2022 年 29 巻 11 号 p. 1613-1624
2022 年 29 巻 11 号 p. 1613-1624
Aims: Mechanical thrombectomy using a standard device has been effective for acute cerebral large-vessel occlusions, particularly those due to cardiogenic embolism. However, evidence for those with underlying atherosclerotic lesions is lacking. In this study, we evaluated the predictive factors, treatment details, and outcomes of acute cerebral large-vessel occlusions with underlying atherosclerotic lesions in patients who underwent mechanical thrombectomy.
Methods: We retrospectively analyzed consecutive patients with acute large-vessel occlusions who underwent mechanical thrombectomy at our institution between August 2014 and May 2021. Predictive factors of underlying atherosclerotic lesions were evaluated using univariate and multivariate analyses. In addition, treatment details and outcomes were evaluated and compared with those of other etiologies.
Results: Among 322 included patients, 202 (62.7%) were males and 65 (20.2%) had underlying atherosclerotic lesions. Multivariate analysis identified dyslipidemia, lack of arterial fibrillation documented on admission, smoking, internal carotid artery lesions, and stenosis ≥ 25% in non-occluded large vessels as predictive factors of underlying atherosclerotic lesions. Regarding treatment for underlying atherosclerotic lesions, the need for percutaneous transluminal angioplasty, stent placement, medical therapy, and longer procedure time were observed, while successful reperfusion rates, favorable outcomes, and mortality rates showed no significant differences with those of other etiologies.
Conclusion: Coexisting diseases and radiological findings were useful for predicting underlying atherosclerotic lesions. Further understanding these characteristics may lead to the early detection of underlying atherosclerotic lesions, optimal treatment strategies, and better outcomes.
Mechanical thrombectomy has been accepted as the gold standard according to five randomized controlled trials that used a stent retriever as the main device1-5). After establishing its effectiveness, studies have shifted toward eligibility criteria expansion and revascularization rate improvement. The aforementioned randomized studies have indicated the effectiveness of the procedure at <6 hours from onset; however, clinical-imaging mismatch has also been observed at >6 hours from onset in two subsequent randomized controlled trials6, 7). Furthermore, the contact aspiration technique using large-bore catheters has been as effective as stent retriever8), with their combination also expected to improve revascularization rates9-12). However, these studies mainly included patients with cardiogenic cerebral embolism, and the evidence for patients with underlying atherosclerotic lesions that often require additional treatments remains to be elucidated.
Underlying atherosclerotic lesions have been detected in 8.3%-71.4% of cerebral large-vessel occlusions, and in higher proportions for Asians13-15). Regarding treatment outcomes, the rates of successful reperfusion, complications, and favorable outcomes have been similar to those of cardiogenic emboli15-17). In contrast, other reports showed lower reperfusion and higher complication rates13, 18, 19). In patients with underlying atherosclerotic lesions, treatment strategies have contributed more toward achieving higher reperfusion rates, lower complication rates, and better outcomes; however, preprocedural prediction of etiology is known to be difficult. Although a higher rate of coexisting diseases and importance of increased d-dimer values have been reported15, 20, 21), the evidence is still lacking because of the small number of patients. We thought that clarifying the predictive factors of patients with underlying atherosclerotic lesions would help select the most suitable device. In this study, we retrospectively evaluated the predictive factors of underlying atherosclerotic lesions in cerebral large-vessel occlusions, as well as treatment details and outcomes compared with those of other etiologies.
This study protocol was approved by the ethics committee of the Saitama Medical University International Medical Center (IRB number 14-196), and all subjects provided informed consent, with an opt-out policy. Consecutive patients of any age who underwent mechanical thrombectomy for acute large-vessel occlusions after magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) from August 2014 to May 2021 were enrolled in this study. Among patients who underwent mechanical thrombectomy ≥ 2 times during the study period at our institution, only the first procedure was evaluated. Patients who were not preprocedurally evaluated using MRI or MRA were excluded. At our institution, indications for mechanical thrombectomy were generally as follows: 1) acute large-vessel (internal carotid artery, ICA; the first and second segments of the middle cerebral artery: M1and M2, respectively; basilar artery, BA; vertebral artery, VA) occlusion within 6 hours from onset; 2) mismatch between hyperintense areas in diffusion-weighted imaging (DWI) and perfusion areas of the occluded vessel from MRI and MRA; 3) NIHSS score22) ≥ 6; 4) DWI-Alberta Stroke Program Early Computed Tomography Score (ASPECTS)23) ≥ 6; and 5) no posterior circulation (pc)-ASPECTS24) based on DWI limit. Patients with a pre-onset modified Rankin Scale (mRS)25) score ≥ 3 or a DWI-ASPECTS score ≤ 5, indicating early ischemic changes, were excluded. In addition, a recombinant tissue-type plasminogen activator (rt-PA) was intravenously administered to patients within 4.5 hours from onset and without contraindications. Stroke subtypes were classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification26). Underlying atherosclerotic lesion was defined as significant fixed focal steno-occlusive lesions proximal to or at the occlusion site that became evident during thrombectomy or on follow-up MRI/MRA13). The following two etiologies were considered for underlying atherosclerotic lesions: 1) intracranial atherosclerotic large vessel occlusion, and 2) embolic large vessel occlusion from extracranial atherosclerotic lesions. The second cause was defined as a tandem lesion; for example, in the case of ICA and M1 tandem lesions, the location of the lesion was identified separately as one ICA lesion and one M1 lesion. The patients with extracranial atherosclerotic steno-occlusive lesions except for tandem lesions were excluded in this study. The definitions of risk factors for atherosclerosis were based on the Japanese criterion27), in reference to the medical history and examination data recorded on admission.
