2022 年 29 巻 12 号 p. 1823-1834
2022 年 29 巻 12 号 p. 1823-1834
Aims: T-cadherin (T-cad) is a specific binding partner of adiponectin (APN), adipocyte-specific secretory protein. APN exhibits organ protection via the T-cad-dependent accumulation onto several tissues such as the aorta, heart, and muscle. Recently, for the first time, we showed that three forms (130, 100, and 30 kDa) of soluble T-cad existed in human serum and correlated with several clinical parameters in patients with type 2 diabetes. Nevertheless, the significance of soluble T-cad has not been elucidated in the acute stage of cardiovascular diseases. We herein examined soluble T-cad concentrations and investigated their clinical significance in patients with emergency hospital admission due to ST-segment elevation myocardial infarction (STEMI).
Methods: This observational study enrolled 47 patients with STEMI who were treated via primary percutaneous coronary intervention (PCI). Soluble T-cad and APN concentrations were measured by using an enzyme-linked immunosorbent assay. This study is registered with the University Hospital Medical Information Network (Number: UMIN 000014418).
Results: Serum concentrations of soluble 130 and 100 kDa T-cad rapidly and significantly decreased after hospitalization and reached the bottom at 72 h after admission (p<0.001 and p<0.001, respectively). The patients with high soluble T-cad and low APN concentrations on admission showed a significantly higher area under the curve of serum creatine kinase-MB (p<0.01).
Conclusion: Serum soluble T-cad concentration changed dramatically in patients with STEMI, and the high T-cad and low APN concentrations on admission were associated with the myocardial infarction size. Further study is needed to investigate the usefulness of categorizing patients with STEMI by serum T-cad and APN for the prediction of severe prognoses.
Adiponectin (APN) is present in the peripheral circulation with 103–106 times higher concentrations compared with other typical hormones or cytokines. APN concentration inversely correlates with body fat mass, especially visceral fat mass although APN is exclusively secreted from adipocytes1-4). There are numerous reports that the low level of serum APN is associated with not only metabolic syndrome but also type 2 diabetes and cardiovascular diseases5-11). Transplantation of APN-producing adipocyte cell-sheet onto myocardium ameliorated cardiac dysfunction in a mouse myocardial infarction model12). We previously investigated the time-dependent change of serum APN in patients with ST-segment elevation myocardial infarction (STEMI) and showed that serum APN concentrations rapidly decreased and reached the bottom at 24 h after recanalization13). Interestingly, such APN reduction was inversely correlated with the area under the curve (AUC) of serum creatine kinase-MB (CK-MB), which is associated with the infarct area and predicts mortality13, 14).
Several molecules have been demonstrated as a receptor and/or a binding protein of APN15, 16). Among them, T-cadherin (T-cad) is a specific binding partner of high molecular weight adiponectin (HMW-APN), which plays a crucial role in exhibiting a biological function of APN, and its serum level is correlated significantly with metabolic syndrome and atherosclerosis17-19). T-cad is mainly expressed in the aorta, heart, and skeletal muscle20-22). T-cad belongs to a cadherin family characterized by highly conserved cadherin repeats, but T-cad is a unique cadherin possessing a glycosylphosphatidylinositol (GPI) anchor and is expressed on the cell surface without prodomain cleavage23). Interestingly, T-cad lacks a transmembrane domain, but T-cad is required to exhibit the protective effect of APN on various organs24-28). Importantly, genome-wide association study demonstrated the significant association of T-cad gene with circulating APN level, metabolic syndrome, and cardiovascular diseases, by several independent groups29-32). We recently showed that the binding of APN and cell surface T-cad promoted the exosome biogenesis and secretion33), and such APN-T-cad-exosome pathway may be accounted for a cardiovascular protective role of APN34, 35).
Recently, we for the first time identified three forms (130, 100, and 30 kDa) of soluble T-cad in human serum and established the new enzyme-linked immunosorbent assay (ELISA), which can separately measure out the three forms of soluble T-cad36). The soluble form is also reported for the other cadherins and suggested as disease biomarkers or functional molecules37-39). In our previous study, each form of soluble T-cad was differently but significantly associated with clinical parameters, and 130 kDa T-cad concentration was correlated with serum APN level in Japanese patients with type 2 diabetes36). However, the clinical significance of soluble T-cad has not been elucidated in the acute stage of cardiovascular diseases.
In the present study, we measured the three forms of soluble T-cad in patients with STEMI to investigate the time-series change of soluble T-cad and the association between serum AUC of CK-MB and soluble T-cad levels.
The present study is an additional analysis of our previous report13). All subjects were patients with ST-segment elevation myocardial infarction (STEMI) and were admitted to Senri Critical Care Medical Center in Osaka Saiseikai Senri Hospital for treatment of emergent PCI from November 2013 to February 2015. Written consent was obtained from all subjects. Subjects with insufficient serum samples were excluded. This study was approved by the human ethics committees of Osaka Saiseikai Senri Hospital (approval number: 250803) and Osaka University Hospital (approval number: 13127) and was also registered with the University Hospital Medical Information Network (UMIN 000014418).
