Abstract
Aim: Familial hypercholesterolemia (FH) is an underdiagnosed autosomal dominant genetic disorder characterized by high levels of plasma low-density lipoprotein cholesterol (LDL-C) from birth. This study aimed to assess the genetic identification of FH in children with high LDL-C levels who are identified in a universal pediatric FH screening in Kagawa, Japan.
Method: In 2018 and 2019, 15,665 children aged 9 or 10 years underwent the universal lipid screening as part of the annual health checkups for the prevention of lifestyle-related diseases in the Kagawa prefecture. After excluding secondary hyper-LDL cholesterolemia at the local medical institutions, 67 children with LDL-C levels of ≥ 140 mg/dL underwent genetic testing to detect FH causative mutations at four designated hospitals.
Results: The LDL-C levels of 140 and 180 mg/dL in 15,665 children corresponded to the 96.3 and 99.7 percentile values, respectively. Among 67 children who underwent genetic testing, 41 had FH causative mutations (36 in the LDL-receptor, 4 in proprotein convertase subtilisin/kexin type 9, and 1 in apolipoprotein B). The area under the curve of receiver operating characteristic curve predicting the presence of FH causative mutation by LDL-C level was 0.705, and FH causative mutations were found in all children with LDL-C levels of ≥ 250 mg/dL.
Conclusion: FH causative mutations were confirmed in almost 60% of the referred children, who were identified through the combination of the lipid universal screening as a part of the health checkup system and the exclusion of secondary hyper-LDL cholesterolemia at the local medical institutions.
See editorial vol. 29: 814-815
Introduction
Familial hypercholesterolemia (FH) is an autosomal dominant genetic disorder with an incidence of 1:200–500 in the general population1-3). Mutations in three genes, low-density lipoprotein receptor (LDLR), apolipoprotein B (APOB), and proprotein convertase subtilisin/kexin type 9 (PCSK9), have been detected in about 80% of FH patients4). Mutations in these genes lead to a high level of plasma LDL-cholesterol (LDL-C), which is one of the most important causes of premature coronary artery disease (CAD) in FH patients2, 5). Since early diagnosis and treatment are important to prevent CAD, screening is recommended in children aged 9–11 years6). However, the diagnosis of pediatric FH is still challenging7, 8), as the clinical features in adult FH, such as xanthoma, and family history of premature CAD or FH are rarely confirmed in children and adolescents, and lipid blood tests are rarely administered.
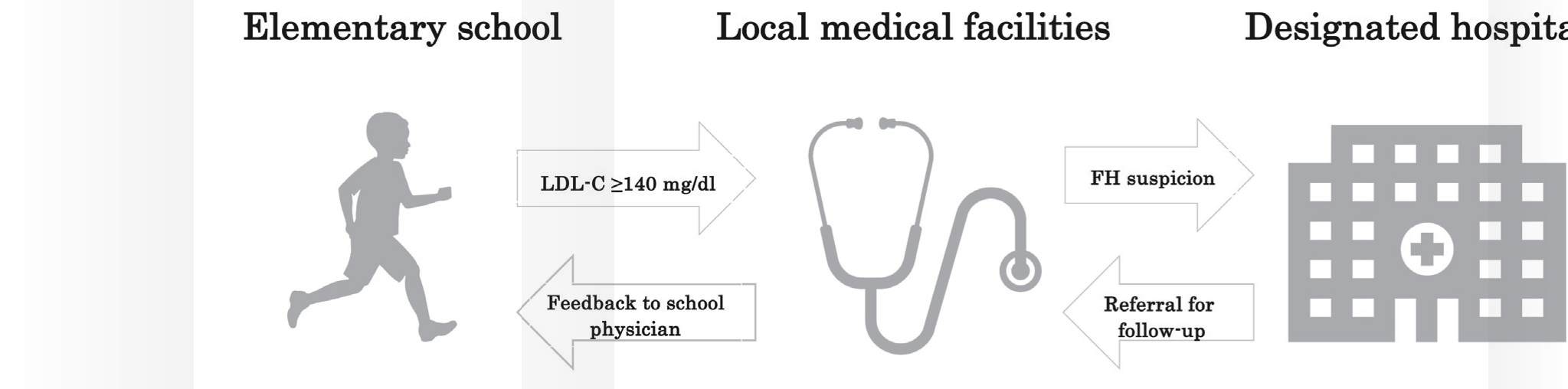
From 2012, the Kagawa prefecture started the “Kagawa health checkups for the prevention of lifestyle-related diseases in children,” which include blood testing of LDL-C in addition to other metabolic parameters. More than 7,000 children aged 9 or 10 years have undergone universal lipid screening in primary schools in each year. Children with LDL-C levels of ≥ 140 mg/dL are recommended to visit their local medical institutions. When they are suspected of FH in these institutions after excluding secondary hypercholesterolemia, they are introduced to either of four designated hospitals for FH genetic testing. This study aimed to assess the genetic identification of FH in children with high LDL-C levels who are identified in a universal pediatric FH screening in the Kagawa prefecture.
Method
Study Population
All 17 local governments in the Kagawa prefecture annually perform health checkups for the prevention of lifestyle-related diseases in children. The procedure, which is performed in elementary schools, includes lipid screening. The Kagawa prefectural government collects and analyzes the medical data obtained from all local governments. A total of 15,665 (male/female=8,051/7,614) children aged 9 or 10 years voluntarily received health checkup in 2018 and 2019. The checkup rate in 2018 and 2019 was 92.2%. Children with LDL-C levels of ≥ 140 mg/dL received the recommendation letter to visit their local medical institutions. Children suspected of having FH at these institutions, after excluding secondary hyper-LDL cholesterolemia, were introduced to four designated hospitals for the diagnosis of FH, namely, Kagawa University Hospital, Shikoku Medical Center for Children and Adults, Mitoyo General Hospital, and Kagawa Prefectural Central Hospital (Fig.1). Among the children assigned to the four hospitals, 67 with high LDL-C levels underwent genetic testing from April 2018 to August 2020.
