2023 年 30 巻 11 号 p. 1622-1634
2023 年 30 巻 11 号 p. 1622-1634
Aims: The study aimed to investigate low-density lipoprotein cholesterol (LDL-C) goal achievement rates in patients receiving LDL-C-lowering therapy using recent real-world data, following the 2017 revision of the Japan Atherosclerosis Society Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases (JAS GL2017).
Methods: Patients with documented LDL-C test results were extracted from the Medical Data Vision claims database between July 2018 and June 2021 and divided into three groups according to JAS GL2017: primary prevention high risk (Group I, LDL-C goal <120 mg/dL), secondary prevention (Group II, LDL-C goal <100 mg/dL), and secondary prevention high risk (Group III, LDL-C goal <70 mg/dL).
Results: The mean LDL-C value was 108.7 mg/dL (n=125,235), 94.4 mg/dL (n=57,910), and 90.6 mg/dL (n=33,850) in Groups I, II, and III, respectively. Intensive statin monotherapy (pitavastatin, rosuvastatin, or atorvastatin) was the most frequently prescribed lipid-lowering treatment (21.6%, 30.8%, and 42.7% in Groups I, II, and III, respectively), followed by ezetimibe (2.5%, 7.1%, and 8.5% in Groups I, II, and III, respectively). LDL-C goals were achieved by 65.5%, 60.6%, and 25.4% of patients overall in Groups I, II, and III, respectively. Achievement rates were 83.9%, 75.3%, and 29.5% in patients prescribed intensive statin monotherapy and 82.3%, 86.4%, and 46.4% in those prescribed statin and ezetimibe combinations in Groups I, II, and III, respectively. In Group III, the proportion of patients with familial hypercholesterolemia prescribed statin and ezetimibe combinations achieving LDL-C goals was low (32.5%).
Conclusions: The proportion of patients achieving LDL-C goals for secondary prevention in the high-risk group remains low even with statin and ezetimibe combination therapy.
Cardiovascular disease is the second most common cause of death in Japan, accounting for 15% of all deaths in 2018 1), and combined heart and cerebrovascular diseases are the most significant contributors to direct medical costs in the country2). For this reason, the prevention of cardiovascular disease is a major priority for public health policy in Japan, as elsewhere. A high circulating level of low-density lipoprotein cholesterol (LDL-C) is one of the best-established modifiable risk factors for atherosclerotic cardiovascular disease (ASCVD).
Historically, the prevalence of cardiovascular disease in Japan has been lower than that in North America and Europe, perhaps for dietary reasons3). However, over recent decades, blood cholesterol levels4) and thus the incidence of coronary heart disease3) have been rising. A number of large prospective cohort studies published over the last decade have reported that LDL-C levels are a major risk factor for morbidity and mortality from coronary artery disease (CAD) in the medium to long terms in Japan5-8). These trends emphasize the need for effective therapeutic measures to control hypercholesterolemia.
The introduction of statins as cholesterol-lowering therapy in the 1980s was one of the most significant therapeutic advances in cardiovascular medicine over the last 50 years. A meta-analysis of data collected over this period suggests that statins reduce the risk of major cardiovascular events by approximately 21%, with a reduction in coronary mortality of 19%9). More recently, it has been demonstrated that further reductions in the cardiovascular event risk can be obtained by combining statins with cholesterol-lowering drugs with different mechanisms of actions, namely, the cholesterol absorption inhibitor ezetimibe10) and the antiproprotein convertase subtilisin/kexin type 9 monoclonal antibodies (anti-PCSK9 mAbs)11, 12).
For these reasons, lipid-lowering treatments are recommended for lowering LDL-C to achieve specified goals in ASCVD primary and secondary prevention in practice guidelines in Europe13), Japan14), and North America15). According to these guidelines, the intensity of treatment (type or dose of statin, or combination with ezetimibe or an anti-PCSK9 mAb) should be increased until the target reduction in LDL-C levels is achieved. European guidelines follow a risk approach including target thresholds and percentage reduction of LDL-C13). In the Japanese guidelines14), the goal is to reduce LDL-C below a given threshold, whereas in the American guidelines15), the goal is to achieve a given percentage reduction in LDL-C. However, many studies worldwide have shown that, in everyday practice, LDL-C goals are not achieved in a large proportion of patients16-22).
In 2017, the Japan Atherosclerosis Society (JAS) published a revised guideline for the Prevention of Atherosclerotic Cardiovascular Diseases (JAS GL2017)14), which set strict LDL-C goals in patients receiving lipid-lowering therapy for secondary prevention of CAD, based on the Suita score derived from the Suita study23). The revised goal is LDL-C <100 mg/dL for patients with a history of CAD and LDL-C <70 mg/dL for patients with CAD and acute coronary syndrome (ACS), familial hypercholesterolemia (FH), or diabetes mellitus (DM) complicated with other high-risk comorbidities for ASCVD14). There have been several reports on LDL-C goal achievement rates in the risk groups classified in accordance with JAS GL2017, although those studies used data prior to the revision of the guideline. For example, in the FAME study, which enrolled 788 patients with FH between June 2006 and December 2012 and followed them for 4 years, 16.4% and 4.8% of patients without CAD and those with CAD achieved the goal of LDL-C <100 and <70 mg/dL, respectively, during the follow-up treatment24). Additionally, a health insurance claims database study reported that 27.3% of patients treated with intensive statin monotherapy in the secondary prevention high-risk setting achieved the goal of LDL-C <70 mg/dL over the period of 2012–2016 25).
The objective of this study was to assess the proportion of patients at high risk for ASCVD who achieved the recommended goals for LDL-C in JAS GL2017 both overall and by the intensity of statin therapy for each prevention level using recent real-world data.
This cross-sectional study was performed using a Japanese hospital-based insurance claims database (Medical Data Vision, MDV; Tokyo, Japan)26, 27). The MDV database compiles all healthcare resource consumption from 2008 in over 400 participating hospitals, representing more than 20% of acute-care hospitals in Japan participating in the Japanese Diagnosis Procedure Combination (DPC) fixed-payment reimbursement system. The database documents medical data from over 30 million patients of all ages. All patient data are anonymized. Both inpatient and outpatient medical services are documented, and the available information covers procedures, tests performed, medications prescribed, the reason for hospitalization, and inpatient deaths. Reasons for hospitalization are identified using a diagnostic code based on the International Classification of Diseases (10th edition, ICD-10). Certain patient outcomes and laboratory test results are also documented. The number of hospitals that provide laboratory test results is approximately 10% of all hospitals participating in the database.
Study PopulationThe study population consisted of adult (≥ 18 years old) patients who met the study definition for high risk of ASCVD and with at least one documented laboratory test result for LDL-C in the database during the selection period, which lasted from July 1, 2018, to June 30, 2021. The index date was the last LDL-C value observed during the selection period. All patients were required to be continuously enrolled in the database for over 1 year before the index date. For this reason, the pre-index period was defined as the 12 months before the index date (exclusive).
