Abstract
Aims: Coronary vasospasm is associated with acute coronary syndrome (ACS) and may persist during primary percutaneous coronary intervention (PCI). We aimed to elucidate the incidence, morphological characteristics, and prognostic impact of residual vasospasm in plaque rupture (PR) and plaque erosion (PE) lesions using optical coherence tomography (OCT).
Methods: We enrolled 142 patients with ACS who underwent OCT-guided primary PCI. All patients received intracoronary vasodilators before OCT examination. Residual vasospasm was identified as intimal gathering and categorised as polygonal- or wavy- patterned depending on the luminal shape. A wavy pattern was defined as a curved intimal surface line. A polygonal pattern was defined as a lumen with multiple angles. The incidence of major cardiovascular events, defined as death, non-fatal myocardial infarction, stroke, and any revascularization, within 1-year of PCI was identified.
Results: The prevalence of residual vasospasm in PR and PE was 15.1% (13 of 86) and 21.4% (12 of 56), respectively. Wavy pattern was the major shape of the residual vasospasm. Polygonal-patterned lumen was more frequently observed in PR than in PE (38.5 vs. 8.3 %). The polygonal-patterned lumens had significantly larger lipid arcs (257.9 vs. 78.0 °; P<0.01), and significantly smaller areas (1.27 vs. 1.88 mm2; P=0.05) than wavy-patterned lumens. Residual vasospasm had a prognostic impact on PR but not PE at 1-year of successful primary PCI.
Conclusion: Considerable proportion of ACS including both PR and PE had residual vasospasm with variable morphological feature and different prognostic impact.
Introduction
Vasospasm plays an important role in the pathogenesis of acute coronary syndrome (ACS)1). Prolonged coronary spasm leads to vessel occlusion and subsequent myocardial ischemia2). However, whether the character of vasospasm differs depending on the aetiology of ACS remains unknown3).
Optical coherence tomography (OCT) is a high-resolution imaging device that provides a maximal axial resolution of 10 µm4). OCT can identify coronary spasm, which is characterised by lumen-deforming intimal bumps and intimal gathering5-7). Several forms of spontaneous coronary artery spasms have been observed8). Furthermore, we often encounter cases where vasospasm persists despite the administration of adequate vasodilators. We have defined these as residual vasospasms, which could be systematically assessed using OCT in patients with ACS undergoing primary percutaneous coronary intervention (PCI).
Aim
We aimed to evaluate the incidence, morphological characteristics, and prognostic impact of residual vasospasm in two dominant aetiologies of ACS: plaque rupture (PR) and plaque erosion (PE).
Methods
Study Participants
A post hoc analysis of a prospective observational study was conducted. Consecutive patients with ACS who underwent OCT-guided primary PCI between April 2018 and March 2022 at Showa University Hospital and who fulfilled the criteria for PR or PE were included (Supplementary Fig.1). Vasodilators such as nitroglycerin were administered into the coronary vessels before OCT. The type and dose of intracoronary vasodilators administered were determined at the discretion of the operator, based on the patient’s hemodynamic status.
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board of Showa University (No. 3045). Informed consent was obtained from all the participants.
OCT Imaging
Following a diagnostic coronary angiography, careful manual thrombectomy with an aspiration catheter or dilatation with a balloon measuring ≤ 2.0 mm was performed if the thrombosis in myocardial infarction (TIMI) flow remained at 0 or 1. OCT images were acquired using a commercially available frequency-domain imaging system (ILUMIEN; Abbott Medical, St. Paul, MN, USA). Under OCT-guidance, a 2.7-Fr catheter (Dragonfly™ OPTIS™; Abbott Medical, St. Paul, MN, USA) was advanced distal to the lesion. Automated pullback was initiated after confirmation of blood clearance of the injected contrast media. All the images were de-identified and digitally stored.