In addition to the significant elevation of LDL-C levels (≥ 140 mg/dL) in children, we obtained medical information on the family history of FH, family history of premature CAD, LDL-C level of a parent, and appearance of xanthoma in the second-degree relatives of the referred children. The weight and standing height were measured, and the percentage of overweight (POW) was determined as previously described9). Premature CAD was defined as CAD with an onset at <55 years of age for males and <65 years of age in females, respectively10). When the children who were referred from local medical institutes underwent genetic testing, the LDL-C levels were measured again, and concomitant endocrine and kidney disorders were excluded. Children were clinically diagnosed with FH according to the Guidance for Pediatric FH 2017 10).
Basically, the doctors at the local medical institutions or four designated hospitals did not directly examine the LDL-C levels or Achilles tendon of the parents of the referred children from elementary school. The information on the parents, including their LDL-C levels and the family history of FH and premature CAD, was collected through interview or from their medical checkup data when parents brought them to the hospitals.
Genetic Testing
Among the children who were assigned to the four designated hospitals, 67 underwent genetic testing. Sequencing of the target genes was performed in Kanazawa University as previously reported1). Briefly, the DNA extracted from peripheral white blood cells was pooled, selected for size, ligated to sequencing adapters, and amplified to enrich for target regions that were sequenced using the KAPA DNA Library Preparation. A custom NimbleGen in-solution DNA capture library (Roche NimbleGen Inc., Madison, WI) was designed to capture all coding exons in 21 dyslipidemia-related Mendelian genes, including three FH genes (LDLR, PCSK9, and APOB). The target coverage for each subject was more than 20-fold in more than 98% of all targeted exons.
Causative mutations for FH were determined in the LDLR, PCSK9, and APOB genes as previously reported1). We defined a causative mutation for FH if it fulfilled any of the following criteria: a) rare protein-truncating mutations in the LDLR gene; b) rare damaging missense mutations in the LDLR gene, defined as those predicted as damaging by five in silico software programs (SIFT, Polyphen2-HDIV, Polyphen2-HVAR, MutationTaster-2, and LRT) as previously described1); c) ClinVar-registered pathogenic or likely pathogenic mutations causing FH in LDLR, PCSK9, or APOB; or d) PCSK9 p.Val4Ile and p.Glu32Lys mutations and APOB p.Arg3527Gln mutation previously reported to cause FH in the Japanese population11).
Statistical Analysis
Continuous variables, such as the patients’ clinical characteristics, were compared using the Student’s t-test, whereas categorical variables, such as sensitivity or specificity, were compared using the chi-squared test. The LDL-C levels measured at four designated hospitals were utilized for statistical analysis. Considering the results of the genetic testing as a reference, the sensitivity, specificity, and mutation detection rate were calculated. Further, 95% confidence intervals were based on binomial probabilities. The area under the curve (AUC) for predicting the presence of FH causative mutations was calculated by analyzing the receiver operating characteristic (ROC) curves. Descriptive statistics, such as means, standard deviation, and ranges, were analyzed for all variables. Statistical analyses were conducted using SPSS version 22.5 (SPSS Inc., Chicago, IL USA). A P value of <0.05 was considered statistically significant.
Ethical Considerations
The protocol was approved by the Ethics and Human Genome Committees at Kagawa University (Heisei 30-187), Shikoku Medical Center for Children and Adults, Mitoyo General Hospital, and Kagawa Prefectural Central Hospital. All procedures were performed in accordance with the ethical standards of the responsible committee on human experimentation and with the latest version of the Declaration of Helsinki. Written informed consent for the genetic testing of children was provided by at least one parent.
Results
Distribution of Pediatric LDL-C Levels in the Kagawa Prefecture, Japan
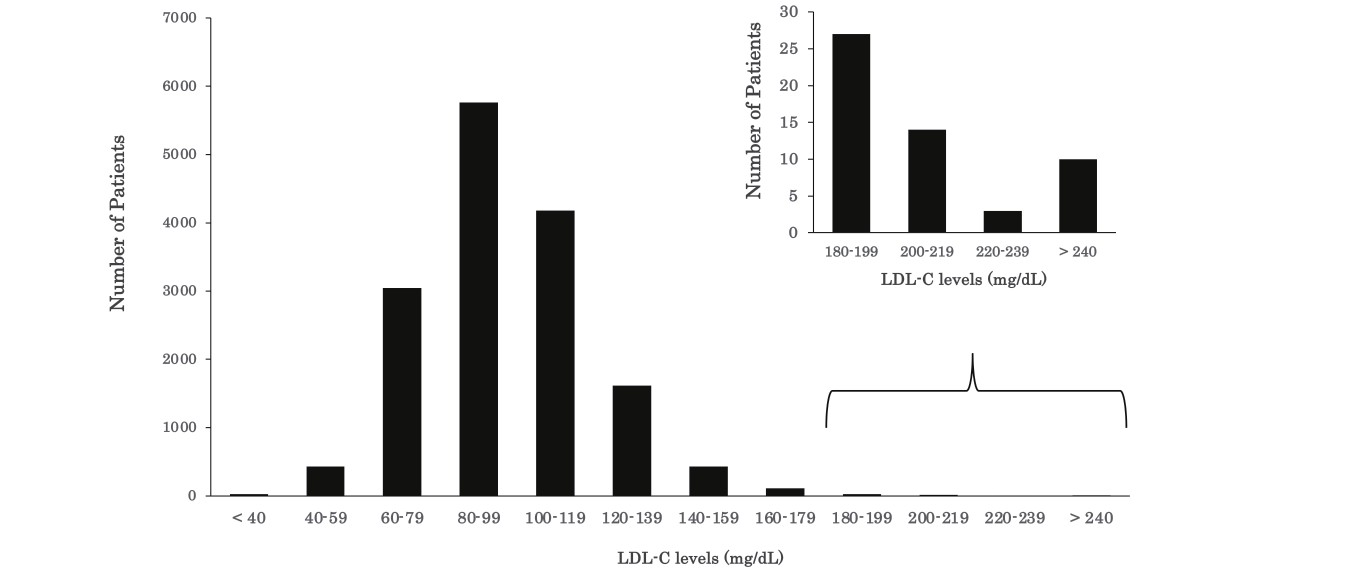
Fig.2 presents the distribution of plasma LDL-C levels in 15,665 children who underwent lipid screening in 2018 and 2019. The 95 percentile value of the LDL-C level was 135 mg/dL, and the value of 140 mg/dL corresponded to the 96.3 percentile in this study. The LDL-C values of 160 and 180 mg/dL corresponded to the 99.0 and 99.7 percentile values, respectively.