A high risk of ASCVD was defined as having at least one claim of disease with a confirmed diagnostic ICD-10 code for angina pectoris, myocardial infarction, ischemic stroke (IS), peripheral artery disease (PAD), DM, chronic kidney disease (CKD), or FH at any time before the index date. These diseases were identified using Level 4 ICD-10 codes (version 2013) listed in Supplementary Table 1.
| Disease | ICD-10 codes |
|---|---|
| Diabetes mellitus | E10.0-9, E11.0-9, E12.0-9, E13.0-9, E14.0-9, O24.4 |
| Angina pectoris | I20.0, I20.1, I20.8, I20.9 |
| Myocardial infarction | I21.0-9, I22.0-9, I23.0-8, I24.0-9, I25.2 |
| Ischaemic stroke | I63.0-9, I65.0, I65.1, I65.2, I65.3, I66.0-9 |
| Peripheral artery disease | I70.0-9, I71.0-9, I72.0-9, I73.0-9, Z95.8 |
| Chronic kidney disease | N18.1-4, N189 |
| Familial hypercholesterolemia | 272001, 8845523, 8845524, 8831271 (disease code) |
Information on age, gender, body mass index (BMI), and smoking status was extracted from the database. The laboratory test results on the index date were documented for lipid-related analytes and other analytes when available. Morbidities documented in the database in the period preceding the index date and FH were identified from the appropriate ICD-10 diagnostic codes (Supplementary Table 1). Medications for cardiovascular and metabolic morbidities were identified from the appropriate European Pharmaceutical Marketing Research Association Anatomical, Therapeutic, and Chemical codes in the database. For lipid-lowering treatments, the dose and formulation of the medication were documented from the prescription record.
Definition of Patient SubgroupsThree patient groups were identified using the definition of risk-prevention groups in JAS GL2017 14), based on the level of prevention for which they were eligible according to the presence of cardiovascular and metabolic comorbidities. Group I (primary prevention, high risk) consisted of patients at high risk for ASCVD (DM, FH, PAD, CKD, and IS) without a history of CAD. Patients with FH without a history of CAD were included in the primary prevention group (Group I), because it was anticipated that patient numbers would be very low. Group II (secondary prevention) consisted of all patients with a history of CAD. Group III (secondary prevention, high risk) consisted of patients in Group II with FH, recent ACS (within 12 months prior to the index date), or DM with complications (IS, PAD, or CKD).
Group III was further divided into three risk subgroups: Group III-FH, Group III-recent ACS, and Group III-complicated DM. These three subgroups were not mutually exclusive, and patients with multiple risks were assigned to more than one risk subgroup.
Definition of Treatment RegimensLipid-lowering treatments of interest included statins, ezetimibe, fixed combinations of statins and ezetimibe, and anti-PCSK9 mAbs. As described previously25), statins were classified into either standard (fluvastatin, pravastatin, and simvastatin) or intensive (atorvastatin, rosuvastatin, and pitavastatin) statins. Additionally, statin treatment regimens were classified into monotherapy with standard statin regimens, monotherapy with intensive statin regimens, monotherapy with intensive statin regimens at the maximum dose, combination therapy with a statin and ezetimibe, and combination therapy with a statin and an anti-PCSK9 mAb. Full definitions of these treatment regimens are provided in Supplementary Table 2.
| Standard statin monotherapy |
Prescription of pravastatin, fluvastatin or simvastatin No concomitant prescription of ezetimibe or an anti-PCSK9 mAb |
|
|---|---|---|
| Intensive statin monotherapy |
Prescription of rosuvastatin, atorvastatin or pitavastatin No concomitant prescription of ezetimibe or an anti-PCSK9 mAb |
|
| Intensive statin monotherapy at maximum dose | Same medications as above, at the following doses: | |
| Patients with FH: | Patients without FH: | |
| Rosuvastatin 20 mg/day | Rosuvastatin 20 mg/day | |
| Atorvastatin 40 mg/day | Atorvastatin 20 mg/day | |
| Pitavastatin 4 mg/day | Pitavastatin 4 mg/day | |
| Combination therapy with a statin and ezetimibe |
Prescription of any statin Concomitant prescription of ezetimibe (fixed or free combination) |
|
| Combination therapy with a statin and an anti-PCSK9 mAb |
Prescription of any statin Concomitant prescription of an anti-PCSK9 mAb (free combination) |
|
FH; familial hypercholesterolemia; anti-PCSK9 mAb: anti-proprotein convertase subtilisin/kexin type 9 monoclonal antibody.
For each regimen, to qualify, patients were required to have received prescriptions covering at least four consecutive weeks up to, and including, the index date, without any gap of longer than 3 days between the estimated end of one prescription (defined as the date of the prescription plus the number of days that the prescription covered) and the beginning of the following one.
OutcomesThe primary outcome of interest was the proportion of patients achieving LDL-C goals on the index date. Three specific LDL-C goals were chosen, namely, <120, <100, and <70 mg/dL, the recommended goals for Groups I, II, and III, respectively, in JAS GL2017 14). The proportions of patients achieving LDL-C goals were determined for each patient group and for each treatment regimen.
Statistical AnalysisThe study was principally descriptive, and no statistical hypotheses were tested. Continuous variables are described using mean values with their standard deviation (SD) or median values with their interquartile range (IQR). Categorical variables are presented as frequency counts and percentages, with their 95% confidential intervals (CI). For two-group comparison, the χ² test was used to compare categorical variables and Student’s t-test (for normally distributed variables, otherwise, the Mann–Whitney U-test) to compare continuous variables. Statistical significance was accepted at P<0.05.
EthicsThe study complied with all relevant international and national legislations on medical research and data privacy. In particular, it complied with the Declaration of Helsinki (Fortaleza Revision, 2013) and the Japanese Act on the Protection of Personal Information (Act No. 57, 2003 and subsequent revisions). Furthermore, because the data were anonymized prior to extraction, the Japanese Pharmaceuticals and Medical Devices Agency guidelines for conducting pharmacoepidemiological research using medical databases, which specify when ethics approval and informed consent are required, do not apply.
The source cohort consisted of 395,314 patients with at least one documented LDL-C measurement during the selection period (Fig.1). Of these patients, 212,169 (53.7%) were ineligible because they were not at high risk for ASCVD, not documented in the database for at least 12 months before the index period, or <18 years old. The remaining 183,145 patients constituted the study population. The number of patients in each group of interest ranged from 33,850 in Group III to 125,235 in Group I and from 389 to 28,512 for the different subgroups of Group III.