OCT Analysis
All OCT images were analysed using an offline review workstation. The sites selected for analysis were cross-sections with the least lumen area and reference sections proximal and distal to it. The images were qualitatively and quantitatively analysed at 0.2-mm intervals. PR was defined as intimal fibrous cap disruption of the plaque9). PE was identified as the attachment of a thrombus to an intact and visualised plaque or thrombus-attenuated underlying plaque without superficial lipid accumulation or calcification immediately proximal or distal to the thrombus10).
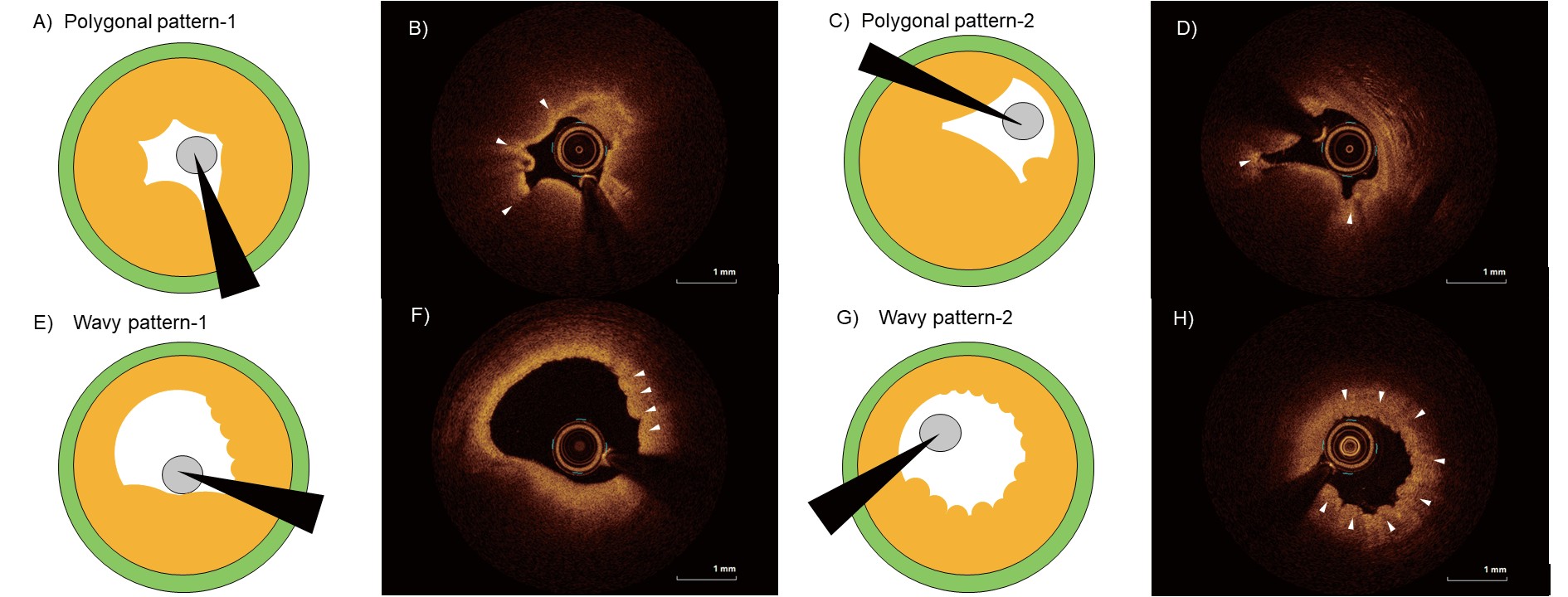
Residual vasospasm was defined as intimal gathering or folding that resulted in multiple kinks in the luminal contour5), despite the administration of vasodilators. A lumen with multiple angles was defined to have a polygonal shape, which was often pentagonal or hexagonal. A wavy pattern was defined as an intimal surface line that was partially or circumferentially curly (Fig.1). The distance from the culprit site at which vasospasm was identified as “-” if it was distal to the vasospasm and “+” if it was proximal to the vasospasm.