Magnetic Resonance Imaging and Angiography EvaluationThe MRI-first policy has been adapted for patients with suspected stroke using the 1.5-T or 3.0-T MR system at our institution. Despite varied evaluation equipment, DWI, MRA, fluid-attenuated inversion recovery imaging, and T2- or T2*-weighted imaging have been routinely performed. In cases of inter-hospital transfer, we used the former hospital’s MRI evaluation as reference. In addition to the evaluation necessary for determination of treatment indication (occluded vessels, DWI-ASPECTS, pc-ASPECTS based on DWI, and etc.), non-occluded large-vessel stenosis ≥ 25% and 50% was evaluated. A typical example of non-occluded large-vessel stenosis ≥ 25% is presented in Fig.1. Furthermore, prominent posterior cerebral artery (PCA) laterality in MRA was evaluated in patients with M1 occlusion according to a previous report28), except for the tandem and bilateral lesions, to assess the presence of collateral flow and its influences. For patients contraindicated for MRI, computed tomography with or without contrast medium was performed; however, these patients were excluded from this study.
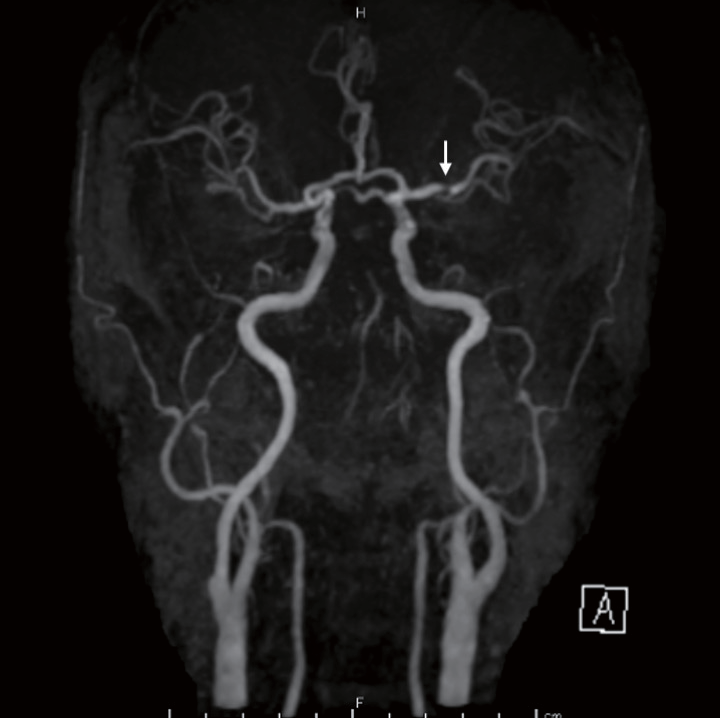
A magnetic resonance angiography scan of a 60-year-old male with basilar artery occlusion showing stenosis ≥ 25% of the left middle cerebral artery (white arrow)
The treatment strategies differed depending on the neuroendovascular surgeons, durations, lesion areas, and access routes. However, all procedures were generally performed using biplane digital subtraction angiography equipment under sedation. The transfemoral approach was adopted, and a catheter was navigated to the proximal portion of the occluded vessel with an inner catheter and guidewire. Then, the microcatheter was navigated to the distal portion of the occluded vessel with a microguidewire, and first-pass devices (stent, aspiration catheter, a combination of both, or other devices) were used. The devices were removed during manual aspiration through the guiding catheter. This process was repeated until successful reperfusion was achieved. Angioplasty, stent placement, and medical therapies (other than rt-PA) were additionally performed for residual spastic, atherosclerotic, or dissected lesions. The degree of reperfusion was evaluated using the Thrombolysis in Cerebral Ischemia (TICI) scale29), with grades >2 B indicating successful reperfusion. We terminated difficult reperfusion procedures, considering the onset time, perfusion area of the occluded vessel, and reperfusion benefits. Treatment outcomes were evaluated using mRS at discharge, with scores ≤ 2 indicating favorable outcomes. In addition, angiographical findings (tapered occlusion, truncal-type occlusion) which are reported to be predictive factors of atherosclerotic lesions were evaluated based on the previous reports30-32).
Statistical AnalysisData were expressed as medians and interquartile ranges (IQR). The Mann-Whitney U test, Fisher’s exact test, and Pearson’s chi-square test were used to compare the characteristics of underlying atherosclerotic lesions with those of other etiologies. About associated factors of underlying atherosclerotic lesions, in univariate analysis, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using simple logistic regression model. The variables were selected considering the results of past reports15) and the p value (<0.10), for comparing characteristics of underlying atherosclerotic lesions with those of other etiologies (Table 1). When two variables were apparently associated, only one factor was selected. Multivariate analyses were also performed to detect the predictive factors using a forward-backward stepwise selection method. The Kruskal-Wallis test and Fisher-Freeman-Halton exact tests were used to compare the three groups. For the items that exhibited statistical significance in three-group comparisons, the Dunn test, Fisher’s exact test, and Pearson’s chi-square test followed by the Bonferroni correction were used to compare each of the two groups (statistical significance was set at p<0.017). All statistical analyses were performed using SPSS software (version 26; IBM Corp., Armonk, NY, USA). Statistical significance was set at p<0.05, except for the post hoc test after three-group comparisons.