Clinical Examinations and Laboratory TestsThe baseline information of the patients including age, gender, body mass index (BMI), coronary risk factors (diabetes, hypertension, dyslipidemia, and smoking), medication, and onset time of STEMI were collected on admission13). Blood samples were collected on admission (0 h) and at 3, 6, 12, 24, 48, 72, 168 h after admission. The estimated glomerular filtration rate (eGFR) was calculated using the simplified Modification of Diet in Renal Disease equation modified by the appropriate coefficient for Japanese populations by gender40). Serum CK-MB and AUC of serum CK-MB were measured and calculated as reported previously13). Serum adiponectin (APN) concentration was measured with human adiponectin ELISA kit (Otsuka Pharmaceutical Co. Ltd.). Serum 130/100/30 kDa T-cadherin (T-cad) concentrations were measured and calculated using Human T-cadherin (130K) Assay kit, Human T-cadherin (100K+130K) Assay kit, and Human T-cadherin (30K+130K) Assay kit (Immuno-Biological Laboratories Co., Ltd.), as reported previously36). Diabetes, hypertension, and dyslipidemia were diagnosed according to the criteria commonly used Japanese guidelines: 1) diabetes: HbA1c(NGSP) ≥ 6.5% and fasting glucose ≥ 126 mg/dL (or continuous high glucose levels are observed), 2) hypertension: systolic blood pressure (BP) ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg, 3) dyslipidemia: low-density lipoprotein cholesterol (LDL-C) concentrations ≥ 140 mg/dL, triglyceride (TG) concentrations ≥ 150 mg/dL, and/or high-density lipoprotein cholesterol (HDL-C) concentrations ≤ 40 mg/dL. Subjects were considered to have diabetes, hypertension, or dyslipidemia if they received each medication.
Statistical AnalysisData are presented as mean±standard deviation for variables with normal distribution, median (lower–higher quartile) for variables with skewed distribution, or frequency (%). In simple linear regression analyses, logarithmic transformation was performed for the variables with skewed distribution. Dunnet’s test was used for multiple comparisons. In all cases, the probability (P) values of <0.05 were considered statistically significant. All analyses were performed with JMP Pro 14.3.0 software (SAS Institute Inc., Cary, NC, USA).
Of the 49 subjects in our previous report13), we excluded two patients whose serum sample volumes were insufficient for the assay of T-cad ELISA, and thus, 47 subjects were enrolled and analyzed in the present study. Table 1 shows their clinical characteristics and serum soluble T-cad concentrations on admission. The mean concentrations of the 130+100, 130+30, and 130 kDa T-cad (directly measured) were 2559.0, 1310.7, and 601.4 pmol/L, respectively. The mean concentrations of the 100 and 30 kDa T-cad (calculated by subtraction of [130k+100k]−[130k] and [130k+30k]−[130k], respectively, as in our previous study36)) were 1957.6 and 709.3 pmol/L, respectively.
| Variables | |
|---|---|
| n [males/females] | 47 [32/15] |
| Age (years) | 66.2±12.7 |
| BMI (kg/m2) | 24.5±3.6 |
| Diabetes (%) | 34 |
| Hypertension (%) | 55 |
| Dyslipidemia (%) | 77 |
| Current smoker (%) | 41 |
| Glucose (mg/dL) | 198.1±66.1 |
| HbA1c (%) | 6.3±0.9 |
| LDL-C (mg/dL) | 131.8±38.5 |
| HDL-C (mg/dL) | 45.3±12.1 |
| TG (mg/dL) | 146 (88-225.5) |
| AST (U/L) | 27 (21-68) |
| ALT (U/L) | 23 (15-43) |
| eGFR (mL/min/1.73m2) | 63.2±20.4 |
| WBC (x1000/μL) | 9.4 (8.1-12.8) |
| Hemoglobin (g/dL) | 13.7±1.9 |
| Albumin (g/dL) | 3.9±0.4 |
| CRP (mg/dL) | 0.12 (0.06–0.59) |
| BNP (pg/mL) | 39.0 (21.7–112) |
| Onset to Door Time (min) | 88 (55-278) |
| Adiponectin (μg/mL) | 6.6 (4.3–9.7) |
| 130-kDa T-cadherin (pmol/L) | 601.4±203.5 |
| 100-kDa T-cadherin (pmol/L) | 1957.6±591.4 |
| 30-kDa T-cadherin (pmol/L) | 709.3±335.0 |
Data are represented as mean±SD for values with normal distribution, median (minimum-maximum) for values with skewed distribution, or frequency. The concentrations of serum soluble T-cadherin are represented as recombinant 130-kDa T-cadherin equivalent.
Abbreviations: BMI; body mass index, LDL-C; low-density lipoprotein cholesterol, HDL-C; high-density lipoprotein cholesterol, TG; triglyceride, AST; aspartate aminotransferase, ALT; alanine aminotransferase, eGFR; estimated glomerular filtration rate, WBC; white blood cell, CRP; C-reactive protein, BNP; brain natriuretic peptide.
First, we examined the time course of serum soluble T-cad concentrations in patients with STEMI (Fig.1). Interestingly, the serum 130 (Fig.1A) and 100 kDa (Fig.1B) T-cad concentrations decreased and reached the bottom at 72 h after admission. Serum 130 and 100 kDa T-cad levels at 12, 24, 48, 72, and 168 h were significantly lower than those on admission. Conversely, 30 kDa T-cad concentration increased and showed the highest value at 48 h after admission (Fig.1C).