Definitions of lumen area, lipid index, thin-cap fibroatheroma (TCFA), cholesterol crystal, calcification index, macrophage accumulation and grade, thrombus, and volume, and the microchannels are shown in Supplementary Material. All OCT images were analysed by two independent investigators (TS and TA) blinded to the angiographic and clinical findings, using an offline review workstation. When discordance in the qualitative analysis of plaque morphology arose between the observers, a consensus was reached with the assistance of a third investigator (HM).
Definition of Clinical Outcomes
Follow-up data were obtained by reviewing the medical records and scripted telephone interviews. Major adverse cardiovascular events (MACE) was defined as cardiac death, non-fatal myocardial infarction, hospitalisation for ischaemic or haemorrhagic stroke, or any coronary revascularization. The prescription of calcium channel blockers and/or nitrates after PCI was left to the discretion of each treating physician, taking into account various factors such as chest symptoms and overall patient condition.
Statistical Analyses
Statistical analyses were performed using JMP (version 16; SAS Institute, Cary, NC, USA). Data are presented as mean±standard deviation, median with interquartile range, or number (percentage) as appropriate. Normality of distribution was determined using the Shapiro–Wilk test. Normally distributed continuous variables were compared using the unpaired Student’s t-test. Non-normally distributed continuous variables were compared using the Mann–Whitney U test or Wilcoxon signed-rank test. Categorical variables were compared using Fisher’s exact test or chi-square test. Inter- and intra-observer reliabilities were assessed using kappa statistics. All statistical analyses were two-tailed with statistical significance set at P<0.05.
Results
A total of 142 patients, including 86 with PR and 56 with PE, were analysed. Residual vasospasm in the culprit lesion was observed in 15.1% (13/86) and 21.4% (12/56) of patients with PR and PE, respectively, even after administration of vasodilators (Fig.2A). The inter- and intra- observer kappa coefficients for residual vasospasm were 0.830 and 0.860, respectively. A wavy pattern was the most common type of residual vasospasm, although there was a dissimilar trend between PR and PE. The polygonal pattern was more likely to occur in PR than in PE (38.4% vs. 8.4%, P=0.06). The representative cases of PR and PE in Fig.2B-K show a polygonal and wavy pattern, respectively, and was accompanied by an angiogram.
Baseline Characteristics and Laboratory Findings
A comparison of the characteristics among patients with and without residual vasospasm in PR and PE are shown in Table 1. Mostly, the patient characteristics did not differ between those with or without residual vasospasm. In PR, initial TIMI grade was better in patients with residual vasospasm than in those without residual vasospasm (TIMI 0: 56.2 vs. 15.4%; P<0.01). The laboratory findings did not differ significantly between those with and without residual spasm (Table 2).
