2022 年 29 巻 11 号 p. 1646-1654
2022 年 29 巻 11 号 p. 1646-1654
Aim: Acute kidney injury (AKI) is an important clinical issue in the diagnosis and treatment of cardiovascular diseases. The association between pretreatment by statins and the occurrence of AKI in patients with peripheral arterial diseases (PAD) remains unclear. Therefore, we examined the association between statin therapy and the occurrence of AKI in patients with PAD.
Methods: We retrospectively analyzed data from the endovascular treatment (EVT) database in our hospital. A total of 295 patients who underwent angiography and/or intervention for PAD between October 2011 and March 2016 were enrolled and divided into two groups: those without statins (control group; N=157) and those with statins (statin group; N=138) for at least 1 month before admission. We examined the occurrence of AKI and its related factors in these patients.
Results: The serum creatinine levels, dose of contrast medium, use of a renin-angiotensin system inhibitor, smoking habit, and blood pressure were similar in both groups. The statin group had more diabetes patients, had patients who were significantly younger, had patients with a higher body mass index (BMI), and had patients with lower low-density lipoprotein cholesterol than the control group. With regard to the occurrence of AKI, there was a significantly lower incidence in the statin group compared with the control group (5% vs. 16%, p<0.05). The result of the multivariate analysis indicated that statin therapy was significantly correlated with lower occurrence rates of AKI (p<0.05).
Conclusions: Our study suggests that statin therapy might prevent the occurrence of AKI after angiography and/or intervention for PAD.
Acute kidney injury (AKI) is associated with increased morbidity and mortality, and patients with severe kidney injury require maintenance hemodialysis. Moreover, even if kidney function is improved, patients with a history of AKI will still have poor outcomes compared with the healthy ones1, 2). Therefore, patients with AKI need a large healthcare cost and lengthened hospital stays3). AKI has several risk factors, such as older age, obesity, anemia, chronic kidney disease (CKD), hypertension, diabetes mellitus, heart failure, cardiovascular disease (CVD), surgery, and diuretics. Another important risk factor is the dose of contrast medium4-6). Particularly, in patients with CKD, the prevalence of CVD is very high, and they often face situations that require the use of contrast medium7, 8). To prevent the occurrence of AKI, several guidelines recommend intravenous hydration with normal saline or sodium bicarbonate before the administration of contrast medium as a standard precaution9-12). However, AKI following contrast medium administration cannot be completely prevented. Multiple meta-analysis reported that the onset of AKI in coronary angiography and intervention was significantly suppressed by pretreatment with statins in addition to hydration by isotonic saline13-15).
The presence of peripheral arterial disease (PAD) is closely associated with amputation, other CVD, and mortality. The coexistence of PAD and impaired kidney function has been reported to additively increase mortality rate16, 17). Thus, preserving kidney function in patients with PAD seems to be essential. However, there is no specific information on the prevention of AKI with regard to PAD, and the effectiveness of pretreatment with statins for AKI in patients with PAD has not been fully elucidated.
Therefore, we conducted a retrospective study that investigated whether the use of statins was related to the occurrence of AKI in patients with PAD.
This was a single-center, retrospective, observational study and was conducted in accordance with the principles stated in the Declaration of Helsinki. Our study protocol was approved by the appropriate institutional review committee (no. 28005).
A total of 377 patients were hospitalized at our institution between October 2011 and March 2016 who underwent angiography and/or intervention for PAD (Fig.1). PAD was diagnosed by subjective and objective complaints and findings arising from leg ischemia and/or ankle-brachial index <0.9. All the patients underwent ultrasound scan, computed tomography, or magnetic resonance angiography to confirm stenosis of peripheral artery. The severity of PAD was evaluated using the Rutherford classification. Patients who underwent hemodialysis (N=69) and those with insufficient clinical data (N=13) were excluded from the study. Those who took statins for less than 3 months were also excluded. Consequently, 295 patients were included in the analysis. These patients were divided into two groups: those who were taking statins (statin group; N=138) and those who were not taking statins (control group; N=157). All the study patients received a standard hydration therapy before and after angiography and/or intervention for PAD. Isotonic saline (0.9% sodium chloride, 1 mL/kg/h) was administered every 12 h before and after the procedure. The low-molar, nonionic contrast medium iopamidol (370 mg iodine/mL, iopamiron® ) was used for all procedures. AKI was defined by the absolute increase in serum creatinine (SCr) of ≥ 0.5 mg/dL or a relative increase of ≥ 25% measured within 1 week after performing the procedures according to the KDIGO guideline10).