Clinical and Biochemical Features of Children who Underwent Genetic Testing
Table 1 presents the clinical and biochemical features of 67 children who underwent genetic testing, along with their family history of FH and premature CAD and the prevalence of xanthoma and high parental LDL-C level (≥ 180 mg/dL). Among 67 children who underwent genetic testing, 41 had an FH causative mutation, and all of them had heterozygous one. In detail, 36 children had 19 mutations in LDLR, 4 children had 2 mutations in PCSK9, and 1 child had 1 mutation in APOB (Supplemental Table 1). The LDL-C levels of the children with an FH causative mutation were significantly higher than those of children without it (184 versus 152 mg/dL, P=0.005). The POW was significantly lower in children with an FH causative mutation than in children without it (−5.2% versus 4.0%, P=0.032), although the POWs of both groups did not meet the criteria for obesity. No significant differences were observed in other clinical and biochemical features between children with and without a FH causative mutation (Table 1).
Table 1.
Clinical and biochemical characteristics of children genetically tested
| Characteristics |
Patients
n = 67
|
mutant (+)
n = 41
|
mutant (-)
n = 26
|
p value mutant (+) vs. (-)
|
| Age (IQR), y |
10 (10-11) |
10 (10-12) |
10 (10-11) |
0.810 |
| Male sex, n (%)
|
33 (49) |
21 (51) |
12 (46) |
0.686 |
| Systolic BP (IQR), mmHg |
96 (88-107) |
97 (90-108) |
95 (88-105) |
0.562 |
| Diastolic BP (IQR), mmHg |
60 (55-66) |
60 (55-66) |
61 (50-66) |
0.483 |
| Height (IQR), cm |
140.8 (133.1-149.0) |
141.1 (133.0-151.0) |
139.7 (135.0-147.5) |
0.997 |
| Weight (IQR), kg |
33.3 (29.0-43.6) |
32.0 (26.0-44.4) |
36.2 (30.4-42.0) |
0.498 |
| POW (IQR), % |
-2.2 (-11.1-5.7) |
-5.2 (-12.2-2.1) |
4.0 (-2.0-7.5) |
0.032*
|
| Cholesterol level (IQR), mg/dl |
|
|
|
|
| LDL-cholesterol |
178 (149-206) |
184 (168-215) |
152 (140-187) |
0.005*
|
| HDL-cholesterol |
55 (50-65) |
55 (50 65) |
56 (51-63) |
0.593 |
| TG |
74 (55-113) |
74 (55 97) |
77 (57-121) |
0.895 |
| Family history of FH, n (%)
|
10 (15) |
8 (20) |
2 (8) |
0.294 |
| Family history of premature CAD, n (%)
|
14 (21) |
10 (24) |
4 (15) |
0.540 |
| Parent’s Xanthoma, n (%)
|
6 (9) |
5 (12) |
1(4) |
0.392 |
| Parent’s LDL-cholesterol ≥ 180 mg/dl, n (%)
|
36 (54) |
26 (63) |
10 (38) |
0.078 |
CAD, coronary artery disease; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; POW: percentage of overweight; TG, Triglyceride;. *P<0.05 vs. mutant (-).
Supplemental Table 1.
Causative mutations detected in 41 children among 67 children who received genetic testing
| Gene |
DNA |
Amino acid |
Number of children |
| LDLR |
c. 301G> A |
p. Glu101Lys |
1 |
| LDLR |
c. 361T> C |
p. Cys121Arg |
1 |
| LDLR |
c. 682G> C |
p. Glu228Gln |
1 |
| LDLR |
c. 939C> A |
p. Cys313Ter |
1 |
| LDLR |
c. 967G> A |
p. Gly323Ser |
1 |
| LDLR |
c. 1056C> A |
p. Cys352Ter |
1 |
| LDLR |
c. 1201C> T |
p. Leu401Phe |
1 |
| LDLR |
c. 1207T> C |
p. Phe403Leu |
6 |
| LDLR |
c. 1252G> A |
p. Glu418Lys |
5 |
| LDLR |
c. 1502C> T |
p. ALa501Val |
1 |
| LDLR |
c. 1702C> G |
p. Leu568Val |
3 |
| LDLR |
c. 1705+1G> C |
|
3 |
| LDLR |
c. 1706A> G |
p. Asp569Gly |
3 |
| LDLR |
c. 1747C> T |
p. His583Tyr |
1 |
| LDLR |
c. 1783C> T |
p. Arg595Trp |
3 |
| LDLR |
c. 1845+2T> C |
|
1 |
| LDLR |
c. 2054C> T |
p. Pro685Leu |
1 |
| LDLR |
c. 2378T> C |
p. Val763Ala |
1 |
| LDLR |
c. 2579C> T |
p. Ala860Val |
1 |
| APOB |
c. 10596_10597insGCATT |
p. Gln3533AlafsTer16 |
1 |
| PCSK9 |
c. 94G> A |
p. Glu32Lys |
3 |
| PCSK9 |
c. 161A> C |
p. Glu54Ala |
1 |
All of them were heterozygous.