Table 1.Baseline characteristics of the patients
| Characteristics |
PR (N = 86) |
PE (N = 56) |
| Residual vasospasm |
Residual vasospasm |
|
–
(N = 73)
|
+
(N = 13)
|
P-value
|
–
(N = 44)
|
+
(N = 12)
|
P-value
|
| Age, years |
68.4±12.2 |
70.9±12.9 |
0.52 |
64.1±12.9 |
69.4±17.3 |
0.19 |
| Sex, female, % |
17 (23.4%) |
5 (38.5%) |
0.26 |
8 (18.2%) |
4 (33.3%) |
0.27 |
| Coronary risk factors, % |
|
|
|
|
|
|
| Hypertension |
37 (50.7%) |
5 (38.5%) |
0.41 |
26 (59.1%) |
8 (66.7%) |
0.63 |
| Diabetes mellitus |
34 (46.6%) |
6 (46.2%) |
0.98 |
18 (40.9%) |
3 (25.0%) |
0.30 |
| Dyslipidemia |
52 (71.2%) |
9 (69.2%) |
0.88 |
29 (65.9%) |
7 (58.3%) |
0.63 |
| Currently smoking |
23 (31.5%) |
4 (30.8%) |
0.96 |
17 (38.6%) |
2 (16.8%) |
0.14 |
| Medications, % |
|
|
|
|
|
|
| Statin |
12 (16.4%) |
3 (23.1%) |
0.57 |
5 (11.4%) |
2 (16.7%) |
0.63 |
| Ca-channel blockers |
18 (24.7%) |
3 (23.1%) |
0.90 |
10 (22.7%) |
4 (33.3%) |
0.46 |
| Β-blockers |
4 (5.5%) |
2 (15.4%) |
0.25 |
3 (6.8%) |
2 (16.7%) |
0.32 |
| ACEI/ ARB |
21 (28.8%) |
2 (15.4%) |
0.29 |
10 (22.7%) |
3 (25.0%) |
0.87 |
| Clinical presentation, % |
|
|
0.27 |
|
|
0.81 |
| STEMI |
51 (69.9%) |
7 (53.9%) |
|
24 (54.6%) |
7 (58.3%) |
|
| NSTE-ACS |
22 (30.1%) |
6 (46.2%) |
|
20 (45.5%) |
4 (41.7%) |
|
| Peak CK, U/L*
|
1282 (345-2990) |
1432 (517-2151) |
0.91 |
695 (166-2634) |
1035 (145-2506) |
0.99 |
| Vessel involved |
|
|
0.50 |
|
|
0.76 |
| LAD |
43 (58.9%) |
6 (46.2%) |
|
32 (72.7%) |
8 (66.7%) |
|
| LCX |
8 (11.0%) |
3 (23.1%) |
|
5 (11.4%) |
1 (8.3%) |
|
| RCA |
22 (30.1%) |
4 (30.8%) |
|
7 (15.9%) |
3 (25.0%) |
|
| Baseline TIMI grade |
|
|
<0.01 |
|
|
0.39 |
| 0 |
41 (56.2%) |
2 (15.4%) |
|
21 (47.7%) |
6 (50.0%) |
|
| 1 |
5 (6.9%) |
1 (7.7%) |
|
1 (2.3%) |
0 (0.0%) |
|
| 2 |
9 (12.3%) |
7 (53.9%) |
|
5 (11.4%) |
4 (33.3%) |
|
| 3 |
18 (24.7%) |
3 (23.1%) |
|
17 (38.6%) |
2 (16.7%) |
|
| Multi-vessel disease, %†
|
40 (54.8%) |
9 (69.2%) |
0.33 |
24 (54.6%) |
9 (75.0%) |
0.19 |
| Culprit lesion PCI variables |
|
|
|
|
|
0.30 |
| DES, % |
72 (98.6%) |
13 (100%) |
|
42 (95.5%) |
11 (91.7%) |
|
| DCB, % |
1 (1.4%) |
0 (0.0%) |
|
2 (4.6%) |
0 (0.0%) |
|
Data are expressed as the mean±standard deviation, median (25% and 75% quartiles), or number (%). Abbreviations:
PR, plaque rupture; PE, plaque erosion; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; STEMI, ST-segment elevation myocardial infarction; NSTE-ACS, non-ST-segment elevation acute coronary syndrome; CK, creatine kinase; LAD, left anterior descending coronary artery; LCX, left circumflex artery; RCA, right coronary artery; TIMI, thrombosis in myocardial infarction; DES, drug-eluting stent; DCB, drug-coated balloon.
*Peak creatine kinase (CK) levels were evaluated every 6 h after symptom onset for the first two days.
†Multivessel disease was defined as luminal stenosis of at least 70% in at least two major coronary arteries or in one coronary artery in addition to a 50% or greater stenosis of the left main trunk.