Enrollment flow diagram for the present study
Data for continuous variables are expressed as values±standard deviation, whereas non-normally distributed variables were expressed as median (quartile). The differences between the groups were analyzed using Students t-test or the Mann–Whitney U test. Categorical variables were expressed as frequencies and percentages, and comparisons were performed using the chi-squared test. Multivariate logistic regression analysis was also performed. For the matching of the clinical characteristics between the two groups, the propensity score was calculated using a logistic regression model from the following factors: sex, age, body mass index (BMI), hypertension, diabetes mellitus, creatinine, use of renin-angiotensin system inhibitors, and dose of contrast medium. Propensity score matching was performed using a nearest-neighbor 1:1 matching between the statin and control groups (Supplementary Table 1). P values <0.05 were considered statistically significant. Statistical analyses were conducted using the JMP software version 9.0.3 (SAS Institute Inc., Cary, NC).
| statin group (n = 113) | control group (n = 113) | P | |
|---|---|---|---|
| Sex (male) | 93 (82) | 92 (81) | 0.734 |
| age (year) | 74±8 | 74±9 | 0.814 |
| BMI (kg/m2) | 22.3±3.1 | 22.2±3.5 | 0.861 |
| Smoking (%) | 86 (76) | 86 (76) | 1.000 |
| Diabetes mellitus (%) | 68 (60) | 66 (58) | 0.788 |
| Hyperlipidemia (%) | 98 (87) | 19 (17) | <0.001 |
| Hypertension (%) | 80 (71) | 80 (71) | 1.000 |
| CKD (%) | 60 (53) | 58 (51) | 0.791 |
| SBP (mmHg) | 157±25 | 153±23 | 0.206 |
| DBP (mmHg) | 76±13 | 75±13 | 0.693 |
| Cr (mg/dL) | 0.98±0.28 | 0.94±0.33 | 0.436 |
| eGFR (mL/min/1.73m2) | 60.0±18.5 | 64.0±22.7 | 0.144 |
| T-cholesterol (mg/dL) | 172±41 | 186±39 | 0.009 |
| TG (mg/dL) | 125±59 | 133±64 | 0.306 |
| HDL-cholesterol (mg/dL) | 49±14 | 49±15 | 0.974 |
| LDL-cholesterol (mg/dL) | 100±33 | 109±32 | 0.039 |
| RAS inhibitor (%) | 64 (57) | 61 (54) | 0.690 |
| Antiplatelet (%) | 113 (100) | 113 (100) | 1.000 |
| Diuretic (%) | 20 (18) | 18 (16) | 0.723 |
| Dose of contrast medium (mL) | 60±39 | 61±45 | 0.724 |
BMI: body mass index; CKD: chronic kidney disease; SBP: systolic blood pressure; DBP: diastolic blood pressure; Cr: creatinine; eGFR: estimated glomerular filtration rate; T-cholesterol: total cholesterol; TG: triglyceride; HDL-cholesterol: high density lipoprotein cholesterol; LDL-cholesterol: low density lipoprotein cholesterol; RAS inhibitor: renin-angiotensin-aldosterone system inhibitor.
Table 1 presents the characteristics and laboratory data of all study patients in the two groups. The percentage of male patients, smoking habit, blood pressure, kidney function, and use of renin-angiotensin system inhibitors were similar between the two groups. However, the BMI and percentage of diabetes mellitus were significantly higher, and the total cholesterol and low-density lipoprotein cholesterol levels were significantly lower in the statin group compared with the control group. The statin group also included relatively younger patients. With regard to the kind of stain used, the number of patients taking each statin is as follows: rosuvastatin (N=59, 42.8%), atorvastatin (N=52, 37.7%), pitavastatin (N=11, 8.0%), pravastatin (N=11, 8.0%), simvastatin (N=3, 2.2%), and fluvastatin (n=2, 1.4%).