| Characteristics | Total | AL-positive | AL-negative | p |
|---|---|---|---|---|
| Number of patients | 322 | 65 | 257 | |
| Age, median (IQR), y | 76 (69-82) | 72 (65-79) | 77 (70-82) | 0.012* |
| Males, n (%) | 202 (62.7) | 48 (73.8) | 154 (59.9) | 0.038* |
| Coexisting disease | ||||
| Hypertension, n (%) | 187 (58.1) | 46 (70.8) | 141 (54.9) | 0.020* |
| Diabetes mellitus, n (%) | 71 (22.0) | 19 (29.2) | 52 (20.2) | 0.118 |
| Dyslipidemia, n (%) | 90 (28.0) | 27 (41.5) | 63 (24.5) | 0.006* |
| Atrial fibrillation†, n (%) | 184 (57.1) | 8 (12.3) | 176 (68.5) | <0.001* |
| Atrial fibrillation documented on admission, n (%) | 165 (51.2) | 6 (9.2) | 159 (61.5) | <0.001* |
| Coronary artery disease, n (%) | 39 (12.1) | 11 (16.9) | 28 (10.9) | 0.183 |
| Smoking, n (%) | 109 (33.9) | 34 (52.3) | 75 (29.2) | <0.001* |
| History of cerebral infarction, n (%) | 50 (15.5) | 10 (15.4) | 40 (15.6) | 0.972 |
| Anticoagulant medication before onset, n (%) | 51 (15.8) | 0 (0) | 51 (19.8) | <0.001* |
| Antiplatelet medication before onset, n (%) | 59 (18.3) | 14 (21.5) | 45 (17.5) | 0.453 |
| Inter-hospital transfer, n (%) | 69 (21.4) | 18 (27.7) | 51 (19.8) | 0.168 |
| Drip-and-ship, n (%) | 21 (6.5) | 6 (9.2) | 15 (5.8) | 0.396 |
| In-hospital onset, n (%) | 20 (6.2) | 5 (7.7) | 15 (5.8) | 0.569 |
| NIHSS score, median (IQR) | 17 (12-23) | 14 (9-21) | 18 (12-23) | 0.009* |
| Location of the lesion | ||||
| ICA, n (%) | 101 (31.4) | 32 (49.2) | 69 (26.8) | <0.001* |
| M1, n (%) | 178 (55.3) | 45 (69.2) | 133 (51.8) | 0.011* |
| M2, n (%) | 40 (12.4) | 8 (12.3) | 32 (12.5) | 0.975 |
| Posterior circulation occlusion, n (%) | 38 (11.8) | 10 (15.4) | 28 (10.9) | 0.316 |
| Tandem lesion, n (%) | 32 (9.9) | 32 (49.2) | 0 (0) | <0.001* |
| ASPECTS, median (IQR)‡ | 8 (6-9) | 8 (7-9) | 8 (6-9) | 0.438 |
| pc-ASPECTS, median (IQR)§ | 7 (6-8.8) | 6.5 (3-8.5) | 7 (6-8.5) | 0.244 |
| Stenosis ≥ 25% in other large vessels, n (%)|| | 107 (33.2) | 31 (47.7) | 76 (29.6) | 0.006* |
| Stenosis ≥ 50% in other large vessels, n (%)|| | 32 (9.9) | 12 (18.5) | 20 (7.8) | 0.010* |
| Prominent posterior cerebral artery laterality, n (%)¶ | 53 (35.6) | 10 (50.0) | 43 (33.3) | 0.147 |
*p<0.05. †Including paroxysmal atrial fibrillation detected during hospitalization. ‡Only anterior circulation occlusion patients. §Only posterior circulation occlusion patients. ||Stenosis ≥ 25% in non-occluded large vessels detected through magnetic resonance angiography. ¶Only in 149 patients. AL, atherosclerotic lesion; IQR, interquartile range; NIHSS, Institutes of Health Stroke Scale; ICA, internal carotid artery; M1, middle cerebral artery first segment; M2, middle cerebral artery second segment; pc-ASPECTS, posterior circulation-Alberta Stroke Program Early Computed Tomography score.
Data were expressed as medians and IQR. The Mann-Whitney U test, Fisher’s exact test, and Pearson’s chi-square test were used to compare the characteristics of underlying atherosclerotic lesions with those of other etiologies.
Among 322 examined patients with a median age of 76 years (IQR, 69-82 years; range, 32-96 years), 202 (62.7%) were males and 65 (20.2%) had underlying atherosclerotic lesions (large-artery atherosclerosis). Of the other 257 patients, 192, 8, 6, and 51 had cardioembolism, stroke of other determined etiology (dissected and cancer-associated), and stroke of undetermined etiology, respectively. Demographic and clinical data of the patients are shown in Table 1. Comparing the two groups, age (72 [65-79] vs. 77 [70-82] years, p=0.012) male sex (48 [73.8%] vs. 154 [59.9%], p=0.038), hypertension (46 [70.8%] vs. 141 [54.9%], p=0.020), dyslipidemia (27 [41.5%] vs. 63 [24.5%], p=0.006), atrial fibrillation (including paroxysmal atrial fibrillation during hospitalization) (8 [12.3%] vs. 176 [68.5%], p<0.001), atrial fibrillation documented on admission (6 [9.2%] vs. 159 [61.5%], p<0.001), smoking (34 [52.3%] vs. 75 [29.2%], p<0.001), anticoagulant medication before onset (0 [0%] vs. 51 [19.8%], p<0.001), NIHSS score (14 [9-21] vs. 18 [12-23], p=0.009), ICA lesion (32 [49.2%] vs. 69 [26.8%], p<0.001), M1 lesion (45 [69.2%] vs. 133 [51.8%], p=0.001), tandem lesion (32 [49.2%] vs. 0 [0%], p<0.001), stenosis ≥ 25% in other large vessels (31 [47.7%] vs. 76 [29.6%], p=0.006), and stenosis ≥ 50% in other large vessels (12 [18.5%] vs. 20 [7.8%], p=0.010) were significantly different, while the other characteristics including diabetes mellitus (19 [29.2%] vs. 52 [20.2%], p=0.118) and coronary artery disease (11 [16.9%] vs. 28 [10.9%], p=0.183) showed no significant differences. Univariate and multivariate analyses were performed to identify the factors associated with atherosclerotic lesions (Table 2). Anticoagulant medication before onset was not