Table 2.Laboratory findings of the patients
| Parameters |
PR (N = 86) |
PE (N = 56) |
| Residual vasospasm |
Residual vasospasm |
|
–
(N = 73)
|
+
(N = 13)
|
P value
|
–
(N = 44)
|
+
(N = 12)
|
P value
|
| TG, mg/dL |
122.0 (84.0-200.5) |
119.0 (72.5-175.0) |
0.40 |
120.5 (81.0-196.8) |
92.0 (42.0-177.0) |
0.16 |
| LDL-C, mg/dL |
119.0 (101.5-143.0) |
122.0 (112.0-156.5) |
0.40 |
111.0 (92.3-140.3) |
122.0 (104.3-139.0) |
0.60 |
| HDL-C, mg/dL |
45.0 (37.0-54.0) |
48.0 (41.0-65.5) |
0.13 |
45.5 (39.3-56.0) |
43.5 (38.5-48.8) |
0.48 |
| Non-HDL-C, mg/dL |
147.0 (122.5-176.0) |
148.0 (138.0-175.5) |
0.66 |
143.0 (117.0-173.8) |
143.5 (121.3-174.8) |
0.92 |
| HbA1c, % |
6.1 (5.7-7.1) |
6.2 (5.8-7.0) |
0.98 |
6.1 (5.7-6.7) |
5.9 (5.4-6.6) |
0.55 |
| Hs-CRP, mg/L |
2.39 (0.90-4.73) |
2.50 (0.66-7.4) |
0.91 |
1.57 (0.48-6.59) |
1.10 (0.49-6.99) |
0.79 |
| eGFR, mL/min/1.73 m2
|
73.7 (54.6-84.3) |
56.4 (54.0-70.6) |
0.08 |
71.3 (58.8-84.5) |
69.8 (53.2-80.9) |
0.69 |
| NT-proBNP, pg/mL |
263 (60-1070) |
438 (75-2082) |
0.28 |
332 (113-1660) |
757 (106-1506) |
0.83 |
Data are expressed as the median (25% and 75% quartiles) and gm/dL, unless specified otherwise. Abbreviations: PR, plaque rupture; PE, plaque erosion; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; Non-HDL-C, non-high- density lipoprotein cholesterol; HbA1c, glycated haemoglobin; Hs-CRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-brain natriuretic peptide.
Table 3 shows the OCT findings at the level of the lesion. Thrombi were significant smaller in PR with residual vasospasm than in PR without residual vasospasm (0.22 mm3 vs. 1.22; P=0.01). The macrophage grade was greater in PE with residual vasospasm than in PE without residual vasospasm (P=0.05). The prevalence of TCFA, cholesterol crystals, calcified plaques, and macrophage accumulation was comparable between patients with and without residual vasospasm in both aetiologies.
Table 3.OCT findings of the patients
| Parameters |
PR (N = 86) |
PE (N = 56) |
| Residual vasospasm |
Residual vasospasm |
|
–
(N = 73)
|
+
(N = 13)
|
P value
|
–
(N = 44)
|
+
(N = 12)
|
P value
|
| Thin-cap fibroatheroma, % |
56 (76.7%) |
11 (84.6%) |
0.51 |
10 (22.7%) |
4 (33.3%) |
0.46 |
| Cholesterol crystals, % |
46 (63.0%) |
9 (69.2%) |
0.66 |
20 (45.5%) |
3 (25.0%) |
0.28 |
| Calcified plaque, % |
40 (54.8%) |
8 (61.5%) |
0.65 |
19 (43.2%) |
8 (66.7%) |
0.15 |
| Thrombus volume, mm3
|
1.22 (0.26-3.38) |
0.22 (0.08-0.42) |
<0.01 |
0.56 (0.04-2.13) |
0.16 (0.03-0.97) |
0.35 |
| Macrophage accumulation, % |
73 (100.0%) |
12 (92.3%) |
0.15 |
38 (86.4%) |
12 (100.0%) |
0.22 |
| Macrophage grades, % |
|
|
0.27 |
|
|
0.05 |
| Grade I |
0 (0.0%) |
1 (7.1%) |
|
8 (18.2%) |
1 (7.7%) |
|
| Grade II |
14 (19.2%) |
3 (23.1%) |
|
12 (27.3%) |
2 (16.7%) |
|
| Grade III |
31 (42.5%) |
6 (46.2%) |
|
10 (22.7%) |
8 (66.7%) |
|
| Grade IV |
28 (38.4%) |
3 (23.1%) |
|
8 (18.2%) |
1 (8.3%) |
|
| Microchannel, % |
34 (46.6%) |
6 (46.2%) |
0.98 |
19 (43.2%) |
7 (58.3%) |
0.35 |
| Minimum lumen area, mm2
|
0.96 (0.82-1.26) |
1.01 (0.79-1.82) |
0.51 |
0.98 (0.72-1.69) |
0.83 (0.67-1.82) |