| statin group (N = 138) | control group (N = 157) | P | |
|---|---|---|---|
| Sex (male) | 114 (83) | 125 (80) | 0.513 |
| age (year) | 72±9 | 76±9 | 0.002 |
| BMI (kg/m2) | 22.6±3.2 | 21.6±3.4 | 0.016 |
| Smoking (%) | 108 (78) | 116 (74) | 0.380 |
| Diabetes mellitus (%) | 82 (59) | 74 (47) | 0.024 |
| Hyperlipidemia (%) | 138 (100) | 24 (15) | <0.001 |
| Hypertension (%) | 92 (67) | 113 (72) | 0.323 |
| CKD (%) | 87 (47) | 65 (55) | 0.155 |
| Stage 3 (%) | 62 (45) | 78 (50) | 0.416 |
| Stage 4-5 (%) | 3 (2) | 9 (6) | 0.113 |
| SBP (mmHg) | 155±25 | 158±35 | 0.451 |
| DBP (mmHg) | 76±14 | 75±14 | 0.641 |
| Cr (mg/dL) | 0.95±0.27 | 0.98±0.38 | 0.539 |
| eGFR (mL/min/1.73 m2) | 59.8±18.0 | 60.2±22.3 | 0.882 |
| T-cholesterol (mg/dL) | 172±40 | 185±38 | 0.006 |
| TG (mg/dL) | 126±57 | 132±70 | 0.425 |
| HDL-cholesterol (mg/dL) | 48±14 | 48±14 | 0.396 |
| LDL-cholesterol (mg/dL) | 100±32 | 108±31 | 0.017 |
| RAS inhibitor (%) | 78 (57) | 76 (48) | 0.164 |
| Antiplatelet (%) | 138 (100) | 157 (100) | 1.000 |
| Diuretic (%) | 24 (17) | 25 (16) | 0.735 |
| Rutherford classification | |||
| 1 (%) | 5 (4) | 3 (2) | 0.377 |
| 2 (%) | 41 (30) | 20 (13) | <0.001 |
| 3 (%) | 59 (43) | 73 (46) | 0.683 |
| 4 (%) | 10 (7) | 14 (9) | 0.600 |
| 5 (%) | 22 (15) | 42 (27) | 0.010 |
| 6 (%) | 1 (1) | 5 (3) | 0.121 |
BMI: body mass index; CKD: chronic kidney disease; SBP: systolic blood pressure; DBP: diastolic blood pressure; Cr: creatinine; eGFR: estimated glomerular filtration rate; T-cholesterol: total cholesterol; TG: triglyceride; HDL-cholesterol: high density lipoprotein cholesterol; LDL-cholesterol: low density lipoprotein cholesterol; RAS inhibitor: renin-angiotensin-aldosterone system inhibitor.
As presented in Table 2, the occurrence of AKI was significantly lower in the statin group compared with the control group (5.1% vs. 15.3%, respectively, p<0.05). The dose of contrast medium administered was similar between the two groups. The changes in the estimated glomerular filtration rate (eGFR) were significantly greater in the control group than in the statin group.
| statin group | control group | P | |
|---|---|---|---|
| AKI (%) | 7 (5.1) | 24 (15.3) | 0.040 |
| Dose of contrast medium (mL) | 62.1±38.6 | 59.8±43.8 | 0.680 |
| post Cr (mg/dL) | 0.99±0.33 | 1.04±0.40 | 0.539 |
| post eGFR (mL/min/1.73 m2) | 57.8±18.0 | 56.1±21.3 | 0.424 |
| ⊿Cr (mg/dL) | 0.04±0.16 | 0.07±0.17 | 0.158 |
| ⊿eGFR (mL/min/1.73 m2) | -1.85±8.57 | -4.07±9.49 | 0.037 |
AKI: acute kidney injury; Cr: creatinine; eGFR: estimated glomerular filtration rate.