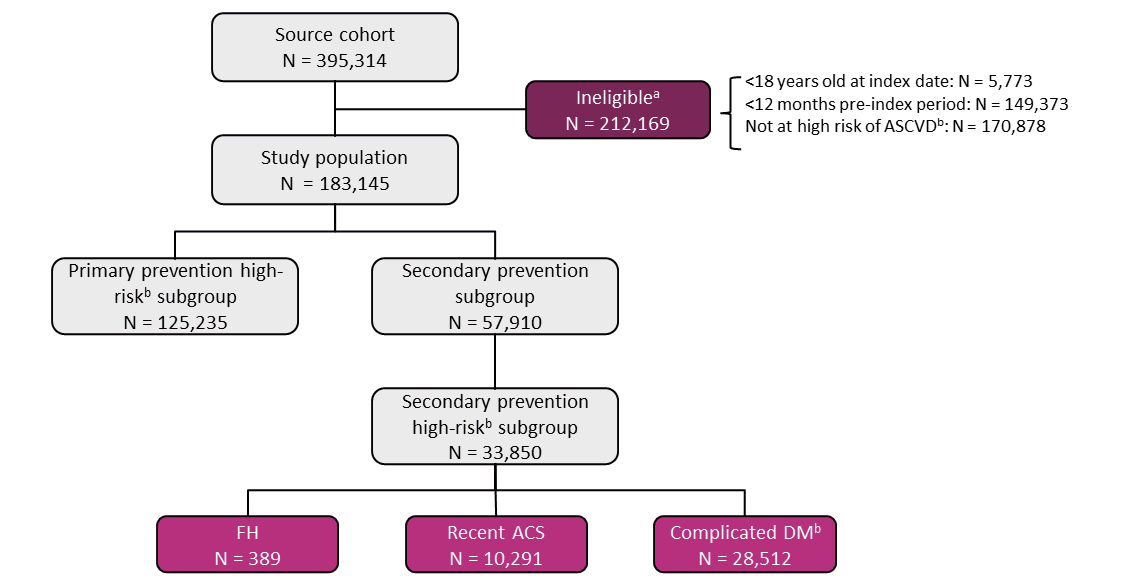
aPatients could be ineligible for more than one reason.
bAs defined by associated comorbidities and described in the Methods.
ACS: acute coronary syndrome; ASCVD: atherosclerotic cardiovascular disease; DM: diabetes mellitus; FH: familial hypercholesterolemia.
The characteristics of the patients in the three risk-prevention groups of interest are presented in Table 1. Compared with those in Groups I and II, patients in Group III were older and more frequently men and smokers and had higher HbA1c and lower eGFR. The BMI in Group III was slightly higher than that in Group I. Less than 1% of patients in both Groups I and II had documented FH. Patients in Group III more frequently presented with hypercholesterolemia, IS, DM, CKD, and hypertension and more frequently received adenosine diphosphate purinergic receptor type P2Y12 inhibitors, antithrombotic drugs, and aspirin than those in Groups I and II. In the subgroups of Group III, patients in Group III-recent ACS were more frequently men and smokers and less frequently presented a history of IS and PAD compared with those in Group III-FH and Group III-complicated DM. Patients in Group III-FH were younger and less frequently had CKD than those in Group III-recent ACS and Group III-complicated DM.
| Variable |
GROUP I Primary prevention high risk (N=125,235) |
GROUP II Secondary prevention (N=57,910) |
GROUP III Secondary prevention high risk (N=33,850) |
GROUP III FH subgroup (N=389) |
GROUP III Recent ACS subgroup (N=10,291) |
GROUP III Complicated DM subgroup (N=28,512) |
|---|---|---|---|---|---|---|
| Male (n, %) | 65,156 (52.0%) | 36,466 (63.0%) | 22,502 (66.5%)*** | 245 (63.0%) | 7,532 (73.2%) | 18,604 (65.2%) |
| Age (years) | ||||||
| Mean±SD | 70.5±13.3 | 74.4±11.4 | 75.0±10.7*** | 68.7±13.5 | 72.8±11.7 | 75.8±10.1 |
| <50 years (n, %) | 9,982 (8.0%) | 1,902 (3.3%) | 819 (2.4%) | 41 (10.5%) | 444 (4.3%) | 463 (1.6%) |
| 50-59 years (n, %) | 14,395 (11.5%) | 4,418 (7.6%) | 2,209 (6.5%) | 55 (14.1%) | 984 (9.6%) | 1,495 (5.2%) |
| 60-69 years (n, %) | 26,176 (20.9%) | 10,049 (17.4%) | 5,686 (16.8%) | 84 (21.6%) | 1,987 (19.3%) | 4,598 (16.1%) |
| 70-79 years (n, %) | 41,428 (33.1%) | 20,684 (35.7%) | 12,661 (37.4%) | 123 (31.6%) | 3,676 (35.7%) | 10,963 (38.5%) |
| ≥ 80 years (n, %) | 33,254 (26.6%) | 20,857 (36.0%) | 12,475 (36.9%) | 86 (22.1%) | 3,200 (31.1%) | 10,993 (38.6%) |
| Body mass index (kg/m2) | N=30,877 | N=21,040 | N=13,937 | N=111 | N=5,374 | N=11,236 |
| Mean±SD | 23.0±4.8 | 23.4±15.9 | 23.5±4.2*** | 23.9±4.3 | 23.7±4.1 | 23.5±4.3 |
| 25-29.9 kg/m2 (n, %) | 6,547 (21.2%) | 5,119 (24.3%) | 3,568 (25.6%) | 31 (27.9%) | 1,429 (26.6%) | 2,839 (25.3%) |
| ≥ 30 kg/m2 (n, %) | 2,101 (6.8%) | 1,258 (6.0%) | 937 (6.7%) | 11 (9.9%) | 359 (6.7%) | 764 (6.8%) |
| Smoking status | N=28,487 | N=19,448 | N=12,800 | N=108 | N=4,960 | N=1,0315 |
| Smoker (n, %) | 10,819 (38.0%) | 9,124 (46.9%) | 6,356 (49.7%)*** | 50 (46.3%) | 2,679 (54.0%) | 4,992 (48.4%) |
| Brinkman smoking index (mean±SD) | 276.4±500.0 | 368.7±565.2 | 398.3±586.2*** | 349.9±529.6 | 409.3±545.5 | 400.9±606.5 |
| Laboratory tests | ||||||
| HbA1c (%) | N=99,564 | N=48,590 | N=30,679 | N=306 | N=9,125 | N=26,246 |
| Mean±SD | 6.4±1.1 | 6.4±1.0 | 6.6±1.1*** | 6.5±1.3 | 6.4±1.0 | 6.7±1.1 |
| eGFR (mL/min/1.73 m2) | N=123,900 | N=57,660 | N=33,760 | N=385 | N=10,267 | N=28,439 |
| Mean±SD | 61.4±24.1 | 56.3±23.8 | 52.1±23.3*** | 61.2±22.4 | 56.6±23.6 | 49.9±22.9 |
| Morbidities | ||||||
| Angina pectoris (n, %) | 0 (0.0%) | 52,487 (90.6%) | 30,274 (89.4%) a | 352 (90.5%) | 8,236 (80.0%) | 26,286 (92.2%) |
| Unstable angina pectoris (n, %) | 0 (0.0%) | 9,587 (16.6%) | 8,412 (24.9%) a | 83 (21.3%) | 6,394 (62.1%) | 5,503 (19.3%) |
| Myocardial infarction (n, %) | 0 (0.0%) | 17,120 (29.6%) | 12,235 (36.1%) a | 163 (41.9%) | 6,459 (62.8%) | 8,831 (31.0%) |
| Recent ACS (n, %) | 0 (0.0%) | 10,291 (17.8%) | 10,291 (30.4%) a | 95 (24.4%) | 10,291 (100%) | 5,086 (17.8%) |
| Ischemic stroke (n, %) | 22,249 (17.8%) | 12,819 (22.1%) | 9,541 (28.2%)*** | 111 (28.5%) | 2,020 (19.6%) | 8,965 (31.4%) |
| Peripheral artery disease (n, %) | 15,946 (12.7%) | 14,779 (25.5%) | 11,053 (32.7%)*** | 116 (29.8%) | 2,536 (24.6%) | 10,348 (36.3%) |
| Aortic aneurysm (n, %) | 2,560 (2.0%) | 2,631 (4.5%) | 1,655 (4.9%)*** | 27 (6.9%) | 460 (4.5%) | 1,471 (5.2%) |
| Diabetes mellitus (n, %) | 72,094 (57.6%) | 34,758 (60.0%) | 29,626 (87.5%)*** | 248 (63.8%) | 6,170 (60.0%) | 28,512 (100%) |
| Hypercholesterolemia (n, %) | 31,075 (24.8%) | 25,618 (44.2%) | 17,216 (50.9%)*** | 389 (100%) | 6,061 (58.9%) | 14,168 (49.7%) |
| FH | 282 (0.2%) | 389 (0.7%) | 389 (1.1%)*** | 389 (100%) | 95 (0.9%) | 202 (0.7%) |