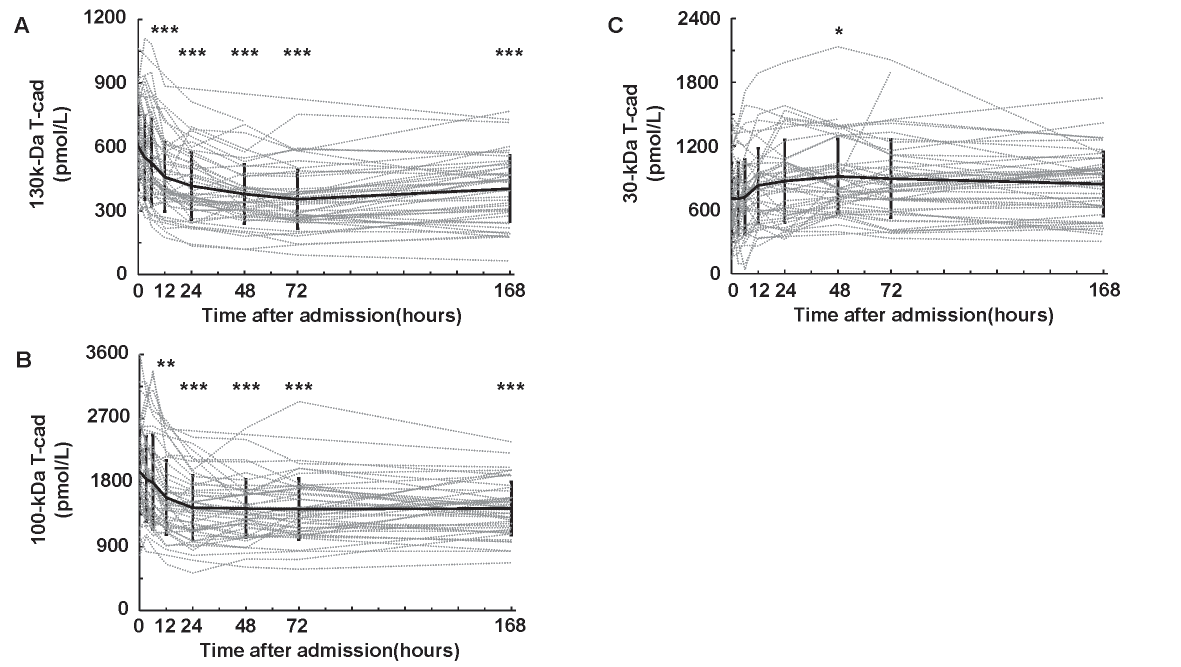
Solid black lines show mean±SD. Dotted gray lines represent the changes of serum soluble T-cadherin in each subject. n=47, *: p<0.05, **: p<0.01, ***: p<0.001, Dunnett’s test (vs. 0 h).
Next, we performed univariate analyses between serum 130/100/30 kDa T-cad and clinical parameters on admission (Table 2). The concentration of 130 kDa T-cad was significantly correlated with WBC (r=−0.349, p=0.016). No significant correlations with the clinical parameters were observed in 100 kDa T-cad. Conversely, the concentration of 30 kDa T-cad showed significant correlations with age (r=0.426, p=0.003), LDL-C (r=−0.355, p=0.015), eGFR (r=−0.514, p<0.001), hemoglobin (r=−0.379, p=0.009), and albumin (r=−0.363, p=0.012).
| vs 130-kDa T-cad | vs 100-kDa T-cad | vs 30-kDa T-cad | ||||
|---|---|---|---|---|---|---|
| Variable | r | p | r | p | r | p |
| Age | 0.026 | 0.864 | 0.135 | 0.365 | 0.426 | 0.003 |
| BMI | 0.042 | 0.782 | 0.153 | 0.306 | -0.140 | 0.349 |
| Glucose | -0.121 | 0.419 | 0.130 | 0.383 | -0.007 | 0.963 |
| HbA1c | -0.169 | 0.255 | 0.073 | 0.625 | -0.013 | 0.933 |
| LDL-C | -0.042 | 0.781 | -0.023 | 0.882 | -0.355 | 0.015 |
| HDL-C | 0.132 | 0.381 | 0.041 | 0.786 | -0.178 | 0.236 |
| log (TG) | -0.212 | 0.161 | -0.075 | 0.623 | -0.004 | 0.980 |
| log (AST) | 0.054 | 0.721 | -0.006 | 0.971 | -0.016 | 0.915 |
| log (ALT) | -0.057 | 0.701 | -0.277 | 0.059 | -0.018 | 0.906 |
| eGFR | -0.116 | 0.438 | -0.147 | 0.325 | -0.514 | <0.001 |
| log (WBC) | -0.349 | 0.016 | -0.275 | 0.062 | -0.125 | 0.403 |
| Hemoglobin | -0.072 | 0.629 | -0.009 | 0.952 | -0.379 | 0.009 |
| Albumin | 0.123 | 0.411 | 0.227 | 0.126 | -0.363 | 0.012 |
| log (CRP) | -0.050 | 0.740 | -0.164 | 0.277 | 0.181 | 0.229 |
| log (BNP) | 0.104 | 0.490 | 0.027 | 0.858 | 0.134 | 0.376 |
| Onset to Door Time | -0.117 | 0.434 | -0.057 | 0.702 | -0.006 | 0.967 |
| log (Adiponectin) | 0.084 | 0.573 | -0.045 | 0.766 | 0.171 | 0.251 |
| AUC of CK-MB | 0.130 | 0.382 | 0.102 | 0.493 | -0.066 | 0.662 |
Pearson correlation coefficient (r) and each probability value (p) are shown. Logarithmic transformation was performed for the skewed values. Abbreviations: BMI; body mass index, LDL-C; low-density lipoprotein cholesterol, HDL-C; high-density lipoprotein cholesterol, TG; triglyceride, AST; aspartate aminotransferase, ALT; alanine aminotransferase, eGFR; estimated glomerular filtration rate, WBC; white blood cell, CRP; C-reactive protein, BNP; brain natriuretic peptide, AUC of CK-MB; area under the concentration-time curve of creatine kinase-myocardial band.