chosen because of its strong association with atrial fibrillation documented on admission. In addition, M1 lesion also not selected, as it sometimes due to atherosclerotic artery to artery embolism from ICA lesion. Furthermore, tandem lesion was also not selected, as it was not usually detected in preprocedural MRA evaluation. Univariate analysis using a simple logistic regression showed that NIHSS score (OR, 0.96; 95% CI, 0.93-0.997; p=0.032), age (OR, 0.97; 95% CI, 0.95-0.997; p=0.026), male sex (OR, 1.89; 95% CI, 1.03-3.47; p=0.040), hypertension (OR, 1.99; 95% CI, 1.11-3.59; p=0.022), dyslipidemia (OR, 2.19; 95% CI, 1.24-3.87; p=0.007), lack of atrial fibrillation documented on admission (OR, 15.95; 95% CI, 6.64-38.34; p<0.001), smoking (OR, 2.66; 95% CI, 1.53-4.64; p=0.001), ICA lesion (OR, 2.64; 95% CI, 1.51-4.62; p=0.001), and stenosis ≥ 25% in other large vessels (OR, 2.17; 95% CI, 1.25-3.79; p=0.006) were significantly associated with underlying atherosclerotic lesions. Multivariate analysis showed that dyslipidemia (OR, 3.41; 95% CI, 1.66-7.00; p=0.001), lack of atrial fibrillation documented on admission (OR, 25.19; 95% CI, 9.46-67.04; p<0.001), smoking (OR, 2.79; 95% CI, 1.42-5.49; p=0.003), ICA lesion (OR, 3.36; 95% CI, 1.68-6.73; p=0.001), and stenosis ≥ 25% in other large vessels (OR, 2.34; 95% CI, 1.19-4.61; p=0.014) were predictive factors for underlying atherosclerotic lesions.
| Characteristic | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |
| NIHSS score | 0.96 (0.93-0.997) | 0.032* | ||
| Age | 0.97 (0.95-0.997) | 0.026* | ||
| Male sex | 1.89 (1.03-3.47) | 0.040* | ||
| Hypertension | 1.99 (1.11-3.59) | 0.022* | ||
| Diabetes mellitus | 1.63 (0.88-3.01) | 0.120 | ||
| Dyslipidemia | 2.19 (1.24-3.87) | 0.007* | 3.41 (1.66-7.00) | 0.001* |
| Lack of atrial fibrillation documented on admission | 15.95 (6.64-38.34) | <0.001* | 25.19 (9.46-67.04) | <0.001* |
| Coronary artery disease | 1.67 (0.78-3.56) | 0.187 | ||
| Smoking | 2.66 (1.53-4.64) | 0.001* | 2.79 (1.42-5.49) | 0.003* |
| ICA lesion | 2.64 (1.51-4.62) | 0.001* | 3.36 (1.68-6.73) | 0.001* |
| Stenosis ≥ 25% in other large vessels (%)† | 2.17 (1.25-3.79) | 0.006* | 2.34 (1.19-4.61) | 0.014* |
*p<0.05
†Stenosis ≥ 25% in other non-occluded large vessels detected through magnetic resonance angiography.
CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; ICA, internal carotid artery.
In univariate analysis, about associated factors of underlying atherosclerotic lesions, odds ratios and 95% CIs were calculated using simple logistic regression model. Multivariate analyses were also performed to detect the predictive factors using a forward-backward stepwise selection method.
As preprocedural collateral flow evaluation, prominent PCA laterality in MRA was examined in a total of 149 patients with M1 occlusion, except for tandem and bilateral lesions. A higher rate of prominent PCA laterality was observed in patients with underlying arteriosclerotic lesions as compared to those with other etiologies, although the difference did not attain statistical significance (10 patients [50.0%] vs. 43 patients [33.3%], p=0.147).
Treatment Details and OutcomesThe treatment details and outcomes are shown in Table 3. Among the 322 patients, 169 (52.5%) were administered with intravenous rt-PA, and in terms of first-pass devices, a stent retriever, aspiration catheter, and a combination of both were used in 99 (30.7%), 105 (32.6%), and 98 (30.4) patients, respectively. Among the other 20 (6.2%) patients, 11 underwent percutaneous transluminal angioplasty (PTA), one underwent stent placement, two underwent microcatheterization with a microguidewire, three underwent suction through a balloon-guiding catheter, and three had inaccessible vessels. PTA, stent placement, and medical therapy other than rt-PA were performed in 39 (12.1%), 17 (5.3%), and 29 (9.0%) patients, respectively. Comparing the patients with and without underlying atherosclerotic lesion, other first-pass devices used (9 [13.8%] vs. 11 [4.3%], p=0.008), PTA (33 [50.8%] vs. 6 [2.3%], p<0.001), stent placement (14 [21.5%] vs. 3 [1.2%], p<0.001), medical therapy other than rt-PA (23 [35.4%] vs. 6 [2.3%], p<0.001), puncture-to-reperfusion time (61 [39.5-95.8] vs. 43 [30-68] min, p<0.001), onset-to-reperfusion time (321.5 [216-463] vs. 252 [186.5-345] min, p=0.015), TICI 3 reperfusion (22 [33.8%] vs. 123 [47.9%], p=0.042), and first-pass TICI 2B-3 reperfusion (15 [23.1%] vs. 132 [51.4%], p<0.001) were significantly different, while the other items, including TICI 2B-3 reperfusion (49 [75.4%] vs. 216 [84.0%], p=0.102), mRS ≤ 2 at discharge (18 [27.7%] vs. 94 [36.6%], p=0.179) and mortality (7 [10.8%] vs. 20 [7.8%], p=0.438), showed no significant differences.