| Chronic kidney disease1 (n, %) | 87,300 (69.7%) | 41,614 (71.9%) | 28,340 (83.7%)*** | 235 (60.4%) | 7,356 (71.5%) | 25,554 (89.6%) |
| Stage III | 48,846 (39.0%) | 23,550 (40.7%) | 15,308 (45.2%) | 133 (34.2%) | 4,110 (39.9%) | 13,572 (47.6%) |
| Stage IV-V | 10,763 (8.6%) | 8,043 (13.9%) | 6,057 (17.9%) | 37 (9.5%) | 1,410 (13.7%) | 5,719 (20.1%) |
| Hypertension (n, %) | 79,882 (63.8%) | 48,962 (84.5%) | 30,237 (89.3%)*** | 333 (85.6%) | 9,247 (89.9%) | 25,640 (89.9%) |
| Medication use | ||||||
| P2Y12 inhibitors (n, %) | 5,877 (4.7%) | 14,899 (25.7%) | 10,885 (32.2%)*** | 135 (34.7%) | 5,543 (53.9%) | 7,961 (27.9%) |
| Anti-thrombotic drugs (n, %) | 22,524 (18.0%) | 21,400 (37.0%) | 14,154 (41.8%)*** | 125 (32.1%) | 5,566 (54.1%) | 11,331 (39.7%) |
| Aspirin (n, %) | 8,215 (6.6%) | 25,601 (44.2%) | 17,173 (50.7%)*** | 231 (59.4%) | 7,340 (71.3%) | 13,238 (46.4%) |
1Staging of chronic kidney failure was only available for the 56,609 patients in Group I, the 31,593 patients in Group II and the 21,365 patients in Group III with chronic kidney failure who had a valid creatinine measure from which the glomerular filtration rate could be estimated (or a detailed ICD-10 code).
Statistical significance is represented by the asterisks: ***p<0.001, compared to Group I. a These variables were exclusion criteria for Group I.
ACS: acute coronary syndrome; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; eGFR: estimated glomerular filtration rate; FH: familial hypercholesterolemia; HbA1c: glycated haemoglobin; P2Y12: adenosine diphosphate purinergic receptor type P2Y12; SD: standard deviation.
Lipid-lowering treatments prescribed to the three risk-prevention groups are presented in Table 2. Statins were the most widely used treatments, most frequently in Group III (46.6%) and least frequently in Group I (25.3%). Most statin prescriptions were for an intensive statin, and 19.0%, 29.9%, and 33.1% of patients in Groups I, II, and III, respectively, used intensive statin monotherapy. The maximum dose of intensive statins was used in <1% of patients in all groups. Statins and intensive statins were used more often in Group III-FH than in Group III-recent ACS and Group III-complicated DM.
| Treatment regimen |
GROUP I Primary prevention high risk (N=125,235) |
GROUP II Secondary prevention (N=57,910) |
GROUP III Secondary prevention high risk (N=33,850) |
GROUP III FH subgroup (N=389) |
GROUP III Recent ACS subgroup (N=10,291) |
GROUP III Complicated DM subgroup (N=28,512) |
|---|---|---|---|---|---|---|
| All statins (n, %) | 31,637 (25.3%) | 24,191 (41.8%) | 15,769 (46.6%)*** | 280 (72.0%) | 5,559 (54.0%) | 12,814 (44.9%) |
| Standard statins (n, %) | 4,563 (3.6%) | 2,191 (3.8%) | 1,318 (3.9%)* | 26 (6.7%) | 275 (2.7%) | 1,186 (4.2%) |
| Standard statin monotherapy | 4,105 (3.3%) | 1,993 (3.4%) | 1,195 (3.5%)* | 22 (5.7%) | 245 (2.4%) | 1,075 (3.8%) |
| Intensive statins (n, %) | 27,074 (21.6%) | 22,000 (38.0%) | 14,451 (42.7%)*** | 254 (65.3%) | 5,284 (51.3%) | 11,628 (40.8%) |
| Intensive statin monotherapy | 23,773 (19.0%) | 17,321 (29.9%) | 11,188 (33.1%)*** | 90 (23.1%) | 3,794 (36.9%) | 9,265 (32.5%) |
| Maximum dose intensive statin monotherapy | 16 (<0.1%) | 75 (0.1%) | 51 (0.2%)*** | 0 (0.0%) | 33 (0.3%) | 32 (0.1%) |
| Ezetimibe (n, %) | 3,133 (2.5%) | 4,083 (7.1%) | 2,871 (8.5%)*** | 151 (38.8%) | 1,298 (12.6%) | 2,097 (7.4%) |
| Ezetimibe and statin combination therapy | 1,203 (1.0%) | 3,106 (5.4%) | 2,231 (6.6%)*** | 117 (30.1%) | 1,115 (10.8%) | 1,550 (5.4%) |
| Anti-PCSK9 mAb (n, %) | 7 (<0.1%) | 55 (0.1%) | 49 (0.1%)*** | 29 (7.5%) | 19 (0.2%) | 28 (0.1%) |
| Anti-PCSK9 mAb/statin combination therapy | 3 (<0.1%) | 25 (<0.1%) | 22 (0.1%)*** | 13 (3.3%) | 8 (0.1%) | 12 (<0.1%) |
| Ezetimibe/Anti-PCSK9 mAb/statin triple therapy | 1 (<0.1%) | 13 (<0.1%) | 13 (<0.1%)*** | 11 (2.8%) | 5 (<0.1%) | 7 (<0.1%) |
| Fibrates (n, %) | 4,189 (3.3%) | 1,249 (2.2%) | 814 (2.4%)*** | 15 (3.9%) | 165 (1.6%) | 740 (2.6%) |
| Nicotinic acid derivatives (n, %) | 1,251 (1.0%) | 498 (0.9%) | 302 (0.9%) | 0 (0.0%) | 58 (0.6%) | 284 (1.0%) |
| Bile acid sequestrants (n, %) | 125 (0.1%) | 47 (0.1%) | 36 (0.1%) | 7 (1.8%) | 9 (0.1%) | 28 (0.1%) |
| Probucol (n, %) | 43 (<0.1%) | 38 (0.1%) | 24 (0.1%)** | 4 (1.0%) | 3 (<0.1%) | 22 (0.1%) |
| Ethyl icosapentate / ω-3-acid ethyl esters (n, %) | 2,940 (2.3%) | 2,430 (4.2%) | 1,654 (4.9%)*** | 58 (14.9%) | 419 (4.1%) | 1,449 (5.1%) |
| No lipid-lowering treatmenta (n, %) | 84,163 (67.2%) | 29,982 (51.8%) | 15,736 (46.5%)*** | 77 (19.8%) | 4,147 (40.3%) | 13,603 (47.7%) |
a No LDL-C lowering treatment in the four weeks preceding the index date. Statistical significance is represented by the asterisks: *p<0.05, **p<0.01, ***p<0.001, compared to Group I.