In our previous report, we showed that serum APN concentration was inversely correlated with AUC of CK-MB in patients with STEMI13) and was strongly associated with serum 130 kDa T-cad concentration in Japanese patients with type 2 diabetes36). However, no correlation was observed between the 130/100/30 kDa T-cad concentrations on admission and APN or AUC of CK-MB (data not shown). There is a possibility that serum soluble T-cad concentrations in the acute phase of STEMI temporarily changed from their stable phase, and we thus examined T-cad concentrations at 168 h after admission and the difference of T-cad levels from baseline to the minimum (or maximum). However, we found no significant correlations of each soluble T-cad form with the AUC of CK-MB (data not shown).
Comparison of AUC of CK-MB in the Four Groups Categorized with Serum Soluble T-Cadherin and Adiponectin ConcentrationsWe previously demonstrated that low APN level on admission was a risk for high AUC of CK-MB when subjects were divided into four groups according to APN concentration on admission13). Finally, we here categorized the subjects into four groups divided by the median concentrations of serum soluble T-cad and APN on admission (Fig.2). We found that the AUC of CK-MB in group I (high T-cad & low APN) was significantly higher than that of other groups, categorized using the 130 kDa T-cad (Fig.2A) and 100 kDa T-cad (Fig.2B). A similar tendency was also observed in 30 kDa T-cad (Fig.2C).
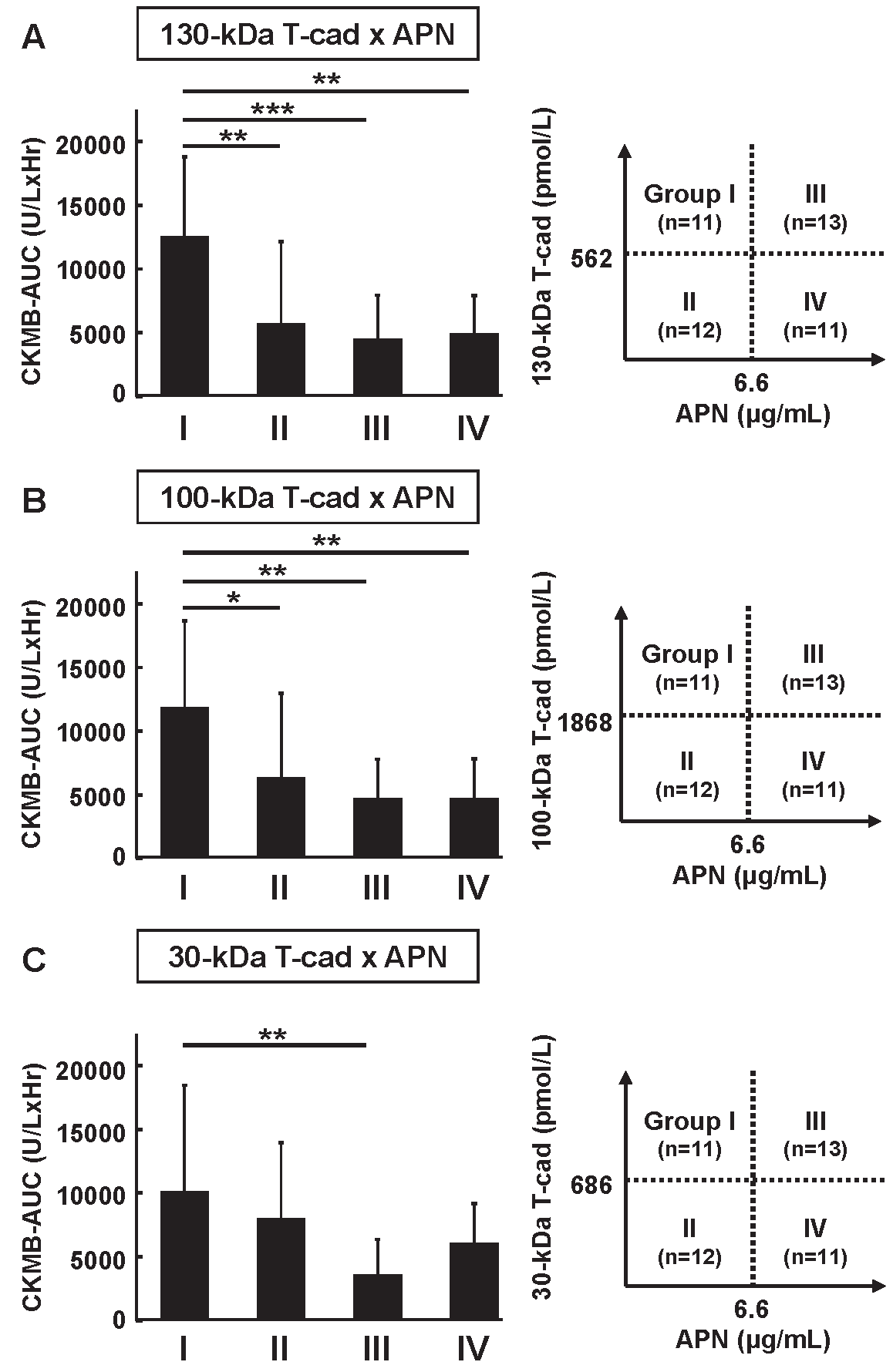
All 47 subjects were divided into four groups according to the concentrations of (A) 130 kDa/(B) 100 kDa/(C) 30 kDa T-cad and APN on admission. The data are represented as mean±SD. The numbers of subjects in each group and the median concentration of T-cad and APN are shown to the right of each bar graph. *: p<0.05, **: p<0.01, ***: p<0.001, Dunnett’s test (vs. Group I).