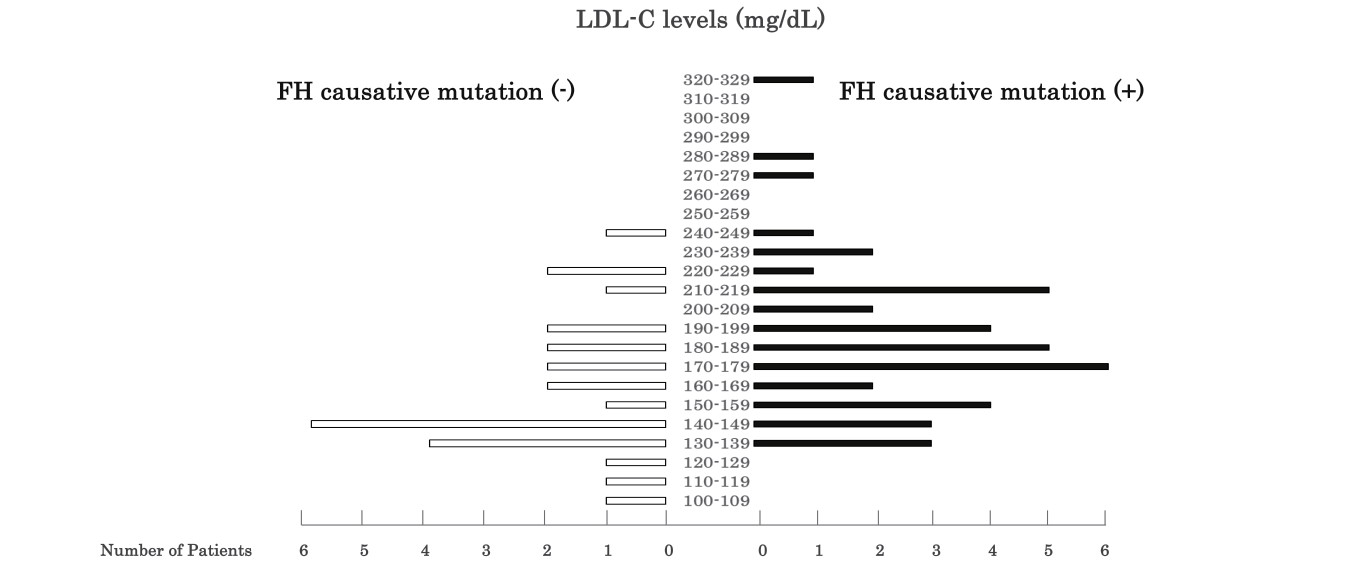
Fig.3 presents the distribution of plasma LDL-C levels in 67 children who underwent genetic testing based on the presence and absence of an FH causative mutation. Although the children exhibited LDL-C levels of ≥ 140 mg/dL in Kagawa health checkups, ten demonstrated LDL-C levels of <140 mg/dL at the four designated hospitals. Notably, although the number of children was small, all three children (male/female ratio: 1/2) with LDL-C levels of ≥ 250 mg/dL had FH causative mutations (Fig.3).
Fig.4A presents the ROC curve for plasma LDL-C levels for predicting the presence of an FH causative mutation. The AUC calculated by the ROC curve was 0.705 (0.572–0.838), indicating the low accuracy of using LDL-C levels for predicting FH causative mutations in children. The sensitivities and specificities of each LDL-C level are presented in Fig.4B.