| Total | AL-positive | AL-negative | p | |
|---|---|---|---|---|
| Intravenous rt-PA, n (%) | 169 (52.5) | 28 (43.1) | 141 (54.9) | 0.089 |
| First-pass devices, n (%) | ||||
| Stent retriever | 99 (30.7) | 16 (24.6) | 83 (32.3) | 0.231 |
| Aspiration catheter | 105 (32.6) | 18 (27.7) | 87 (33.9) | 0.344 |
| Combined approach | 98 (30.4) | 22 (33.8) | 76 (29.6) | 0.503 |
| Other devices | 20 (6.2) | 9 (13.8) | 11 (4.3) | 0.008* |
| PTA, n (%) | 39 (12.1) | 33 (50.8) | 6 (2.3) | <0.001* |
| Stent placement, n (%) | 17 (5.3) | 14 (21.5) | 3 (1.2) | <0.001* |
| Medical therapy other than rt-PA, n (%) | 29 (9.0) | 23 (35.4) | 6 (2.3) | <0.001* |
| Time course | ||||
| Onset-to-door time (min), median (IQR) | 108.5 (58-241.5) | 162.5 (59.3-312.8) | 97.5 (57-208.8) | 0.072 |
| Door-to-puncture time (min), median (IQR) | 72 (49-103) | 66 (48.5-109) | 74 (48.8-102.3) | 0.930 |
| Puncture-to-reperfusion time (min), median (IQR) | 46 (31-72.5) | 61 (39.5-95.8) | 43 (30-68) | <0.001* |
| Door-to-reperfusion time (min), median (IQR) | 127 (93-164) | 146 (97-207) | 126 (91.5-157) | 0.061 |
| Onset-to-reperfusion time (min), median (IQR) | 264 (189.5-365.5) | 321.5 (216-463) | 252 (186.5-345) | 0.015* |
| TICI 2B-3 reperfusion, n (%) | 265 (82.3) | 49 (75.4) | 216 (84.0) | 0.102 |
| TICI 3 reperfusion, n (%) | 145 (45.0) | 22 (33.8) | 123 (47.9) | 0.042* |
| First-pass TICI 2B-3 reperfusion, n (%) | 147 (45.7) | 15 (23.1) | 132 (51.4) | <0.001* |
| Symptomatic complications, n (%) | ||||
| Ischemic | 1 (0.3) | 0 (0) | 1 (0.4) | 1.000 |
| Hemorrhagic | 17 (5.3) | 2 (3.1) | 15 (5.8) | 0.540 |
| Puncture-related | 8 (2.5) | 3 (4.6) | 5 (1.9) | 0.205 |
| Hospitalization period (days), median (IQR) | 28 (18-40) | 29 (18-41) | 28 (18-40) | 0.853 |
| mRS at discharge, median (IQR) | 3 (2-5) | 4 (2-5) | 3 (2-5) | 0.254 |
| mRS ≤ 2 at discharge, n (%) | 112 (34.8) | 18 (27.7) | 94 (36.6) | 0.179 |
| Mortality, n (%) | 27 (8.4) | 7 (10.8) | 20 (7.8) | 0.438 |
| Angiographical findings, n (%) | ||||
| Tapered occlusion† | 61 (20.1) | 22 (46.8) | 39 (15.2) | <0.001* |
| Truncal-type occlusion‡ | 86 (33.1) | 27 (65.9) | 59 (26.9) | <0.001* |
*p<0.05. †Only in 304 patients. ‡Only in 260 patients. AL, atherosclerotic lesion; rt-PA, recombinant tissue-type plasminogen activator; PTA, percutaneous transluminal angioplasty; IQR: interquartile range; TICI, Thrombolysis in Cerebral Ischemia; mRS, modified Rankin Scale.
Data were expressed as medians and IQR. The Mann-Whitney U test, Fisher’s exact test, and Pearson’s chi-square test were used to compare the therapeutic data of underlying atherosclerotic lesions with those of other etiologies.
In addition, the data of angiographical findings which were reported to be predictive factors of atherosclerotic lesions, obtained during the procedure were also evaluated. Eighteen patients with extracranial lesions were apparently confirmed for atherosclerotic lesions through angiographical findings. Excluding these 18 patients, tapered occlusion and truncal-type occlusion were evaluated. Upon comparison of underlying atherosclerotic lesion and other etiologies in 304 patients, a higher rate of tapered occlusion was observed in the patients with underlying atherosclerotic lesions (22 [46.8%] vs. 39 [15.2%], p<0.001). Regarding truncal-type occlusion, the occlusion type could not be determined in many cases due to a lack of angiography data just after stent retriever deployment. Upon comparison of the patients with underlying atherosclerotic lesions and those with other etiologies in 260 patients who could be determined the occlusion type, a higher rate of truncal-type occlusion was observed in the patients with underlying atherosclerotic lesions (27 [65.9%] vs. 59 [26.9%], p<0.001).