We also determined the clinical features in each group (Supplemental Table 1, 2, 3). Using 130 or 100 kDa T-cad for the categorization (Supplemental Table 1, 2), the subjects in Group I were relatively young and obese and showed relatively high blood glucose levels compared with the subjects of the other groups. However, there was no significant clinical feature in the categorization with 30 kDa T-cad (Supplemental Table 3).
| Group | P value | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Ⅰ | Ⅱ | Ⅲ | Ⅳ | Ⅰ vs Ⅱ | Ⅰ vs Ⅲ | Ⅰ vs Ⅳ |
| n (males/females) | 11 (8/3) | 12 (12/0) | 13 (6/7) | 11 (6/5) | 0.093 | 0.240 | 0.659 |
| Age (years) | 59.2±13.5 | 60.4±13.0 | 73.3±10.0 | 71.3±8.2 | 0.987 | 0.011 | 0.043 |
| BMI (kg/m2) | 26.9±4.0 | 24.6±3.3 | 22.7±2.1 | 24.2±3.9 | 0.179 | 0.010 | 0.109 |
| Diabetes (n (%)) | 6 (55) | 4 (33) | 3 (23) | 3 (27) | 0.414 | 0.206 | 0.387 |
| Hypertension (n (%)) | 4 (37) | 5 (42) | 9 (69) | 8 (73) | 1.000 | 0.217 | 0.198 |
| Dyslipidemia (n (%)) | 9 (82) | 9 (75) | 9 (69) | 9 (82) | 1.000 | 0.649 | 1.000 |
| Current smoker (n (%)) | 4 (36) | 7 (58) | 5 (38) | 3 (27) | 0.414 | 1.000 | 1.000 |
| Glucose (mg/dL) | 243.9±74.5 | 197.9±55.1 | 165.9±61.6 | 199.5±61.7 | 0.368 | 0.029 | 0.420 |
| HbA1c (%) | 6.7±1.3 | 6.5±1.1 | 5.8±0.5 | 6.2±0.5 | 0.952 | 0.038 | 0.524 |
| LDL-C (mg/dL) | 136.8±40.7 | 140.8±46.7 | 134.0±38.5 | 114.8±23.7 | 0.990 | 0.996 | 0.417 |
| HDL-C (mg/dL) | 40.9±7.3 | 41.3±5.7 | 51.2±17.4 | 46.5±11.1 | 1.000 | 0.101 | 0.544 |
| TG (mg/dL) | 154 (115-238) | 204 (124-274) | 83.5 (69-149) | 141 (84-228) | 0.860 | 0.059 | 0.649 |
| AST (U/L) | 33 (22-70) | 43 (27-74.8) | 22 (18-65.5) | 23 (15-66) | 0.926 | 0.628 | 0.428 |
| ALT (U/L) | 21 (18-57) | 29.5 (24-43) | 16 (13.5-30.5) | 23 (12-72) | 0.958 | 0.093 | 0.960 |
| eGFR (mL/min/1.73m2) | 61.1±16.2 | 72.2±23.7 | 59.7±22.2 | 59.4±17.5 | 0.417 | 0.997 | 0.995 |
| WBC (x1000/μL) | 10.4 (8-12.1) | 12.0 (9.5-13.5) | 8.3 (7.3-9.8) | 9.2 (8.3-13.1) | 0.440 | 0.508 | 0.960 |
| Hemoglobin (g/dL) | 14.9±1.3 | 14.8±1.1 | 12.7±2.4 | 12.6±1.4 | 0.998 | 0.005 | 0.005 |
| Albumin (g/dL) | 4.1±0.5 | 4.1±0.3 | 3.9±0.5 | 3.7±0.3 | 1.000 | 0.456 | 0.078 |
| CRP (mg/dL) | 0.10 (0.05-0.24) | 0.09 (0.06-0.40) | 0.19 (0.07-1.12) | 0.14 (0.07-2.26) | 0.985 | 0.362 | 0.332 |
| BNP (pg/mL) | 23.6 (6.1-28.7) | 38.6 (14.8-161) | 78.4 (38.4-160.2) | 39.0 (22.7-104.8) | 0.442 | 0.009 | 0.297 |
| Onset to Door Time (min) | 68 (55-190) | 214 (60.3-470) | 57 (44-310) | 98 (41-160) | 0.402 | 0.999 | 0.982 |
Data are represented as mean±SD for values with normal distribution, median (minimum-maximum) for values with skewed distribution, or frequency. Dunnet’s test was used for values with normal distribution, Steel’s test was used for values with skewed distribution, and, Fisher’s exact
test was used for analysis of data classified into two categories. P value <0.05 is considered as statistically significant and shown in bold font.