Relation between the Current Clinical Diagnostic Criteria and FH Causative Mutations
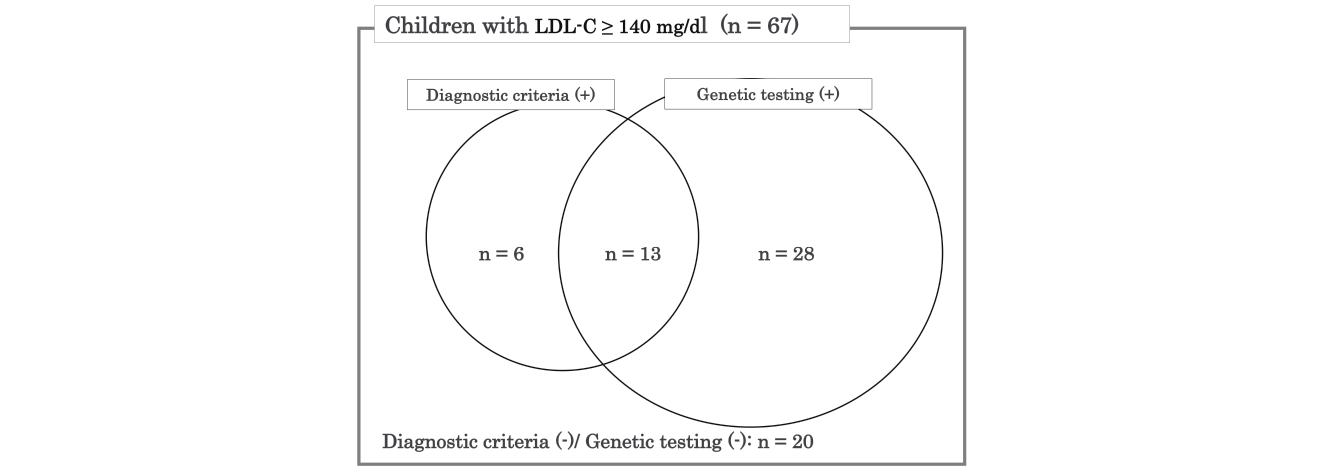
Fig.5 presents a Venn diagram representing the number of children diagnosed with FH according to the clinical diagnostic criteria (Guidance for Pediatric FH 201710)) and genetic testing. Among the 67 children who demonstrated LDL-C levels of ≥ 140 mg/dL and underwent the genetic testing, 19 met the clinical diagnostic criteria, 41 had FH causative mutations, and 13 fulfilled the clinical diagnostic criteria and had FH causative mutations (Fig.5). Six children without FH causative mutations were clinically diagnosed with FH. Furthermore, 20 children with LDL-C levels of ≥ 140 mg/dL did not meet the clinical diagnostic criteria and did not have FH causative mutations. According to the clinical diagnostic criteria (Guidance for Pediatric FH 2017), the sensitivity and specificity regarding FH causative mutations were 32% and 77%, respectively. Furthermore, we demonstrated the clinical and biochemical characteristics of 67 children who were divided into four groups based on the results of the clinical diagnostic criteria and genetic testing (Supplemental Table 2). No obvious clinical and biochemical characteristics to identify four groups could be observed. In a group negative for clinical diagnostic criteria and genetic testing, there was one child who was diagnosed with sitosterolemia by examining the ABCG5/ABCG8 gene.
Supplemental Table 2.
Clinical and biochemical characteristics of 67 children who received genetic testing
| Characteristics |
Diagnostic criteria (-)/ Genetic testing (-)
n = 20
|
Diagnostic criteria (+)/ Genetic testing (-)
n = 6
|
Diagnostic criteria (-)/ Genetic testing (+)
n = 28
|
Diagnostic criteria (+)/ Genetic testing (+)
n = 13
|
| Age (IQR), y |
10 (10-11) |
11 (10-11) |
10 (10-11) |
12 (10-13) |
| Male sex, n (%)
|
8 (40) |
4 (67) |
12 (43) |
9 (69) |
| Systolic BP (IQR), mmHg |
92 (88-107) |
96 (92-97) |
96 (90-102) |
105 (87-115) |
| Diastolic BP (IQR), mmHg |
60 (50-66) |
64 (53-66) |
60 (55-66) |
60 (56-69) |
| Height (IQR), cm |
138.7 (135.0-148.0) |
140.1 (137.7-145.8) |
141.1 (135.4-145.8) |
149.0 (130.0-155.3) |
| Weight (IQR), kg |
36.5 (30.8-42.7) |
33.3 (29.4-35.6) |
31.5 (29.4-41.2) |
32.6 (24.0-50.2) |
| POW (IQR), % |
5.2 (-0.1-10.3) |
-2.3 (-11.8-4.0) |
-4.6 (-12.0-1.1) |
-7.2 (-12.2-5.4) |
| Cholesterol level (IQR), mg/dl |
|
|
|
|
| LDL-cholesterol |
152 (141-189) |
156 (120-176) |
183 (163-215) |
189 (171-214) |
| HDL-cholesterol |
56 (52-64) |
54 (49-56) |
57 (50-67) |
54 (48-57) |
| TG |
77 (59-127) |
74 (55-115) |
75 (55-124) |
67 (49-95) |
| Family history of FH, n (%)
|
0 (0) |
2 (33) |
0 (0) |
8 (62) |
| Family history of premature CAD, n (%)
|
0 (0) |
4 (67) |
0 (0) |
10 (77) |
| Parent’s Xanthoma, n (%)
|
0 (0) |
1 (17) |
1 (4) |
4 (31) |
| Parent’s LDL-cholesterol ≥ 180 mg/dl, n (%)
|
6 (30) |
4 (67) |
16 (57) |
10 (77) |
Diagnostic criteria (+)/(-) and genetic testing (+)/(-) indicate positive or negative for clinical diagnostic criteria and genetic testing, respectively. IQR, interquartile range; POW: percentage of overweight; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, Triglyceride; CAD, coronary artery disease.
Discussion
This is the first study on the topic of universal screening for pediatric FH based on Kagawa health checkups for childhood lifestyle-related diseases prevention in Japan.
From 2012, Kagawa health checkups for the prevention of lifestyle-related diseases in children, including the measurement of LDL-C levels, is annually conducted in cooperation with the local government, elementary schools, and medical association in the Kagawa prefecture. In 2018, four designated hospitals, including Kagawa University Hospital, participated in this system focusing on the diagnosis and follow-up of FH. In addition to the performance of genetic testing at the four designated hospitals, we made and distributed the flyer about FH to the elementary schools and hosted a group study for elementary school nurses who play key roles in the introduction of children with high LDL-C levels to the local medical institutions/hospitals. Moreover, Kagawa pediatric association has recently made a comprehensive guidance program for doctors at the local medical institutions on how to follow-up the children who demonstrated abnormal values during the Kagawa health checkups. The tight cooperation among the local government, elementary schools, prefectural medical association, hospitals, and academia makes it possible to build up and polish the systems to find and follow-up both the FH children and their parents/relatives.