For subgroup analysis, factors including anticoagulant and antiplatelet medication before stroke onset, puncture-to-reperfusion time, TICI 2B-3 reperfusion rates, intracranial symptomatic complications, favorable outcomes, and mortality due to first-pass devices were evaluated (Table 4) (stent retriever vs. aspiration catheter vs. combined approach). In patients with underlying atherosclerotic lesions, no items showed statistical significance in the three-group comparisons. However, a relatively shorter puncture-to-reperfusion time was observed in the combined approach (64 [32.5-101.5] vs. 91 [52.5-151.5] vs. 50 [36-72] min, p=0.054), although the difference was not statistically significant. In patients with other etiologies, anticoagulant medication before onset (20 [24.1] vs. 10 [11.5] vs. 19 [25.0] patients, p=0.043), puncture-to-reperfusion time (45.5 [29-62] vs. 36.5 [26.3-59.5] vs. 54 [38.5-77.8] min, p<0.001), and intracranial symptomatic complications (2 [2.4] vs. 3 [3.4] vs. 9 [11.8], p=0.028) showed significant difference in the three-group comparisons. In two-group comparisons for items that showed statistical significance in three-group comparisons, puncture-to-reperfusion time was significantly different in the stent retriever vs. combined approach (p=0.008) and aspiration catheter vs. combined approach (p<0.001).
| Disease subtype | First-pass device | p | ||||||
|---|---|---|---|---|---|---|---|---|
| 1. Stent retriever | 2. Aspiration catheter | 3. Combined approach | 1 vs. 2 vs. 3 | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||
| Number of patients | AL-positive | 16 | 18 | 22 | ||||
| AL-negative | 83 | 87 | 76 | |||||
| Anticoagulant medication before onset, n (%) | AL-positive | 0 (0) | 0 (0) | 0 (0) | 1.000 | |||
| AL-negative | 20 (24.1) | 10 (11.5) | 19 (25.0) | 0.043* | 0.031 | 1.000 | 0.024 | |
| Antiplatelet medication before onset, n (%) | AL-positive | 2 (12.5) | 5 (27.8) | 3 (13.6) | 0.499 | |||
| AL-negative | 14 (16.9) | 15 (17.2) | 12 (15.8) | 0.977 | ||||
| Puncture-to-reperfusion time (min), median (IQR) | AL-positive | 64 (32.5-101.5) | 91 (52.5-151.5) | 50 (36-72) | 0.054 | |||
| AL-negative | 45.5 (29-62) | 36.5 (26.3-59.5) | 54 (38.5-77.8) | <0.001* | 0.222 | 0.008** | <0.001** | |
| TICI 2B-3 reperfusion, n (%) | AL-positive | 12 (75.0) | 12 (66.7) | 18 (81.8) | 0.501 | |||
| AL-negative | 71 (85.5) | 75 (86.2) | 62 (81.6) | 0.701 | ||||
| Intracranial symptomatic complications, n (%) | AL-positive | 0 (0) | 0 (0) | 0 (0) | 1.000 | |||
| AL-negative | 2 (2.4) | 3 (3.4) | 9 (11.8) | 0.028* | 1.000 | 0.019 | 0.041 | |
| mRS ≤ 2 at discharge, n (%) | AL-positive | 4 (25.0) | 4 (22.2) | 5 (22.7) | 1.000 | |||
| AL-negative | 30 (36.1) | 37 (42.5) | 23 (30.3) | 0.273 | ||||
| Mortality, n (%) | AL-positive | 1 (6.3) | 2 (11.1) | 2 (9.1) | 1.000 | |||
| AL-negative | 4 (4.8) | 7 (8.0) | 7 (9.2) | 0.558 | ||||
*p<0.05. **p<0.017. IQR, interquartile range; AL, atherosclerotic lesion; TICI, Thrombolysis in Cerebral Ischemia; mRS, modified Rankin Scale.
Three group comparisons of therapeutic outcomes were performed using the Kruskal-Wallis test and Fisher-Freeman-Halton exact test (statistical significance was set at p<0.05). For factors that attained statistical significance, the Dunn test, Fisher’s exact test, and Pearson’s chi-square test followed by the Bonferroni correction were used to compare each of the two groups (statistical significance was set at p<0.017).
The demographic and clinical data of the patients with intracranial and extracranial atherosclerotic lesions are shown in Table 5. In comparative analysis of patients with intracranial and extracranial atherosclerotic lesions, ICA lesion (2 [6.1%] vs. 30 [93.8%], p<0.001), PTA (9 [27.3%] vs. 24[75.0%], p<0.001), and stent placement (2 [6.1%] vs. 12 [37.5%], p=0.002) were found to be significantly different between the two groups. On the other hand, the other items including NIHSS score, age, male sex, hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation documented on admission, coronary artery disease, smoking, and stenosis ≥ 25% in other large vessels, did not show any significant difference.
| Characteristics | Intracranial atherosclerotic lesion | Extracranial atherosclerotic lesion | p |
|---|---|---|---|
| Number of patients | 33 | 32 | |
| Age, median (IQR) | 70 (61-80) | 74 (68-77.5) | 0.309 |
| Males, n (%) | 24 (72.7) | 24 (75.0) | 0.835 |
| Coexisting disease | |||
| Hypertension, n (%) | 23 (69.7) | 23 (71.9) | 0.847 |
| Diabetes mellitus, n (%) | 11 (33.3) | 8 (25.0) | 0.460 |
| Dyslipidemia, n (%) | 13 (39.4) | 14 (43.8) | 0.722 |
| Atrial fibrillation documented on admission, n (%) | 2 (6.1) | 4 (12.5) | 0.427 |
| Coronary artery disease, n (%) | 6 (18.2) | 5 (15.6) | 0.783 |
| Smoking, n (%) | 21 (63.6) | 13 (40.6) | 0.063 |
| NIHSS score, median (IQR) | 13 (7-21) | 14 (9-20.3) | 0.990 |
| Location of the lesion | |||
| ICA, n (%) | 2 (6.1) | 30 (93.8) | <0.001* |
| Posterior circulation, n (%) | 8 (24.2) | 2 (6.3) | 0.082 |
| Stenosis ≥ 25% in other large vessels, n (%)† | 18 (54.5) | 13 (40.6) | 0.261 |
| Intravenous rt-PA, n (%) | 13 (39.4) | 15 (46.9) | 0.543 |
| Puncture-to-reperfusion time (min), median (IQR) | 55.5 (37-90) | 64 (49.3-106.3) | 0.451 |
| PTA, n (%) | 9 (27.3) | 24 (75.0) | <0.001* |
| Stent placement, n (%) | 2 (6.1) | 12 (37.5) | 0.002* |
| Medical therapy other than rt-PA, n (%) | 10 (30.3) | 13 (40.6) | 0.384 |
| Intracranial symptomatic complications, n (%) | 0 (0) | 2 (6.3) | 0.238 |
| TICI 2B-3 reperfusion, n (%) | 25 (75.8) | 24 (75.0) | 0.943 |
| TICI 3 reperfusion, n (%) | 10 (30.3) | 12 (37.5) | 0.540 |
| First-pass TICI 2B-3 reperfusion, n (%) | 9 (27.3) | 6 (18.8) | 0.415 |
| mRS ≤ 2 at discharge, n (%) | 10 (30.3) | 8 (25.0) | 0.633 |
| Mortality, n (%) | 4 (12.1) | 3 (9.4) | 1.000 |
*p<0.05. †Stenosis ≥ 25% in non-occluded large vessels detected through magnetic resonance angiography.