Abbreviations: BMI; body mass index, LDL-C; low-density lipoprotein cholesterol, HDL-C; high-density lipoprotein cholesterol, TG; triglyceride, AST; aspartate aminotransferase, ALT; alanine aminotransferase, eGFR; estimated glomerular filtration rate, WBC; white blood cell, CRP; C-reactive protein, BNP; brain natriuretic peptide.
| Group | P value | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Ⅰ | Ⅱ | Ⅲ | Ⅳ | Ⅰ vs Ⅱ | Ⅰ vs Ⅲ | Ⅰ vs Ⅳ |
| n (males/females) | 11 (8/3) | 12 (12/0) | 13 (6/7) | 11 (6/5) | 0.093 | 0.240 | 0.659 |
| Age (years) | 61.4±13.6 | 58.4±12.8 | 73.5±9.8 | 71.1±8.4 | 0.864 | 0.033 | 0.123 |
| BMI (kg/m2) | 27.2±3.7 | 24.4±3.3 | 22.5±2.1 | 24.4±3.8 | 0.109 | 0.003 | 0.129 |
| Diabetes (n (%)) | 8 (73) | 2 (17) | 2 (15) | 4 (36) | 0.012 | 0.011 | 0.198 |
| Hypertension (n (%)) | 5 (45) | 4 (33) | 10 (77) | 7 (64) | 0.680 | 0.206 | 0.670 |
| Dyslipidemia (n (%)) | 9 (82) | 9 (75) | 9 (69) | 9 (82) | 1.000 | 0.649 | 1.000 |
| Currentsmoker (n (%)) | 3 (27) | 8 (67) | 5 (38) | 3 (27) | 0.060 | 0.289 | 1.000 |
| Glucose (mg/dL) | 251.9±66.3 | 182.3±47.7 | 177.8±63.1 | 185.5±64.9 | 0.024 | 0.013 | 0.037 |
| HbA1c (%) | 7.0±1.4 | 6.2±0.7 | 5.7±0.4 | 6.3±0.5 | 0.041 | 0.001 | 0.095 |
| LDL-C (mg/dL) | 134.7±40.2 | 142.5±46.8 | 129.6±37.5 | 120.0±28.7 | 0.933 | 0.978 | 1.000 |
| HDL-C (mg/dL) | 41.3±7.3 | 40.9±5.7 | 47.8±12.2 | 50.5±17.8 | 1.000 | 0.406 | 0.184 |
| TG (mg/dL) | 212 (116-292) | 163 (117-227) | 88 (69-159) | 141 (82-203) | 0.737 | 0.050 | 0.173 |
| AST (U/L) | 40 (27-70) | 34 (26-70) | 23 (19-114) | 22 (17-66) | 0.926 | 0.378 | 0.149 |
| ALT (U/L) | 24 (18-57) | 30 (21-43) | 15 (14-31) | 23 (13-72) | 1.000 | 0.121 | 0.743 |
| eGFR (mL/min/1.73m2) | 57.9±16.2 | 75.1±21.7 | 55.6±22.2 | 64.3±16.2 | 0.100 | 0.982 | 0.780 |
| WBC (x1000/μL) | 11.5 (8-12.1) | 11.1 (9.0-13.5) | 8.7 (8-13.7) | 8.4 (7.4-11) | 0.778 | 0.983 | 0.493 |
| Hemoglobin (g/dL) | 15.0±1.3 | 14.8±1.1 | 12.6±2.4 | 12.7±1.3 | 0.985 | 0.003 | 0.006 |
| Albumin (g/dL) | 4.1±0.5 | 4.1±0.3 | 3.8±0.5 | 3.7±0.3 | 1.000 | 0.345 | 0.102 |
| CRP (mg/dL) | 0.10 (0.05-0.24) | 0.09 (0.06-0.50) | 0.19 (0.07-2.23) | 0.14 (0.07-1.35) | 0.993 | 0.330 | 0.493 |
| BNP (pg/mL) | 23.2 (6.1-28.7) | 39.2 (19.1-161.4) | 78.4 (28.8-201.7) | 44.0 (23.1-95.8) | 0.241 | 0.011 | 0.131 |
| Onset to Door Time (min) | 68 (46-278) | 119 (60-470) | 144 (49-483) | 88 (41-146) | 0.421 | 0.873 | 0.864 |
Data are represented as mean±SD for values with normal distribution, median (minimum-maximum) for values with skewed distribution, or frequency. Dunnet’s test was used for values with normal distribution, Steel’s test was used for values with skewed distribution, and, Fisher’s exact
test was used for analysis of data classified into two categories. P value <0.05 is considered as statistically significant and shown in bold font.