Univariate analysis revealed that the occurrence of AKI was significantly correlated with age (r=0.149, p<0.05), BMI (r=−0.118, p<0.05), hyperlipidemia (r=−0.147, p<0.05), diastolic blood pressure (r=−0.147, p<0.05), and statin use (r=−0.166, p<0.05) in all the study patients. Interestingly, as presented in Table 3, the eGFR before the angiography and/or intervention was comparable between the AKI and non-AKI groups, and it was not significantly associated with the occurrence of AKI. Multivariate analysis, including age, BMI, diastolic blood pressure, and statin use, revealed that the occurrence of AKI was significantly and negatively associated with the use of statin (Table 4). A similar result was observed in patients with diabetes or who were older than 70 years. Furthermore, we conducted multivariate analysis after the propensity score matching. The result also indicated that statin therapy had a significantly positive influence on AKI (odds ratio 0.367; 95% CI 0.144–0.934; p<0.05).
| AKI group (n = 31) | Not AKI group (n = 264) | P | |
|---|---|---|---|
| Sex (male) | 23 (74) | 216 (82) | 0.367 |
| age (year) | 78±9 | 74±9 | 0.008 |
| BMI (kg/m2) | 21±3.2 | 22±3.3 | 0.070 |
| Smoking (%) | 24 (77) | 201 (76) | 0.838 |
| Diabetes mellitus (%) | 12 (39) | 145 (55) | 0.087 |
| Hyperlipidemia (%) | 8 (27) | 135 (68) | 0.012 |
| Hypertension (%) | 26 (84) | 135 (68) | 0.066 |
| CKD (%) | 21 (68) | 132 (50) | 0.056 |
| Stage 3 (%) | 19 (61) | 121 (46) | 0.109 |
| Stage 4-5 (%) | 2 (6) | 11 (4) | 0.569 |
| SBP (mmHg) | 149±26 | 157±31 | 0.108 |
| DBP (mmHg) | 70±17 | 76±13 | 0.059 |
| Cr (mg/dL) | 1.00±0.41 | 0.96±0.32 | 0.567 |
| eGFR (mL/min/1.73m2) | 60.4±24.5 | 62.1±19.6 | 0.703 |
| T-cholesterol (mg/dL) | 173±36 | 179±40 | 0.342 |
| TG (mg/dL) | 124±50 | 130±65 | 0.563 |
| HDL-cholesterol (mg/dL) | 45±14 | 49±14 | 0.190 |
| LDL-cholesterol (mg/dL) | 106±33 | 104±32 | 0.763 |
| Statin (%) | 7 (23) | 132 (50) | 0.020 |
| RAS inhibitor (%) | 20 (65) | 135 (51) | 0.146 |
| Antiplatelet (%) | 31 (100) | 264 (100) | 1.000 |
| Diuretic (%) | 9 (29) | 40 (15) | 0.115 |
Acute kidney injury: AKI; BMI: body mass index; CKD: chronic kidney disease; SBP: systolic blood pressure; DBP: diastolic blood pressure; Cr: creatinine; eGFR: estimated glomerular filtration rate; T-cholesterol: total cholesterol; TG: triglyceride; HDL-cholesterol: high density lipoprotein cholesterol; LDL-cholesterol: low density lipoprotein cholesterol; RAS inhibitor: renin-angiotensin-aldosterone system inhibitor.
| OR | 95% CI | P | |
|---|---|---|---|
| Statin | 0.342 | 0.139-0.839 | 0.019 |
| Age | 1.050 | 1.002-1.101 | 0.039 |
| BMI | 0.961 | 0.855-1.080 | 0.505 |
| DBP | 0.970 | 0.942-0.999 | 0.044 |
OR; odds ratio, CI; confidential interval, BMI; body mass index, DBP; diastolic blood pressure
Although the dose of contrast medium was comparable between the two groups, we evaluated the relationship between the dose of contrast medium and the changes in kidney function. In all the study patients, as presented in Fig.2, the dose of contrast medium was significantly correlated with the changes in the eGFR (r=−0.163, p<0.05). This significant correlation was preserved if analyzed in each group independently.
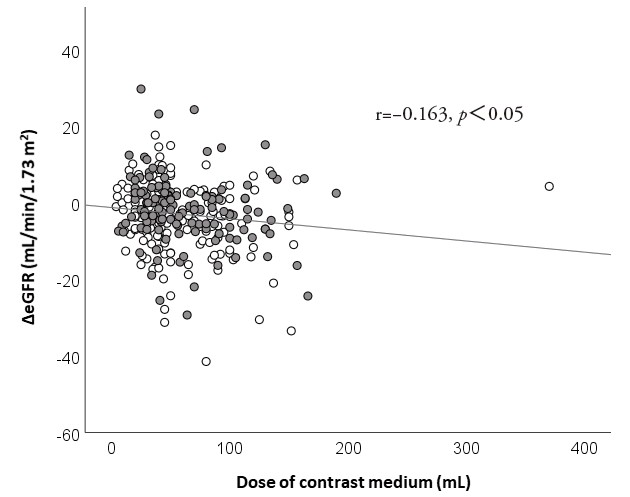
eGFR, estimated glomerular filtration rate
White circle, control group; gray circle, statin group
The present study demonstrated that (1) the use of statin was significantly associated with lower prevalence of AKI following angiography and/or intervention for PAD, and (2) the dose of contrast medium was significantly correlated with the changes in the eGFR.