IQR, interquartile range; NIHSS, Institutes of Health Stroke Scale; ICA, internal carotid artery; rt-PA, recombinant tissue-type plasminogen activator; PTA, percutaneous transluminal angioplasty; IQR: TICI, Thrombolysis in Cerebral Ischemia; mRS, modified Rankin Scale.
Data were expressed as medians and IQR. The Mann-Whitney U test, Fisher’s exact test, and Pearson’s chi-square test were used to compare the characteristics of intracranial atherosclerotic lesion with extracranial atherosclerotic lesion.
In this study, we evaluated the predictive factors for underlying atherosclerotic lesions. Multivariate analysis identified dyslipidemia, lack of atrial fibrillation documented on admission, smoking, ICA lesion, and stenosis ≥ 25% in non-occluded large vessels as significant predictors of underlying atherosclerotic lesions. A previous meta-analysis of 1,967 thrombectomy-treated patients showed that 496 patients with underlying atherosclerotic lesions had a higher prevalence of hypertension, diabetes mellitus, dyslipidemia, and smoking, and a lower prevalence of arterial fibrillation15). Our findings support these results with real-world clinical data in a single Asian medical institution, with the addition that MRA-detected mild stenosis in other non-occluded large vessels may predict underlying atherosclerotic lesions.
As predictive factors of underlying atherosclerotic lesions, coexistent associated diseases were considered as important, according to results of previous studies and ours as well. In addition, MRA-detected mild stenosis of other vessels may also be an important finding. A meta-analysis of Asian and American populations showed that hypertension (OR, 1.46; 95% CI, 1.10-1.93), diabetes mellitus (OR, 1.68; 95% CI, 1.29-2.20), dyslipidemia (OR, 1.94; 95% CI, 1.04-3.62), smoking (OR, 2.11; 95% CI, 1.40-3.17), and arterial fibrillation (OR, 0.20; 95% CI, 0.13-0.31) were associated with underlying atherosclerotic lesions15). Our results showed similar ORs for similar items, except for hypertension and diabetes mellitus. In addition, a lower tendency of arterial fibrillation documented on admission had the highest OR; therefore, it was considered as the most important factor in distinguishing underlying atherosclerotic lesions. In this manner, in patients with cerebral large-vessel occlusions, an interview of medical history and electrocardiography are necessary, despite limited time. In radiological findings, mild stenosis in large non-occluded vessels was considered as important in our study. Patients with symptomatic atherosclerotic lesions had a higher rate of other lesions, although the reports also included non-cerebral vessels33). Since atherosclerosis is a systemic disease, the findings of other vessels are also important. In addition, the d-dimer value has also played a role in detecting cardiac emboli20, 21), although we could not evaluate this in our study. Regarding the location of the lesion, ICA lesions showed a higher prevalence in our study; however, this is conflicting with the aforementioned meta-analysis that reported a lower prevalence of ICA lesions compared to MCA lesions15). In contrast, most atherosclerotic tandem lesions were reported to include ICA stenosis or occlusion; therefore, considering the possibility of ICA lesions might be important regardless of frequency.
In addition, collateral flow, and occlusion type evaluations were also performed in our study, and its association with underlying atherosclerotic lesions was observed. The existence of collateral flow from the PCA via the leptomeningeal anastomosis was assessed with prominent PCA laterality in preprocedural MRA, only in patients with M1 occlusion, based on a previous report28). An earlier study had indicated a better collateral flow in patients with underlying atherosclerotic lesions32), whereas our study did not show any significant difference in the collateral flow. Furthermore, we evaluated the data of angiographical findings obtained during the procedure, which were reported to be predictive factors of atherosclerotic lesions. In our study, tapered occlusion and truncal-type occlusion were significantly associated with underlying atherosclerotic lesions as the past reports30-32). These findings might help to predict the underlying atherosclerotic lesions during the procedure.