0.53 |
| Mean reference lumen area, mm2
|
7.64 (5.57-9.90) |
8.11 (5.21-9.20) |
0.87 |
6.74 (4.85-9.33) |
6.11 (4.46-7.56) |
0.60 |
| Percentage of stenosis, % |
85.8 (80.6-90.1) |
82.2 (79.7-87.0) |
0.24 |
85.4 (75.5-90.1) |
83.6 (74.7-88.4) |
0.60 |
| Lesion length, mm |
23.4 (18.2-29.1) |
26.2 (22.2-30.7) |
0.30 |
22.2 (18.6-29.0) |
23.1 (17.3-27.4) |
0.87 |
| Lipid index |
4263 (2550-5379) |
4924 (1858-6730) |
0.90 |
2555 (1106-4657) |
2255 (1106-4000) |
0.56 |
| Calcification index |
31 (0-254) |
150 (0-539) |
0.31 |
0 (0-168) |
73 (0-344) |
0.20 |
Data are expressed as number (%) or median (25% and 75% quartiles). Abbreviations: PR, plaque rupture; PE, plaque erosion.
Fig.3 shows the comparison of the OCT findings between the polygonal and wavy patterns at the site of the vasospasm. Polygonal lumens had larger maximum lipid arcs (257.9 vs. 78.0 °; P<0.01) and smaller areas (1.27 vs. 1.88 mm2; P=0.05) than wavy lumens did. The longitudinal length of the residual vasospasm was comparable between the two patterns (2.0 vs. 2.8 mm, polygonal vs. wavy pattern, P=0.26). A polygonal pattern was commonly observed at the site of vasospasm, while a wavy pattern was observed distal to it (+0.3 vs. -7.0 mm, P=0.03).
Clinical Outcomes
MACE had occurred in 7.7% of all patients by the one-year (322±96 days) follow-up. In patients with PR, those with residual vasospasm had an increased risk of MACE than those without residual vasospasm (log-rank 3.692, P=0.05; Fig.4A). In contrast, in patients with PE, the frequency of MACE was comparable among those with and without residual spasm (log-rank 0.774, P=0.39; Fig.4B). Supplementary Table 1 shows that in both PR and PE, there were no statistically significant differences in various types of MACE based on the presence or absence of residual vasospasm. However, within the PR, although not statistically significant, there was a somewhat higher number of cardiac deaths in the residual vasospasm group. There were no significant differences in MACE with or without calcium channel blocker use prior to the onset of ACS in both PR and PE (9.5% vs. 9.2%, p=0.97; 7.1% vs. 4.8%, p=0.74, respectively). The prescription of calcium channel blockers and/or nitrates after PCI was given to 12 out of the 25 patients who exhibited residual vasospasm. Of the 12 patients prescribed these medications, 3 experienced MACE, while none of the 13 patients who were not prescribed these medications experienced MACE.

Supplementary Table 1.Comparison of cardiovascular events between the PR and PE with/without residual vasospasm
| Characteristics |
PR (N = 86) |
PE (N = 56) |
| Residual vasospasm |
Residual vasospasm |
|
–
(N = 73)
|
+
(N = 13)
|
P-value
|
–
(N = 44)
|
+
(N = 12)
|
P-value
|
|
|
|
0.40 |
|
|
Not detectable |
| Cardiac death |
1 (1.4%) |
2 (15.4%) |
|
1 (2.3%) |
0 (0.0%) |
|
| Non-fatal MI |
1 (1.4%) |
0 (0.0%) |
|
0 (0.0%) |
0 (0.0%) |
|
| Stroke |
1 (1.4%) |
0 (0.0%) |
|
1 (2.3%) |
0 (0.0%) |
|
| Any revascularization |
2 (2.8%) |
1 (7.7%) |
|
1 (2.3%) |
0 (0.0%) |
|
Abbreviations: PR, plaque rupture; PE, plaque erosion; MI, myocardial infarction.