PAD is an important complication as its progression is related to critical problems, such as claudication, rest pain, ischemic ulcers, amputation, and hospitalization11). Since most patients with PAD have diabetes and dyslipidemia, they are at a high risk for atherosclerotic CVD progression18). In fact, it has been reported that patients with PAD have a higher risk for myocardial infarction, stroke, and mortality compared with those without PAD19-21). Our study participants also frequently had complications associated with CVD, such as coronary artery disease, stroke, and aortic disease. Furthermore, although kidney function is also an important risk factor for CVD, this study included 152 patients with CKD at baseline (51.5%). Therefore, preventing the further progression of kidney dysfunction seems to be very important to improve the patients quality of life and prognosis.
Although several etiologies of AKI following angiography and/or intervention in patients with PAD have been proposed, its pathophysiological mechanisms are very complex. The dose of contrast media, cholesterol embolization, infection, and anemia due to bleeding has been suggested to contribute to the occurrence of AKI following angiography and/or intervention22-26). Among them, the dose of contrast media is the most crucial risk factor in clinical settings. The mechanisms of AKI due to the dose of contrast media involve endothelial cell injury, tubular epithelial cell injury, and impaired kidney microcirculation by exerting direct toxic effects and altering the kidney hemodynamics27-29). The dose of contrast media is reported to enhance the formation of reactive oxygen species and thereby reduce microcirculation by decreasing nitric oxide production30). Furthermore, many studies reported that the dose of contrast media is associated with the occurrence of AKI31, 32). Therefore, contrast media reduction is recommended by current guidelines10-12). Our data also indicated that the dose of contrast media was significantly associated with changes in the eGFR. Furthermore, this significant correlation was observed in both the control and statin groups.
Statins are commonly used medicine and prescribed extensively for lowering cholesterol levels to prevent CVD. Several recent studies showed that statins did not only have lipid-lowering effects but also pleiotropic effects33-35). Several randomized controlled trials demonstrated the effectiveness of statins in the prevention of AKI after contrast media-induced nephropathy in patients undergoing coronary angiography36-38). The occurrence rates of AKI in patients with and without statins were 3.7% and 8.3%, respectively. Nevertheless, there were no reports on the prevention of AKI following angiography and/or intervention using statins in patients with PAD. Thus, we investigated the possible effectiveness of statins on the occurrence of AKI in these patients. The result of our study indicated that the occurrence rates of AKI in patients with and without statins were 5.0% and 15.0%, respectively. The number of patients with AKI who were undergoing coronary angiography was greater in the present study than in the previous studies. A previous systematic review that included 11,311 patients with PAD has reported that the median incidence of AKI in the studies was 10%39). This result corresponded with our study results. We speculated that the difference in the AKI rate was caused by the other underlying diseases experienced by these patients, because patients with PAD might have more severe atherosclerotic lesions18). In addition, the dose of statins was lower in this study than in other previous studies, given that the dose admitted by health insurance providers in Japan is different from that in other countries. Therefore, this finding would be valuable because the findings from our study suggested that a low-dose statin therapy might prevent the occurrence of AKI even in patients with PAD who are undergoing angiography and/or intervention.
This study had several limitations. The number of enrolled patients was relatively small. Therefore, there was a possibility that we could not perform statistically sufficient adjustments. In addition, long-term evaluation was not conducted in this study. A long-term study is needed to ascertain the influence of statins on kidney function.
Our data suggests that the use of statins might reduce the occurrence of AKI after angiography and/or intervention for PAD. However, a further detailed study is needed to prove the reno-protective effects of statins.
This study was presented in part at the American Society of Nephrology, 2018 in New Orleans, U.S.A.
None.
H.F. received lecture fees from AstraZeneca PLC.