Treatment for Underlying Atherosclerotic LesionRegarding treatment for underlying atherosclerotic lesions, the need for PTA, stent placement, and medical therapy, as well as a longer procedure time were observed, while successful reperfusion rates, favorable outcomes, and mortality showed no significant differences compared to those of other etiologies. Baek et al. reported similar successful reperfusion rates (80.4% vs. 88.5%, p=0.097), favorable outcomes (46.4% vs. 46.9%, p=0.097), symptomatic intracranial hemorrhage, and mortality rates between patients with and without atherosclerosis, although atherosclerotic patients had longer procedure times (45.0 vs. 73.0 min, p<0.001) and 84.3% required rescue treatments, including PTA, stent placement, and glycoprotein IIb/IIIa inhibitor infusion17). Furthermore, Jia et al. reported similarly high successful reperfusion rates (95.7% vs. 96.8%, p=0.757), favorable outcomes (63.8% vs. 51.6%, p=0.169), symptomatic hemorrhage, and mortality rates between patients with and without underlying atherosclerotic lesions, although 57.4% of patients with underlying atherosclerotic lesions received rescue treatment34). In contrast, Al Kasab et al. reported a relatively low successful reperfusion rate (64.7%) and high postprocedural intracranial hemorrhage rate (11%) in patients with underlying atherosclerotic lesions, with a similar favorable outcome (42.4%) to that of other reports13). In addition, patients with tandem lesions, mainly including atherosclerosis, were reported to have a lower successful reperfusion rate (58% vs. 82%) and higher intracranial hemorrhage rate (13% vs. 5%) than those without tandem lesions, although favorable outcomes were similar (34% vs. 43%)19). In this manner, patients with underlying atherosclerotic lesions might have higher intracranial hemorrhage rates, higher need for rescue treatment, longer procedure time; similar successful reperfusion rates, favorable outcomes, and mortality rates compared to those without lesions.
Regarding treatment strategies, the combined procedure as a first-pass device decreased the procedure time, although the difference did not attain statistical significance. In contrast, longer procedure time and more symptomatic hemorrhagic complications were observed in patients without underlying atherosclerotic lesions. Successful reperfusion rates, favorable outcomes, and mortality rates were not significantly different among first-pass devices. PTA, stent placement, and medical therapy were performed in 50.8%, 21.5%, and 35.4% of patients with underlying atherosclerotic lesions, respectively. Although rescue treatment is eventually needed to achieve successful reperfusion17, 34), a first-pass device causing partial reperfusion and detection of underlying atherosclerotic lesions may be important. In a past report, thrombectomy using a stent retriever was shown to be less successful in patients with underlying atherosclerotic lesions17). In contrast, other reports showed that the stent retriever as a first-line device achieved a higher successful reperfusion ratio than the aspiration catheter for patients with intracranial atherosclerosis-related occlusions35). The structure of atherosclerotic occlusive lesions is different from that of other etiologies, including cardiogenic emboli; therefore, the effect of standard thrombectomy devices, such as stent retrievers and aspiration catheters, might be less expected. However, preprocedural prediction of etiologies is sometimes difficult in real-world clinical situations. A combined procedure as a first-pass device may decrease the procedure time due to multiple mechanisms, such as the radial force of the stent retriever, supportiveness, and aspiration function of the aspiration catheter, in patients with underlying atherosclerotic lesions, although optional procedures, such as PTA, stent placement, and medical therapy, should be performed once the etiology is identified.
The Difference between Intracranial and Extracranial Atherosclerotic LesionConsidering the different etiologies of intracranial and extracranial atherosclerotic lesions, a subgroup analysis was performed to assess their difference. A lower rate of ICA lesions was observed in intracranial atherosclerotic lesions. On the other hand, PTA and stent placement were performed more frequently in patients with extracranial atherosclerotic lesions. Other factors including NIHSS score, age, male sex, hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation documented on admission, coronary artery disease, smoking, and stenosis ≥ 25% in other large vessels did not show any significant difference. As previously mentioned, a lower prevalence of ICA lesions compared to MCA lesions was reported in a previous meta-analysis15); however, as per the reports, only extracranial atherosclerotic lesions showed a higher rate of ICA lesions13, 19). In addition, a higher rate of intracranial atherosclerotic lesions was observed in studies of patients with posterior circulation occlusion than in those with anterior circulation occlusion15), although there was no significant difference in our study. As for the differences in treatment strategies, most neuroendovascular surgeons hesitate to perform PTA and stent placement due to smaller diameters of intracranial vessels, as it can be a relatively risky procedure. On the other hand, the items selected as predictive factors of underlying atherosclerotic lesions showed no significant difference between the two groups, except for the ICA lesion. This may support the results of our study, although these two etiologies originally might have been analyzed separately.
Limitations and Future WorkThis study had several limitations. The first treatment strategy differed depending on neuroendovascular surgeons, durations, locations of lesions, and access routes, among others, which might have affected the results. Second, the clinical outcome was based on the patient condition at discharge, and the median hospitalization period was only 28 days (range, 18-40 days), which was considerably short compared to most studies that evaluated the patient’s condition at 90 days after the procedure, which might have affected our results. Third, imaging evaluation equipment was not constant. Fourth, the underlying atherosclerotic lesion might be overestimated in patients who received endovascular treatment such as PTA and stent placement, although some of those who received these strategies were diagnosed with other etiologies. Finally, the retrospective study design and inadequate sample size might have weakened our statement. We deem it necessary to confirm our results with future prospective studies with large sample sizes.
In this study, we evaluated the predictive factors for underlying atherosclerotic lesions. Multivariate analysis identified dyslipidemia, lack of atrial fibrillation, smoking, ICA lesion, and stenosis ≥ 25% in non-occluded large vessels as predictive factors of underlying atherosclerotic lesions. Regarding treatment outcomes, the need for PTA, stent placement, medical therapy other than rt-PA, and longer procedure time were observed, while successful reperfusion rates, favorable outcomes, and mortality showed no significant differences with those of other etiologies. In addition, the combined procedure as a first-pass device decreased the procedure time, although the difference did not attain statistical significance. Further understanding of these characteristics may lead to the early detection of underlying atherosclerotic lesions, optimal treatment strategies, and better outcomes.
This work was supported in part by JSPS KAKENHI (Grant No. 20K15933, grant received by T.K.). We would like to thank all medical staff at the Saitama Medical University International Medical Center and Saitama Medical University Research Administration Center.
None to declare.
None to declare.