Abbreviations: BMI; body mass index, LDL-C; low-density lipoprotein cholesterol, HDL-C; high-density lipoprotein cholesterol, TG; triglyceride, AST; aspartate aminotransferase, ALT; alanine aminotransferase, eGFR; estimated glomerular filtration rate, WBC; white blood cell, CRP; C-reactive protein, BNP; brain natriuretic peptide.
| Group | P value | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Ⅰ | Ⅱ | Ⅲ | Ⅳ | ⅠvsⅡ | ⅠvsⅢ | ⅠvsⅣ |
| n (males/females) | 11 (8/3) | 12 (12/0) | 13 (5/8) | 11 (7/4) | 0.093 | 0.123 | 1.000 |
| Age (years) | 64.5±15.3 | 55.5±9.0 | 73.4±7.4 | 71.2±11.0 | 0.053 | 0.054 | 0.160 |
| BMI (kg/m2) | 24.8±3.0 | 26.6±4.2 | 22.8±2.2 | 24.1±3.9 | 0.227 | 0.150 | 0.632 |
| Diabetes (n (%)) | 5 (45) | 5 (42) | 3 (23) | 3 (27) | 1.000 | 0.391 | 0.659 |
| Hypertension (n (%)) | 3 (27) | 6 (50) | 9 (69) | 8 (73) | 0.400 | 0.100 | 0.086 |
| Dyslipidemia (n (%)) | 8 (73) | 10 (83) | 9 (69) | 9 (82) | 0.640 | 1.000 | 1.000 |
| Currentsmoker (n (%)) | 7 (64) | 4 (33) | 3 (23) | 7 (64) | 0.220 | 0.095 | 1.000 |
| Glucose (mg/dL) | 218.3±77.3 | 213.2±57.9 | 181.5±66.9 | 181.2±60.6 | 0.995 | 0.388 | 0.414 |
| HbA1c (%) | 6.7±1.3 | 6.5±1.0 | 5.9±0.6 | 6.0±0.6 | 0.855 | 0.110 | 0.162 |
| LDL-C (mg/dL) | 140.1±42.2 | 138.0±45.7 | 115.1±27.5 | 137.2±36.9 | 0.998 | 0.285 | 0.996 |
| HDL-C (mg/dL) | 38.7±4.9 | 43.1±6.9 | 45.5±12.0 | 53.3±17.1 | 0.683 | 0.343 | 0.014 |
| TG (mg/dL) | 172 (115-249) | 180 (132-253) | 141 (72-159) | 86 (76-172) | 0.981 | 0.321 | 0.086 |
| AST (U/L) | 27 (26-48) | 48 (30-97) | 21 (18-82) | 23 (20-34) | 0.440 | 0.328 | 0.448 |
| ALT (U/L) | 29 (21-43) | 24 (18-85) | 17 (14-74) | 16 (13-36) | 0.999 | 0.488 | 0.207 |
| eGFR (mL/min/1.73m2) | 59.9±13.3 | 73.3±24.7 | 51.4±14.9 | 69.2±21.1 | 0.233 | 0.558 | 0.526 |
| WBC (x1000/μL) | 10.4 (8.1-12.9) | 11.7 (9.2-13.0) | 8.5 (7.8-12.1) | 8.5 (7.4-12.8) | 1.000 | 0.711 | 0.722 |
| Hemoglobin (g/dL) | 14.7±1.3 | 15.0±1.1 | 12.2±2.0 | 13.2±1.8 | 0.922 | 0.001 | 0.078 |
| Albumin (g/dL) | 4.0±0.4 | 4.1±0.4 | 3.7±0.4 | 3.9±0.4 | 0.736 | 0.116 | 0.899 |
| CRP (mg/dL) | 0.09 (0.06-0.24) | 0.10 (0.05-0.50) | 0.31 (0.07-2.80) | 0.11 (0.06-0.35) | 0.985 | 0.146 | 0.912 |
| BNP (pg/mL) | 23.2 (6.1-51.8) | 28.3 (16.8-94.9) | 56.6 (25.6-699.7) | 47.7 (31.2-100.8) | 0.818 | 0.086 | 0.451 |
| Onset to Door Time (min) | 61 (55-397) | 128 (69-402) | 88 (49-175) | 98 (41-152) | 0.673 | 0.983 | 1.000 |
Data are represented as mean±SD for values with normal distribution, median (minimum-maximum) for values with skewed distribution, or frequency. Dunnet’s test was used for values with normal distribution, Steel’s test was used for values with skewed distribution, and, Fisher’s exact
test was used for analysis of data classified into two categories. P value <0.05 is considered as statistically significant and shown in bold font.
Abbreviations: BMI; body mass index, LDL-C; low-density lipoprotein cholesterol, HDL-C; high-density lipoprotein cholesterol, TG; triglyceride, AST; aspartate aminotransferase, ALT; alanine aminotransferase, eGFR; estimated glomerular filtration rate, WBC; white blood cell, CRP; C-reactive protein, BNP; brain natriuretic peptide.
In the present study, we showed that serum concentrations of soluble T-cad in the patients with STEMI were remarkably changed after the hospital admission and the AUC of CK-MB was significantly high in the subjects with high T-cad and low APN concentrations on admission.