ACS: acute coronary syndrome; DM: diabetes mellitus; FH: familial hypercholesterolemia; anti-PCSK9 mAb: anti-proprotein convertase subtilisin/ kexin type 9 monoclonal antibody.
Ezetimibe was prescribed most frequently in Group III (8.5%) and was commonly used in combination with a statin (6.6%). An anti-PCSK9 mAb was prescribed in only 0.1% of patients, principally in Groups II and III. Within Group III, patients with FH were more frequently prescribed ezetimibe (38.8% overall and 30.1% in combination) compared with those with recent ACS and complicated DM. The use of anti-PCSK9 mAbs was more frequent in Group III-FH (7.5%) than in Group III-recent ACS (0.2%) and Group III-complicated DM (0.1%).
None of the other lipid-lowering treatments were prescribed in >5% of patients in any study group; the most commonly prescribed of these other treatments except for statins, ezetimibe, and anti-PCSK9 mAb were fibrates in Group I and ethyl icosapentate/ω-3-acid ethyl esters in Groups II and III, respectively. Overall, 114,145 patients (62.3%) were not prescribed any lipid-lowering treatment for 4 weeks prior to the index date. Patients in Group III (46.5%) were less frequently untreated than those in Groups I (51.8%) and II (67.2%). In the subgroups of Group III, the proportion of patients who were not treated with lipid-lowering drugs was lower in Group III-FH (19.8%) than in Group III-recent ACS (40.3%) and Group III-complicated DM (47.7%).
Lipid ControlAt the index date, LDL-C values (mean±SD) were 108.7±31.9, 94.4±31.1, and 90.6±30.8 mg/dL in Groups I, II, and III, respectively. Median (IQR) values were 107.0 (87.0–128.0), 91.0 (72.3–113.0), and 87.0 (69.0–108.0) mg/dL in Groups I, II, and III, respectively. LDL-C and non-high-density lipoprotein cholesterol (HDL-C) were the lowest in Group III, although HDL-C and triglyceride values were comparable in all three groups (Table 3). The distribution of LDL-C by the risk group and principal treatment class is presented in Supplementary Fig.1.
| Lipid measure |
GROUP I Primary prevention high risk (N= 125,235) |
GROUP II Secondary prevention (N= 57,910) |
GROUP III Secondary prevention high risk (N= 33,850) |
GROUP III FH subgroup (N= 389) |
GROUP III Recent ACS subgroup (N= 10,291) |
GROUP III Complicated DM subgroup (N= 28,512) |
|---|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | N= 83,286 | N= 39,116 | N= 23,391 | N= 223 | N= 7,246 | N= 19,812 |
| Mean±SD | 190.3±40.6 | 172.4±40.5 | 167.6±40.7*** | 174.3±55.3 | 161.5±39.3 | 168.2±40.8 |
| LDL cholesterol (mg/dL) | N= 125,235 | N= 57,910 | N= 33,850 | N= 389 | N= 10,291 | N= 28,512 |
| Mean±SD | 108.7±31.9 | 94.4±31.1 | 90.6±30.8*** | 95.6±45.5 | 85.0±29.3 | 91.3±30.9 |
| HDL cholesterol (mg/dL) | N= 114,982 | N= 54,955 | N= 32,315 | N= 379 | N= 9,908 | N= 27,183 |
| Mean±SD | 58.9±17.9 | 55.3±16.8 | 53.6±16.4*** | 55.4±15.6 | 52.3±15.2 | 53.4±16.6 |
| Non-HDL cholesterol (mg/dL) | N= 83,262 | N= 39,105 | N= 23,386 | N= 223 | N= 7,246 | N= 19,807 |
| Mean±SD | 132.0±38.0 | 117.5±37.1 | 114.3±37.5*** | 120.0±50.9 | 109.7±37.0 | 115.0±37.5 |
| Triglycerides (mg/dL) | N= 120,969 | N= 55,528 | N= 32,463 | N= 377 | N= 9,928 | N= 27,321 |
| Mean±SD | 136.4±99.1 | 132.8±93.5 | 135.2±88.8 | 131.9±69.5 | 135.8±90.0 | 135.6±88.8 |
Statistical significance is represented by the asterisks: ***p<0.001, compared to Group I.
ACS: acute coronary syndrome; DM: diabetes mellitus; FH: familial hypercholesterolemia; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; SD: standard deviation.
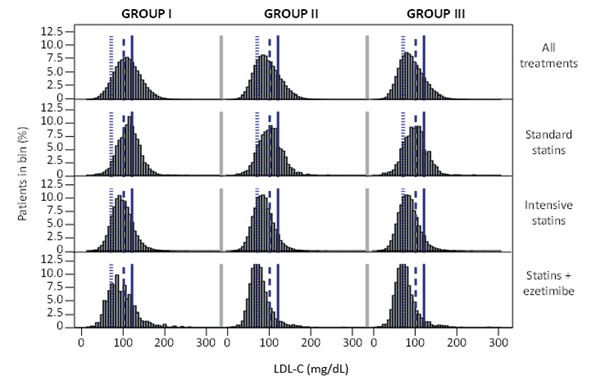
Reference lines were set at 70, 100, and 120 mg/dL.
Irrespective of treatment, 65.5% [95%CI 65.2–65.8] of patients in Group I achieved their LDL-C goal of <120 mg/dL. In Groups II and III, 60.6% [60.2–61.0] and 25.4% [24.9–25.9] of patients achieved their goal of <100 and <70 mg/dL, respectively (Fig.2). The patients in Group III-recent ACS more frequently achieved their goal (31.8% [30.9–32.7]) than those in Group III-FH (26.7% [22.3–31.1]) and Group III-complicated DM (24.6% [24.1–25.1]).