Discussion
The main findings of this study are summarised as follows: 1) the prevalence of residual vasospasm in PR and PE were 15.1% and 21.4%, respectively; 2) the wavy pattern was the major form of residual vasospasm, and polygonal patterns were observed more frequently in PR than in PE; 3) polygonal lumens had larger maximum lipid arcs and smaller areas than wavy lumens; and 4) residual vasospasm had a prognostic impact on PR but not on PE.
Systemic assessment of residual vasospasm using OCT may be challenging. However, the detailed conformational changes in the coronary artery structure was described using OCT in one study on patients with coronary artery spasm5). They assessed 19 coronary arteries (10 spastic and 9 non-spastic lesions) using OCT during spasm provocation tests and found that spastic lesions frequently show an intimal bump even at the baseline5). The OCT images of the baseline lesion were similar to that of the wavy-patterned residual spasm in our study. The OCT images of the lesion during spasm were similar to that of the polygonal-patterned residual spasm in our study. Therefore, we believe that the systemic assessment of residual vasospasm using OCT is not inappropriate. Polygonal-patterned spasms had more severe luminal narrowing than wavy-patterned spasms in our study. The wavy pattern might be a relatively mild vasospasm, whereas the polygonal pattern might be a severe spasm.
Residual vasospasm can be explained by several factors. We speculated that spontaneous vasospasm may be severe enough to persist even after the administration of a vasodilator. Alternatively, the vasodilator may not reach the lesions because of flow limitation due to ACS itself or haemodynamic instability. Furthermore, PCI itself may also induce local vasospasm8). The significantly greater lipid arc in the polygonal-patterned spasm than in the wavy-patterned spasm cannot be explained. Advanced lipid plaques often show disruption of the media, resulting in its thinning3). In such cases, vasospasm of the smooth muscle of the deeper media might occur, leading to intimal gathering. Alternatively, polygonal-patterned spasms may gather foamy macrophages, which may increase the lipid arc. In contrast, smooth muscle cells are generally rich in the intimal layer of PE lesions, which might induce superficial vasospasm.
The prevalence of coronary artery spasm is reportedly 45–73%11, 12). Vasospasm is reportedly a contributing factor to ACS pathophysiology. However, its precise mechanism remains unelucidated2). Some studies and pathologists have suggested that PE is often observed in patients with coronary artery spasm3, 6, 7). In our study, residual vasospasm was observed in both PE and PR, with a slightly greater prevalence in PE. As the aetiology of ACS is explained by PR (two-thirds) and PE (one-third), vasospasm is expected to involve both. Our findings of lower thrombus volumes in PR and PE lesions with residual vasospasm indicate that vasospasm might have an additional impact on their thrombogenicity. The mechanism by which vasospasm affects PR or PE may not always be similar; PE is generally thought to be highly affected by vasospasm3, 7). We further compared PE lesions with and without persistent spasm. Macrophage grade based on OCT images is an assessment of foamy macrophages; this differed between PE lesion with and without residual vasospasm in our study. Vasospasm was more severe in PE lesions with more foamy macrophages.