To date, many universal lipid screening studies have been conducted to detect pediatric FH12). The distribution of plasma LDL-C levels in pediatric health checkups in Kagawa is comparable to other universal lipid screening results13, 14) (Fig.2). This indicates that our results are applicable to other regions in Japan and other countries. In this study, approximately 4% of children were found to have high plasma LDL-C levels (≥ 140 mg/dL) (Fig.2). Among these children with high LDL-C levels, 67 underwent genetic testing for FH after excluding secondary hyper-LDL cholesterolemia. In addition, we found 41 patients who carried FH causative mutations (Fig.5). Overall, 61% of the patients suspected with FH tested positive for FH causative mutations, a finding that is in agreement with those of other universal lipid screening studies15, 16). The pediatric healthcare system for FH in Kagawa, Japan, is an effective universal lipid screening method.
In this study, we employed a panel sequencing approach focusing on genes associated with Mendelian lipid disorders. Although the definition of our causative mutation may not be perfect, most of the mutations found in this study have already been shown to be pathogenic in ClinVar. This study provides useful insights into the motivation for genetic testing of pediatric FH patients. First, genetic testing using panel sequencing can identify pediatric FH patients even when family history is not available. Second, patients with deleterious genetic variations other than the FH gene can be identified via panel sequencing, leading to their accurate diagnosis and risk stratification. Third, reverse cascade screening can be facilitated through the genetic testing of pediatric FH patients.
No significant difference was observed in the height and weight between the children with or without FH causative mutations. Although a significant difference was observed in the POW between children with and without an FH causative mutation, the POWs of these two groups were within the normal range (−20%–20%) (Table 1)9). A high level of LDL-C in children with normal POW should be noted for FH diagnosis and treatment. Other clinical and biochemical characteristics did not differ between the children with and without an FH causative mutation, suggesting that it is difficult to detect pediatric FH using these characteristics.
Consistent with previous reports17), the LDL-C levels were high in children with FH causative mutations (Table 1), indicating that FH causative mutations can result in an increase in LDL-C levels. Khera et al. reported an association between the presence of an FH mutation and a 50 mg/dL increase in LDL-C levels and a 3.8-fold (95% CI: 2.6 to 5.4) increase in the odds of CAD18). Therefore, early diagnosis and treatment are important to prevent premature CAD in children. Based on the results of genetic testing, we attempted to establish the threshold LDL-C level to diagnose pediatric FH. However, LDL-C level of 160 to 240 mg/dL did not demonstrate a precise correlation with FH causative mutations (Fig.4). Among the 67 patients of this study, a remarkably high LDL-C level (≥ 250 mg/dl) was observed only in children with an FH causative mutation (Fig.3). Therefore, it is likely that children with LDL-C levels of ≥ 250 mg/dL have FH causative mutations after excluding secondary hyper-LDL cholesterolemia.
Supplemental Table 2 presents the clinical and biochemical characteristics after 67 children were divided into four groups positive or negative in the clinical diagnostic criteria and genetic testing. However, other than the high LDL-C levels in children positive in the genetic testing and the high prevalence of family history and parent xanthoma in children positive in the clinical diagnostic criteria, we could not find any other differences in the clinical and biochemical characteristics among children in the four groups. Caution must be required when we see clinical characteristics of patients in Supplemental Table 2, because the number of children in this study is small and patient selection bias in the introduction to four designated hospitals from local medical institutes is highly possible.
In a study by Hori, the LDLR and PCSK9 causative mutations were found in 46% and 7.8% of patients diagnosed with FH, respectively19). Compared with these ratios, our data provide the impression that the proportion of LDLR mutations is higher compared with the previous report. Although LDLR, PCSK9, and APOB have been proposed as the main FH causative genes, FH due to LDLR mutations may be associated with more typical FH symptoms in childhood. Importantly, we found the second Japanese case of a causative APOB gene mutation, namely, pGln3533AlafsTer16. The mutation is located outside the LDL-binding region and close to the first Japanese case11, 20).
In addition, the present study suggests that even if the LDL-C level and diagnostic criteria are used, it may not be easy to detect FH causative mutations (Figs.4, 5). The main reason may be attributable to the absence of a family history of FH, as the parents of pediatric FH patients are relatively young to suffer from CAD. Because genetic testing via panel sequencing can identify pediatric FH patients, genetic testing may be a powerful method for diagnosing FH21) in children whenever family history is not available.
When the children were diagnosed with FH, the pediatric doctors at the four designated hospitals started to treat them according to the Guidance for Pediatric FH 2017. The children treated with statins were mainly followed up at the four designated hospitals, and those who did not need statins but do need exercise or diet therapy were mainly followed up at local medical institutes. In addition, when the parents were diagnosed with FH via reverse cascade screening, the cardiologists corrected their LDL-C levels by intensive medical treatment and actively examined whether they have CAD. Indeed, we were able to diagnose many parents with FH who could not be diagnosed by the diagnostic criteria for adult FH and thus started to treat them. In the near future, we need to establish the systems to follow-up the children and their parents with the cooperation of pediatricians and cardiologists in order to efficiently strengthen the motivation of patients with FH to fight.