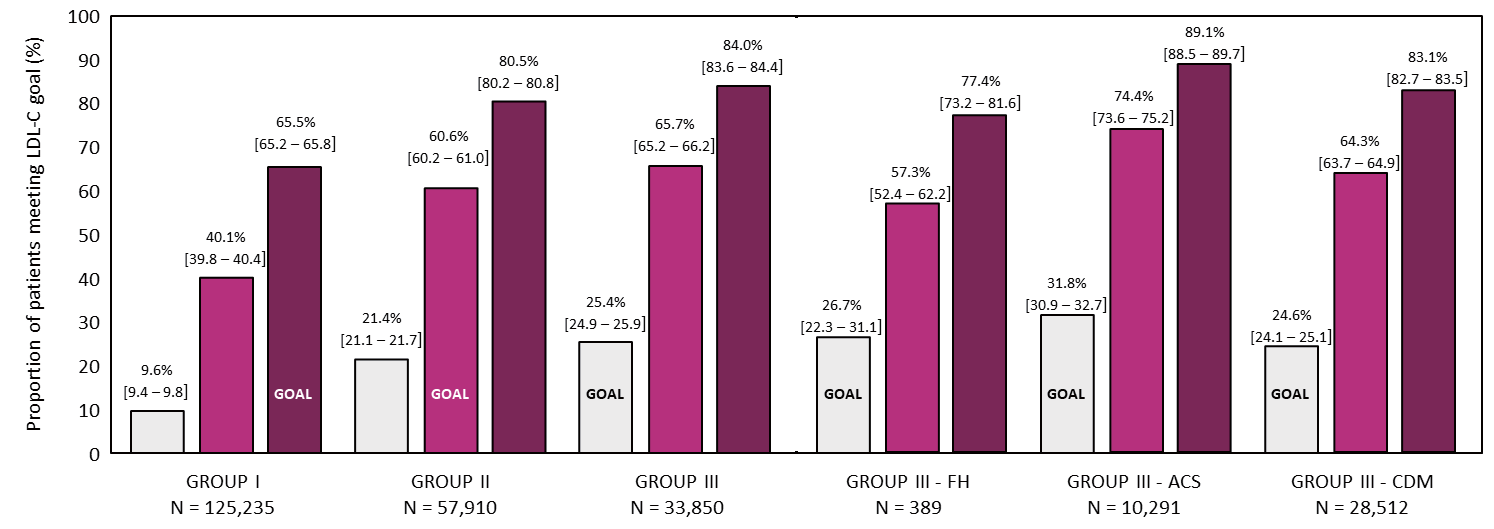
ACS: acute coronary syndrome; DM: diabetes mellitus; FH: familial hypercholesterolemia; LDL-C: low-density lipoprotein cholesterol.
Light-violet–red column, LDL-C goal <70 mg/dL; middle-violet–red columns, LDL-C goal <100 mg/dL; dark-violet–red columns, LDL-C goal <120 mg/dL.
The proportion of patients achieving their LDL-C goals as a function of treatment before the index date is presented in Table 4. In Group I, more patients receiving intensive statin monotherapy achieved the LDL-C goal of <120 mg/dL (83.9% [95%CI 83.4–84.4]) compared with those receiving standard statin monotherapy (63.9% [62.4–65.4]). Additionally, a high proportion of patients receiving a combination of a statin with ezetimibe achieved their goal (82.3% [80.1–84.5]).
| LDL-C <70 mg/dL | LDL-C <100 mg/dL | LDL-C <120 mg/dL | ||
|---|---|---|---|---|
| N | n (% [95% CI]) | n (% [95% CI]) | n (% [95% CI]) | |
| GROUP I: primary prevention high risk (n, % [95%CI]) ; LDL-C goal <120 mg/dL | ||||
| Any lipid-lowering treatment | 38,307 | 4,106 (10.7% [10.4% – 11.0%] | 19,066 (49.8% [49.3% – 50.3%]) | 29,315 (76.5% [76.1% – 76.9%]) |
| Standard statin monotherapy | 4,105 | 175 (4.3% [3.7% – 4.9%]) | 1,261 (30.7% [29.3% – 32.1%]) | 2,623 (63.9% [62.4% – 65.4%]) |
| Intensive statin monotherapy | 23,773 | 2,950 (12.4% [12% – 12.8%]) | 13,802 (58.1% [57.5% – 58.7%]) | 19,957 (83.9% [83.4% – 84.4%]) |
| Maximum dose intensive statin | 16 | 4 (25% [3.8% – 46.2%]) | 6 (37.5% [13.8% – 61.2%]) | 11 (68.8% [46.1% – 91.5%]) |
| Statin + ezetimibe | 1,203 | 274 (22.8% [20.4% – 25.2%]) | 747 (62.1% [59.4% – 64.8%]) | 990 (82.3% [80.1% – 84.5%]) |
| Statin + anti-PCSK9 mAb | 3 | 1 (33.3% [0.0% – 86.6%]) | 2 (66.7% [13.4% – 100.0%]) | 2 (66.7% [13.4% – 100.0%]) |
| GROUP II: secondary prevention (n, % [95%CI]); LDL-C goal <100 mg/dL | ||||
| Any lipid-lowering treatment | 26,418 | 6,917 (26.2% [25.7% – 26.7%]) | 18,845 (71.3% [70.8% – 71.8%]) | 23,431 (88.7% [88.3% – 89.1%]) |
| Standard statin monotherapy | 1,993 | 235 (11.8% [10.4% – 13.2%]) | 987 (49.5% [47.3% – 51.7%]) | 1,544 (77.5% [75.7% – 79.3%]) |
| Intensive statin monotherapy | 17,321 | 4,579 (26.4% [25.7% – 27.1%]) | 13,044 (75.3% [74.7% – 75.9%]) | 15,942 (92.0% [91.6% – 92.4%]) |
| Maximum dose intensive statin | 75 | 16 (21.3% [12.0% – 30.6%]) | 54 (72.0% [61.8% – 82.2%]) | 65 (86.7% [79.0% – 94.4%]) |
| Statin + ezetimibe | 3,106 | 1,379 (44.4% [42.7% – 46.1%]) | 2,685 (86.4% [85.2% – 87.6%]) | 2,931 (94.4% [93.6% – 95.2%]) |
| Statin + anti-PCSK9 mAb | 25 | 21 (84.0% [69.6% – 98.4%]) | 24 (96.0% [88.3% – 100.0%]) | 25 (100.0% [100.0% – 100.0%]) |
| GROUP III: secondary prevention high risk (n, % [95%CI]); LDL-C goal <70 mg/dL | ||||
| Any lipid-lowering treatment | 17,112 | 5,041 (29.5% [28.8% – 30.2%]) | 12,724 (74.4% [73.7% – 75.1%]) | 15,409 (90.0% [89.6% – 90.4%]) |
| Standard statin monotherapy | 1,195 | 162 (13.6% [11.7% – 15.5%]) | 636 (53.2% [50.4% – 56.0%]) | 968 (81.0% [78.8% – 83.2%]) |
| Intensive statin monotherapy | 11,188 | 3,298 (29.5% [28.7% – 30.3%]) | 8,702 (77.8% [77% – 78.6%]) | 10,378 (92.8% [92.3% – 93.3%]) |
| Maximum dose intensive statin | 51 | 10 (19.6% [8.7% – 30.5%]) | 36 (70.6% [58.1% – 83.1%]) | 46 (90.2% [82% – 98.4%]) |
| Statin + ezetimibe | 2,231 | 1,035 (46.4% [44.3% – 48.5%]) | 1,941 (87.0% [85.6% – 88.4%]) | 2,106 (94.4% [93.4% – 95.4%]) |
| Statin + anti-PCSK9 mAb | 22 | 19 (86.4% [72.1% – 100.0%]) | 21 (95.5% [86.8% – 100.0%]) | 22 (100% [100.0% – 100.0%]) |
ACS: acute coronary syndrome; CI: confidence interval; DM: diabetes mellitus; FH: familial hypercholesterolemia; LDL-C: low-density lipoprotein cholesterol; anti-PCSK9 mAb: anti-proprotein convertase subtilisin/kexin type 9 monoclonal antibody.
