A, Stefanadis C, Kallikazaros I. Association between retinal microcirculation and aortic stiffness in hypertensive patients. Int J Cardiol, 2012; 157: 370-373
- 38) De Silva DA, Woon FP, Manzano JJ, Liu EY, Chang HM, Chen C, Wang JJ, Mitchell P, Kingwell BA, Cameron JD, Lindley RI, Wong TY, Wong MC; behalf of the Multi-Centre Retinal Stroke Study Collaborative Group. The relationship between aortic stiffness and changes in retinal microvessels among Asian ischemic stroke patients. J Hum Hypertens, 2012; 26: 716-722
- 39) Triantafyllou A, Anyfanti P, Gavriilaki E, Zabulis X, Gkaliagkousi E, Petidis K, Triantafyllou G, Gkolias V, Pyrpasopoulou A, Douma S. Association between retinal vessel caliber and arterial stiffness in a population comprised of normotensive to early-stage hypertensive individuals. Am J Hypertens, 2014; 27: 1472-1478
- 40) Yannoutsos A, Levy BI, Safar ME, Slama G, Blacher J. Pathophysiology of hypertension: interactions between macro and microvascular alterations through endothelial dysfunction. J Hypertens, 2014; 32: 216-224
- 41) Climie RE, Gallo A, Picone DS, Di Lascio N, van Sloten TT, Guala A, Mayer CC, Hametner B, Bruno RM. Measuring the Interaction Between the Macro- and Micro-Vasculature. Front Cardiovasc Med, 2019; 6: 169
- 42) Sugiura T, Dohi Y, Takase H, Fujii S, Seo Y, Ohte N. Relationship of pulmonary function with myocardial micro-damage and oxidative stress in the Japanese population without a history of cardiopulmonary disease. Medicine (Baltimore), 2020, 99: e21945
- 43) Chatterjee S, Chattopadhyay S, Hope-Ross M, Lip PL. Hypertension and the eye: changing perspectives. J Hum Hypertens, 2002; 16: 667-675
- 44) Nokiba H, Takei T, Suto C, Nitta K. Association between Ophthalmological Changes and Cardiovascular Diseases in Patients with Chronic Kidney Disease Undergoing Hemodialysis. J Atheroscler Thromb, 2015; 22: 1248-1254