Limitations
In this study, although the primary health checkup rate was relatively high (90%–95%), the follow-up system was not well established. The second medical examination rate at the local medical facilities or at a designated hospital was only about 50%–70%, indicating that about half of the children with lifestyle-related diseases or propensities did not receive medical consultation and treatment. Indeed, according to the results of the Kagawa health checkups for the prevention of lifestyle-related diseases in children in 2018 and 2019, approximately 580 children with LDL-C levels of ≥ 140 mg/dL received the recommendation letter from elementary schools to visit the local medical institutes. However, no official data are available on the exact number of children who visited the local medical institutes, who were excluded due to secondary hypercholesterolemia, or who did not agree with further examinations, including genetic testing, at local medical institutes. With the cooperation of the local government and medical association in the Kagawa prefecture, we are now working on establishing the follow-up systems to obtain the exact number of children with LDL-C levels of ≥ 140 mg/dL who actually visit the local medical institutes and four designated hospitals.
Further improvement of the pediatric healthcare system in Kagawa requires the cooperation of all 17 local governments in the Kagawa prefecture, the Kagawa Medical Association, the Kagawa Pediatric Association, and the four designated hospitals in Kagawa.
Future Perspective
This healthcare system of Kagawa identified 41 children with FH causative mutations and six children clinically diagnosed with FH without FH causative mutations over a 2-year period. It was reported that for every 1000 children of 1 to 2 years of age who undergo a child–parent screening, 8 (4 parents along with 4 children) are positive for FH13). Further improvement of the pediatric healthcare system in Kagawa would contribute to the reduction of CAD events in FH patients.
Acknowledgements
This work was supported by a research grant from AMED (No. 20gk0110048h0002), a grant from Ministry of Health, Labor and Welfare Sciences Research Grant for Research on Rare and Intractable Diseases, and a grant from Japanese Circulation Society (project for genome analysis in cardiovascular diseases). We appreciate the cooperation of officials in Kagawa Prefecture and 17 Municipalities (Takamatsu-City, Marugame-City, Sakaide-City, Zentsuji-City, Kanonji-City, Sanuki-City, Higashikagawa-City, Mitoyo-City, Tonosho-Town, Shodoshima-Town, Miki-Town, Naoshima-Town, Utazu-Town, Ayagawa-Town, Kotohira-Town, Tadotsu-Town, Manno-Town). We appreciate the members of the Review Committee of Kagawa health checkups for childhood lifestyle-related diseases prevention, Kagawa Medical Association, and Kagawa Pediatric Association for support of this research. We appreciate the assistance of Tanii Terue and Akiko Kamikura for clinical research coordinators, Masako Fujita for her experimental assistant and Hidehito Nagai for his assistance with the study management (Unmet Research, Inc.). Finally, we appreciate Hanako Jimi, a pediatrician and a member of Diet, for her advice and encouragement.
Conflicts of Interest
Hayato Tada; Bayer Yakuhin, Ltd, and Sanofi K.K.
References
- 1) Tada H, Kawashiri M, Nomura A, Teramoto R, Hosomichi K, Nohara A, Inazu A, Mabuchi H, Tajima A, Yamagishi M. Oligogenic familial hypercholesterolemia, LDL cholesterol, and coronary artery disease. J Clin Lipidol, 2018; 12: 1436-1444
- 2) Harada-Shiba M, Arai H, Ishigaki Y, Ishibashi S, Okamura T, Ogura M, Dobashi K, Nohara A, Bujo H, Miyauchi K, Yamashita S, Yokote K, Working Group by Japan Atherosclerosis Society for Making Guidance of Familial Hypercholesterolemia. Guidelines for Diagnosis and Treatment of Familial Hypercholesterolemia 2017. J Atheroscler Thromb, 2018; 25: 751-770
- 3) Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Borén J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjærg-Hansen A, European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J, 2013; 34: 3478-3490a
- 4) Talmud PJ, Futema M, Humphries SE. The genetic architecture of the familial hyperlipidaemia syndromes: rare mutations and common mutations in multiple genes. Curr Opin Lipidol, 2014; 25: 274-281
- 5) Sugiyama D, Turin TC, Yeasmin F, Rumana N, Watanabe M, Higashiyama A, Takegami M, Kokubo Y, Okamura T, Miyamoto Y. Hypercholesterolemia and Lifetime Risk of Coronary Heart Disease in the General Japanese Population: Results from the Suita Cohort Study. J Atheroscler Thromb, 2020; 27: 60-70
- 6) Mihalopoulos NL, Stipelman C, Hemond J, Brown LL, Young PC. Universal Lipid Screening in 9- to 11-Year-Olds Before and After 2011 Guidelines. Acad Pediatr, 2018; 18: 196-199
- 7) Martin AC, Gidding SS, Wiegman A, Watts GF. Knowns and unknowns in the care of pediatric familial hypercholesterolemia. J Lipid Res, 2017; 58: 1765-1776