Data in bold indicate the target LDL-C goal for each Group.
In Group II, the proportion of patients achieving LDL-C <100 mg/dL was 49.5% [95%CI 47.3–51.7] with standard statin monotherapy, 75.3% [74.7–75.9] with intensive statin monotherapy, 86.4% [85.2–87.6] with statin and ezetimibe combination therapy, and 96.0% [88.3–100.0] with statin and anti-PCSK9 mAb combination therapy.
In Group III, the proportion of patients achieving LDL-C <70 mg/dL was 29.5% [95%CI 28.7–30.3] with intensive statin monotherapy, 46.4% [44.3–48.5] with statin and ezetimibe combination, and 86.4% [72.1–100.0] with statin and anti-PCSK9 mAb combination (Table 4). In the subgroups of Group III (Table 5), patients in Group III-FH responded the least well, with 21.1% [12.7–29.5] treated with intensive statin monotherapy and 32.5% [24.0–41.0] treated with a statin and ezetimibe combination achieving their goal.
| LDL-C < 70 mg/dL | LDL-C < 100 mg/dL | LDL-C < 120 mg/dL | ||
|---|---|---|---|---|
| N | n (% [95% CI]) | n (% [95% CI]) | n (% [95% CI]) | |
| GROUP III FH subgroup (n, % [95%CI]); LDL-C goal <70 mg/dL | ||||
| Any lipid-lowering treatment | 298 | 85 (28.5% [23.4% – 33.6%]) | 177 (59.4% ([53.8% – 65.0%]) | 239 (80.2% [75.7% – 84.7%]) |
| Standard statin monotherapy | 22 | 1 (4.5% [-4.2% – 13.2%]) | 8 (36.4% [16.3% – 56.5%]) | 16 (72.7% [54.1% – 91.3%]) |
| Intensive statin monotherapy | 90 | 19 (21.1% [12.7% – 29.5%]) | 55 (61.1% [51.0% – 71.2%]) | 79 (87.8% [81.0% – 94.6%]) |
| Statin + ezetimibe | 117 | 38 (32.5% [24.0% – 41.0%]) | 73 (62.4% [53.6% – 71.2%]) | 92 (78.6% [71.2% – 86.0%]) |
| Statin + anti-PCSK9 mAb | 13 | 10 (76.9% [54.0% – 99.8%]) | 12 (92.3% [77.8% – 100.0%]) | 13 (100.0% [100.0% – 100.0%]) |
| GROUP III Recent ACS subgroup (n, % [95%CI]); LDL-C goal <70 mg/dL | ||||
| Any lipid-lowering treatment | 5,796 | 2,081 (35.9% [34.7% – 37.1%]) | 4,703 (81.1% [80.1% – 82.1%]) | 5,412 (93.4% [92.8% – 94.0%]) |
| Standard statin monotherapy | 245 | 33 (13.5% [9.2% – 17.8%]) | 141 (57.6% [51.4% – 63.8%]) | 210 (85.7% [81.3% – 90.1%]) |
| Intensive statin monotherapy | 3,794 | 1,308 (34.5% [33.0% – 36.0%]) | 3,115 (82.1% [80.9% – 83.3%]) | 3,584 (94.5% [93.8% – 95.2%]) |
| Statin + ezetimibe | 1,115 | 566 (50.8% [47.9% – 53.7%]) | 1,015 (91.0% [89.3% – 92.7%]) | 1,080 (96.9% [95.9% – 97.9%]) |
| Statin + anti-PCSK9 mAb | 8 | 7 (87.5% [64.6% – 100.0%]) | 7 (87.5% [64.6% – 100.0%]) | 8 (100.0% [100.0% – 100.0%]) |
| GROUP III Complicated DM subgroup (n, % [95%CI]); LDL-C goal <70 mg/dL | ||||
| Any lipid-lowering treatment | 14,065 | 4,025 (28.6% [27.9% – 29.3%]) | 10,275 (73.1% [72.4% – 73.8%]) | 12,557 (89.3% [88.8% – 89.8%]) |
| Standard statin monotherapy | 1,075 | 149 (13.9% [11.8% – 16.0%]) | 577 (53.7% [50.7% – 56.7%]) | 869 (80.8% [78.4% – 83.2%]) |
| Intensive statin monotherapy | 9,265 | 2,695 (29.1% [28.2% – 30.0%]) | 7,141 (77.1% [76.2% – 78.0%]) | 8,551 (92.3% [91.8% – 92.8%]) |
| Statin + ezetimibe | 1,550 | 714 (46.1% [43.6% – 48.6%]) | 1,335 (86.1% [84.4% – 87.8%]) | 1,454 (93.8% [92.6% – 95.0%]) |
| Statin + anti-PCSK9 mAb | 12 | 11 (91.7% [76.1% – 100.0%]) | 12 (100.0% [100.0% – 100.0%]) | 12 (100.0% [100.0% – 100.0%]) |
ACS: acute coronary syndrome; CI: confidence interval; DM: diabetes mellitus; FH: familial hypercholesterolemia; LDL-C: low-density lipoprotein cholesterol.
In general, patients receiving intensive statins at the maximal dose did not perform better than patients receiving intensive statin monotherapy in each group (Table 4).
In this study, we investigated the achievement rates for LDL-C goals defined in JAS GL2017 14) for primary and secondary prevention in patients at high risk for ASCVD in a recent real-world setting based on Japanese insurance claims data subsequent to 2018. We found that these goals were not being met in 34.4%, 39.4%, and 74.6% of patients in the primary prevention high-risk (Group I, LDL-C goal <120 mg/dL), secondary prevention (Group II, LDL-C goal <100 mg/dL), and secondary prevention high-risk (Group III, LDL-C goal <70 mg/dL) settings, respectively.
In all three prevention subgroups, less than half of patients had been prescribed a statin, even though they were eligible for such a treatment according to JAS GL2017 14). Most statin users were prescribed these drugs as intensive statin monotherapy (85%–92%). In all three prevention settings, the proportion of patients achieving the LDL-C goal was higher when an intensive statin in monotherapy was prescribed than that when a standard statin in monotherapy was prescribed. Although the proportion of achievers in the primary prevention high-risk setting (Group I) treated with intensive statin monotherapy and secondary prevention (Group II) could be considered relatively high (83.9% and 75.3%, respectively), this was not the case in the secondary prevention high-risk setting (Group III), of whom only 29.5% achieved their goal. These findings indicate that, although intensive statin monotherapy is the most frequently prescribed LDL-C-lowering therapy in Group III, it is insufficient to achieve LDL-C goals. Nonetheless, very few patients were taking an intensive statin at the maximum dose even in Group III (0.2%), suggesting clinical inertia on the part of prescribing physicians, who do not adapt the statin dose if the LDL-C goal is not achieved.