Compared with our previous report36), there were little or no associations between the serum soluble T-cad levels and metabolic parameters, such as HDL-C, CRP, and APN levels, at the hospitalization (Table 2). A similar result was observed at 168 h after admission, in which time-point could be regarded as a completely recovered state (data not shown). The systemic change in the acute phase and the treatment for STEMI might influence their associations. Reportedly, serum soluble T-cad levels were associated with angiographic severity of acute coronary syndrome41), HMW-APN in stable coronary artery disease42), and obese type 2 diabetes43). However, in these reports, it is unclear which forms of T-cad (130, 100, or 30 kDa) concentration were associated with those factors because their T-cad ELISA systems were different from our newly developed ELISA system.
As shown in Fig.1, serum 130 and 100 kDa T-cad levels significantly decreased and serum 30 kDa T-cad levels slightly increased within 24–48 h after hospitalization. Those changes (approximately 30%–40% from baseline) were larger than the APN change (~20% from baseline) in our previous study13). Compared with the hospitalized patients with type 2 diabetes in our previous report36), patients with STEMI showed higher 100 kDa (STEMI 1957.6±591.4 pmol/L vs. diabetes 1136.2±419.2 pmol/L) and lower 30 kDa (STEMI 709.3±335.0 pmol/L vs. diabetes 1182.9±685.9 pmol/L) T-cad levels, suggesting a possibility that serum soluble T-cad levels could be changed in the acute clinical condition such as STEMI. Such acute and large changes of soluble T-cad could be derived from not only the injured cardiac tissue but also various tissues in the whole body, such as vascular endothelium, skeletal muscle, and nerve tissue, in which T-cad is abundantly expressed22-23). A precise mechanism for the circulating T-cad dynamics will be clarified in the future using further experimental investigations.
The present study did not show the significant associations of AUC of CK-MB with serum soluble T-cad levels or their changes (data not shown), whereas serum APN was significantly associated with the AUC of CK-MB13). Nevertheless, GPI-anchored tissue T-cad plays crucial roles in cardiovascular protection, muscle regeneration, and increasing exosome biogenesis24-28, 33-35). Baltrūnienė et al demonstrated that the decreased myocardial T-cad levels can indicate the severity of heart failure in human subjects44). Although we could not measure the amount of tissue T-cad in the present study protocol, there is a possibility that the myocardial T-cad level sequentially decreases in the acute phase of STEMI.
T-cad is originally expressed on the cell surface of the heart, muscle, and endothelial cells as GPI-anchored form22, 23). We previously demonstrated that APN bound to T-cad on the cell surface with a considerable high affinity and preferentially increased 130 kDa T-cad in tissues and cells21). Interestingly, tissue T-cad protein level, not mRNA level, was dramatically decreased in APN-null mice and APN supplementation significantly recovered such T-cad reduction22). The importance of T-cad on cardiovascular protection is reported by our group and other investigators24-26). We thus hypothesized that circulating T-cad level might reflect the tissue amount of GPI-anchored T-cad. As shown in Fig.2, Group I (high T-cad and low APN) showed the highest AUC of CK-MB level among all groups. In high T-cad groups (Groups I and III), low APN level (Group I) may be one of the risks for the enlargement of infarct area, as reported previously13). Even in low APN groups (Groups I and II), subjects with high T-cad (Group I) could be recognized as having a higher risk for severe prognosis. There is a possibility that the increase of serum soluble T-cad reflects the reduction of myocardial T-cad protein level especially in ischemia or infarct area and thus APN and T-cad-dependent cardioprotective effect is weakened, finally resulting in a large AUC of CK-MB (Group I vs. II). It will be needed to elucidate whether circulating soluble T-cad concentration reflects the amount of tissue T-cad in the future. Also, Group I included relatively young and obese subjects with relatively high blood glucose levels. This clinical feature of Group I may affect the AUC of CK-MB. A larger prospective study will be needed to investigate the usefulness of categorizing patients with STEMI with serum soluble T-cad and APN on admission for the prediction of severe prognoses, in comparison with the other well-known biomarkers (such as CK-MB, cardiac troponin, brain natriuretic peptide, and a ratio of lymphocyte to white cells) for STEMI.
This study has several limitations. First, the study was observational, has a relatively small population, and was performed in a single institution. The influence of gender, culprit lesions of the coronary artery, medication, treatment for STEMI, and other residual or unmeasured factors on the concentration and the time course of serum soluble T-cad cannot be fully excluded. Further investigations are needed to clarify whether serum soluble T-cad predicts the severity or prognosis of STEMI.
In conclusion, we showed that serum soluble T-cad concentration dramatically changed with time in patients with STEMI, and the high T-cad and low APN concentration on admission was associated with the myocardial infarction size.
We thank Yasue Fukushima for excellent technical assistance and all members of the Third Laboratory (Adiposcience Laboratory, Department of Metabolic Medicine, Osaka University) for their helpful discussion of the project. This work was supported in part by a Joint Research with Kowa Pharmaceutical Co., Ltd. (to IS), by Grants-in-Aid for Scientific Research (C) No. 18K16229 (to YF), No.19K09023 (to HN), No. 19K08980 (to NM), and No. 19K08978 (to SK), Grants-in-Aid for Scientific Research (B) No. 18H02863 (to IS), Japan Foundation for Applied Enzymology (to YF), MSD Life Science Foundation (to YF), Japan Heart Foundation & Astellas Grant for Research on Atherosclerosis Update (to YF), and Uehara Memorial Life Science Foundation (to IS).
All authors declared to have no conflict of interests in this study.