- 8) McGowan MP, Hosseini Dehkordi SH, Moriarty PM, Duell PB. Diagnosis and Treatment of Heterozygous Familial Hypercholesterolemia. J Am Heart Assoc, 2019; 8: e013225
- 9) Dobashi K. Evaluation of Obesity in School-Age Children. J Atheroscler Thromb, 2016; 23: 32-38
- 10) Harada-Shiba M, Ohta T, Ohtake A, Ogura M, Dobashi K, Nohara A, Yamashita S, Yokote K. Hypercholesterolemia, Joint Working Group by Japan Pediatric Society and Japan Atherosclerosis Society for Making Guidance of Pediatric Familial Hypercholesterolemia. Guidance for Pediatric Familial Hypercholesterolemia 2017. J Atheroscler Thromb, 2018; 25: 539-553
- 11) Hori M, Takahashi A, Son C, Ogura M, Harada-Shiba M. The first Japanese cases of familial hypercholesterolemia due to a known pathogenic APOB gene variant, c.10580 G>A: p.(Arg3527Gln). J Clin Lipidol, 2020; 14: 482-486
- 12) Wilemon KA, Patel J, Aguilar-Salinas C, Ahmed CD, Alkhnifsawi M, Almahmeed W, Alonso R, Al-Rasadi K, Badimon L, Bernal LM, Bogsrud MP, Braun LT, Brunham L, Catapano AL, Cillíková K, Corral P, Cuevas R, Defesche JC, Descamps OS, de Ferranti S, Eiselé JL, Elikir G, Folco E, Freiberger T, Fuggetta F, Gaspar IM, Gesztes Á, Grošelj U, Hamilton-Craig I, Hanauer-Mader G, Harada-Shiba M, Hastings G, Hovingh GK, Izar MC, Jamison A, Karlsson GN, Kayikçioglu M, Koob S, Koseki M, Lane S, Lima-Martinez MM, López G, Martinez TL, Marais D, Marion L, Mata P, Maurina I, Maxwell D, Mehta R, Mensah GA, Miserez AR, Neely D, Nicholls SJ, Nohara A, Nordestgaard BG, Ose L, Pallidis A, Pang J, Payne J, Peterson AL, Popescu MP, Puri R, Ray KK, Reda A, Sampietro T, Santos RD, Schalkers I, Schreier L, Shapiro MD, Sijbrands E, Soffer D, Stefanutti C, Stoll M, Sy RG, Tamayo ML, Tilney MK, Tokgözoglu L, Tomlinson B, Vallejo-Vaz AJ, Vazquez-Cárdenas A, de Luca PV, Wald DS, Watts GF, Wenger NK, Wolf M, Wood D, Zegerius A, Gaziano TA, Gidding SS, Community R. Reducing the Clinical and Public Health Burden of Familial Hypercholesterolemia: A Global Call to Action. JAMA Cardiol, 2020; 5: 217-229
- 13) Wald D, Bestwick JP, Morris JK, Whyte K, Jenkins L, Wald NJ. Child-Parent Familial Hypercholesterolemia Screening in Primary Care. N Engl J Med, 2016; 375: 1628-1637
- 14) Ruel I, Brisson D, Aljenedil S, Awan Z, Baass A, Bélanger A, Bergeron J, Bewick D, Brophy JM, Brunham LR, Couture P, Dufour R, Francis GA, Frohlich J, Gagné C, Gaudet D, Grégoire JC, Gupta M, Hegele R A, Mancini, GBJ, McCrindle BW, Pang J, Raggi P, Tu JV, Watts GF, Genest J. Simplified Canadian Definition for Familial Hypercholesterolemia. Can J Cardiol, 2018;, 34; 1210-1214
- 15) Groselj U, Kovac J, Sustar U, Mlinaric M, Fras Z, Podkrajsek KT, Battelino T. Universal screening for familial hypercholesterolemia in children: The Slovenian model and literature review. Atherosclerosis, 2018; 277: 383-391
- 16) Mickiewicz A, Chmara M, Futema M, Fijalkowski M, Chlebus K, Galaska R, Bandurski T, Pajkowski M, Zuk M, Wasag B, Limon J, Rynkiewicz A, Gruchala M. Efficacy of clinical diagnostic criteria for familial hypercholesterolemia genetic testing in Poland. Atherosclerosis, 2016; 249: 52-58
- 17) Gidding SS, Champagne MA, de Ferranti SD, Defesche J, Ito MK, Knowles JW, McCrindle B, Raal F, Rader D, Santos RD, Lopes-Virella M, Watts GF, Wierzbicki AS, American Heart Association Atherosclerosis, Hypertension, and Obesity in Young Committee of Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and Council on Lifestyle and Cardiometabolic Health. The Agenda for Familial Hypercholesterolemia: A Scientific Statement From the American Heart Association. Circulation, 2015; 132: 2167-2192
- 18) Khera AV, Won HH, Peloso GM, Lawson KS, Bartz TM, Deng X, van Leeuwen EM, Natarajan P, Emdin CA, Bick AG, Morrison AC, Brody JA, Gupta N, Nomura A, Kessler T, Duga S, Bis JC, van Duijn CM, Cupples LA, Psaty B, Rader DJ, Danesh J, Schunkert H, McPherson R, Farrall M, Watkins H, Lander E, Wilson JG, Correa A, Boerwinkle E, Merlini PA, Ardissino D, Saleheen D, Gabriel S, Kathiresan S. Diagnostic Yield and Clinical Utility of Sequencing Familial Hypercholesterolemia Genes in Patients With Severe Hypercholesterolemia. J Am Coll Cardiol, 2016; 67: 2578-2589
- 19) Hori M, Ohta N, Takahashi A, Masuda H, Isoda R, Yamamoto S, Son C, Ogura M, Hosoda K, Miyamoto Y, Harada-Shiba M. Impact of LDLR and PCSK9 pathogenic variants in Japanese heterozygous familial hypercholesterolemia patients. Atherosclerosis, 2019; 289: 101-108
- 20) Alves AC, Etxebarria A, Soutar AK, Martin C, Bourbon M. Novel functional APOB mutations outside LDL-binding region causing familial hypercholesterolaemia. Hum Mol Genet, 2014; 23: 1817-1828
- 21) Wang H, Yang H, Liu Z, Cui K, Zhang Y, Zhao K, Yin K, Li W, Zhou Z. Targeted Genetic Analysis in a Chinese Cohort of 208 Patients Related to Familial Hypercholesterolemia. J Atheroscler Thromb, 2020; 27: 1288-1298