Apart from statins, ezetimibe was prescribed relatively frequently in Group III (8.5%). Conversely, the prescription of an anti-PCSK9 mAb was very infrequent, even in Group III (0.1%). Given that these treatments have been shown in meta-analyses to provide more robust reductions in LDL-C than statins alone28, 29), as well as benefits in terms of cardiovascular outcomes28, 30, 31), the fact that they are not more widely used in patients failing to meet LDL-C goals on intensive statin monotherapy may represent a loss of treatment opportunity for these patients. In this study, although more patients who were prescribed combination therapy with a statin and either ezetimibe or an anti-PCSK9 mAb (or both) achieved their LDL-C goals than did patients receiving statin monotherapy, the proportion of patients receiving such a combination therapy was <10%, principally in the secondary prevention context. Again, this indicates that treatment intensification is not being offered systematically to patients who fail to achieve their goals.
Preventing the development of cardiovascular morbidity and mortality is a major public health issue, and lowering blood LDL-C is a powerful measure to move in this direction. The findings of this study highlight the fact that the use of intensive LDL-C-lowering drugs was not yet sufficient as it should be in real-world medical practice in Japan. It is thus essential to raise physicians’ awareness of the importance of monitoring LDL-C levels in their patients receiving statin monotherapy regardless of its intensity and the benefit that can be gained by changing the therapy of nonachievers to combination therapy, as recommended in JAS GL2017 14). Additionally, these guidelines should be widely disseminated and understood by prescribers to ensure achievement of LDL-C goals in patients at high risk of ASCVD. A recent physician survey regarding attitudes to treatment of dyslipidemia showed that awareness of goal values for lipid management and of adverse reactions to drug therapy appeared to vary somewhat depending on the physician’s medical specialty, although most physicians recognized elevated LDL-C to be an important risk factor for ASCVD regardless of their speciality32). This study suggests the importance of an educational approach that is tailored to the physician’s specialty.
The present findings can be compared to some extent with those of previous studies in Japan. For example, in a study dating from 2013, also performed in the MDV database, Teramoto et al.19) evaluated lipid-lowering treatment use in patients with high cardiovascular risk. Since then, the proportion of patients receiving a statin has remained relatively stable (57% in secondary prevention for patients with recent ACS in 2013 compared with 54% in this study). However, the proportion of statin monotherapy users prescribed an intensive statin has increased somewhat, from 80% in high-risk primary prevention and 85% in secondary prevention in a previous study based on data collected prior to 2016 25), to 85% and 90%, respectively, in this study. This suggests that prescription of intensive statins rather than standard statins has become more widespread over time because of the dissemination of updated guidelines.
In the Group III, the highest risk group, only 25.4% of patients achieved the LDL-C goal of <70 mg/dL. These results suggest that this group of patients is extremely poorly protected against the risk of ASCVD and recurrence of cardiovascular events. Across all the treatment groups, except for the patients prescribed a combination of a statin and an anti-PCSK9 mAb (which concerned <1% of patients), the proportion of goal achievers was never higher than 50%. More effective lipid-lowering treatments are still needed to protect this group of patients, who are intrinsically at very high risk of ASCVD. In an earlier 2012–2016 study, it has been reported that achievement rates for the LDL-C goal of <70 mg/dL in the context of secondary prevention in high-risk patients classified by JAS GL2017 were 25.6%, 3.8%, 24.9%, and 27.5% for overall, FH, ACS, and complicated diabetes, respectively25). Importantly, the findings of this study indicate that goal achievement rates have improved in FH (26.7%) and recent ACS (31.8%), although they have declined somewhat in complicated DM (24.6%). In a large Japanese cohort of patients with heterozygous or homozygous FH (enrolled from June 2006 to December 2012), followed for 4 years, only 4.8% of patients with secondary prevention of FH achieved the LDL-C goal of <70 mg/dL under treatment24), which is even lower than the rate observed in this study, suggesting that LDL-C control in these patients has improved over time.
The strengths of the study include the size of the population included, the diversity of information recorded in the MDV database, and the use of a standardized coding system for documenting the study variables. Limitations include the fact that the MDV database only covers relatively large acute-care hospitals using the DPC reimbursement payment system, which may compromise the representativeness of the study sample with respect to all patients in Japan at risk for ASCVD. Notably, patients who are managed and prescribed lipid-lowering drugs by their home doctors will not be recorded, and this may lead to imprecision in the estimates of prescription rates, notably for anti-PCSK9 mAbs, which are prescribed principally in hospitals. Additionally, patients in the database do not have a unique patient identifier allowing their care to be tracked between different hospitals, which might lead to underestimation of the use of LDL-C-lowering therapy. Second, the number of hospitals that provide data on laboratory tests is also relatively low (around 10%), which limits the total size of the source population for the study. Third, given the small number of patients with FH without CAD history in primary prevention, these were included in Group I with the other primary prevention patients, even though the treatment target for FH in primary prevention is more stringent than that for others (100 mg/dL vs 120 mg/dL)14). This would not be expected to change the findings significantly. Finally, the data on medication use correspond to medication prescription, and there is no information on whether the medication was actually taken. For this reason, it is not possible to differentiate between nonadherence and treatment ineffectiveness for patients not achieving their LDL-C goals.
In conclusion, this study demonstrates that the proportion of patients who achieved LDL-C goals in Group III (secondary prevention high risk group) remains low after the updates of JAS GL2017, which set stricter LDL-C goals. Moreover, the proportion of patients who are prescribed advanced treatments (maximum dose of an intensive statin or combination with ezetimibe or an anti-PCSK9 mAb) is still low, which suggests the importance of initiating advanced LDL-C-lowering treatment regimens to achieve their LDL-C goals and thus improve their prognosis.
The authors thank Creativ-Ceutical for data acquisition and analysis for preparing this manuscript. The authors also thank Dr. Adam Doble (Foxymed, Paris) for medical writing support.
Hironobu Mitani, Kota Suzuki, and Kazuma Iekushi are employees of Novartis Pharma K.K. Junya Ako has received research funding from Bristol Meyers Squib K.K., Pfizer Japan Inc., Boehringer Ingelheim Co., Ltd., Bayer Yakuhin, Ltd., and Daiichi-Sankyo Co., Ltd., and honoraria from Sanofi K.K., Bristol-Meyers K.K., Pfizer Japan Inc., Boehringer Ingelheim Co., Ltd., Bayer Yakuhin Ltd., and Daiichi-Sankyo Co., Ltd., and Novartis Pharma K.K. Renata Majewska is an employee of Creativ-Ceutical, Cracow, Poland. Salsabil Touzeni in an employee of Creativ-Ceutical, Tunis, Tunisia. Shizuya Yamashita has received honoraria from Kowa Pharmaceutical Co., Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd. and Skylight Biotech. Co., Ltd.