The prognostic impact of vasospasm in patients with ACS is controversial. In a study of 240 patients with ACS, the incidence of MACE (death, ACS, or revascularization) was 47.1% (82 of 174) in the positive group and 27.3% (18 of 66) in the negative group (p<0.01) during a mean follow-up period of 43 months11). In contrast, no such association was detected in another study of 437 patients with ACS who underwent a provocation test12). There is a fundamental difference between previously conducted studies and our study; our findings were based on residual vasospasm detected on OCT and not on a provoked test. Even then, the prognostic impact of residual vasospasm on PR and PE differed. This could be explained by the fact that coronary spasm itself could affect lesions; organic stenosis at the provoked spasm site reportedly leads to the rapid progression of atherosclerosis13, 14). In our study, cardiac death was predominantly occurred in patients with PR and residual vasospasm, particularly in the early stage. The haemodynamic status at onset, such as physiologically enhanced vasoconstriction for maintenance of coronary blood flow, might have influenced the spasm.
Calcium channel blockers which inhibit calcium inflow into smooth muscle cells and stimulate nitric oxide production, leading to vasodilation, are generally recommended as first-line treatment for coronary artery spasm15). Deyama et al. reported that calcium channel blocker use is associated with better clinical outcomes in ACS survivors with inducible coronary artery spasms (log-rank test, P=0.001)12). As our study was performed retrospectively, calcium channel blockers may have been infrequently used as the status of residual vasospasm was unknown. Thus, the benefits of administration of calcium channel blockers to patients with residual spasm remains a question. OCT could help us recognise not only the ACS aetiology but also residual vasospasm, which can be beneficial in optimising medical therapy.
Limitations
This study has some limitations. First, this was a single-centre study with a relatively small sample size. Especially given the limited number of patients taking calcium channel blockers and/or nitrates after PCI and the small number of events, we cannot conclusively determine their effectiveness for residual vasospasm, and believe that further investigation is required. Second, we did not evaluate vasospasm before vasodilator administration. These may have revealed morphological differences and changes in vasospasm frequency. In particular, spasm-induced medial contraction reportedly causes an increase in medial thickness5). Third, the type and dose of intracoronary administration of vasodilators were left to the discretion of the operator based on the patient’s haemodynamic status, which may result in inconsistencies. Fourth, thrombectomy may have modified the morphology of the spastic lesion. Furthermore, residual thrombi may have affected the analysis of plaque characteristics. Fifth, we did not categorize PE based on the underlying plaque. Studies using OCT have shown that PE can be further categorized based on the underlying plaque into PE with lipid-rich and fibrous plaque16, 17). The presence of lipid-rich plaque has been suggested to potentially relate to the prognosis of PE16, 18). The lack of a prognostic difference in PE with or without vasospasm might be due to the influence of the underlying plaque. Sixth, wire manipulation to recanalize the occluded lesion may provoke vasospasm. Seventh, residual vasospasm might be considered as a direct cause of PR or PE, possibly related to the PCI procedure itself, or potentially occurring as a compensatory response to hemodynamic instability. However, any interpretation remains speculative. Finally, only patients who underwent OCT-guided PCI were included; therefore, a selection bias might have occurred. OCT-guided PCI was attempted in majority of the patients with ACS. However, it was not performed it in patients with renal dysfunction, severe heart failure, or ostial lesions. Thus, our findings may not be applicable to patients who are not good candidates for OCT examinations.
Conclusion
Residual vasospasm was not rare in PR and PE with variable morphological features. The prognostic impact of residual vasospasm differed between PR and PE.
Conflict of Interest
Shinji Koba received research funding from Denka Seiken Co. Ltd. Toshiro Shinke received research funding from Abbott Medical. The other authors have no conflicts of interest to declare.
Financial Support
The funding agencies had no role in the preparation of the manuscript.
Author Contributions
TS and HM contributed to the study design, preparation of the manuscript, data analysis, data interpretation, and critical revision of the manuscript. TA, NM, RS, YY, SS, HT (Hideaki Tanaka), and RM contributed to data acquisition and analysis. YO, KO, KA, KN, KS, HT (Hiroaki Tsujita), SK (Seita Kondo), and ST contributed to the data acquisition. HM contributed to the data analysis and interpretation. SK (Shinji Koba), HS, and TS (Toshiro Shinke) contributed to the data interpretation, critical revision, and manuscript editing.
Acknowledgments
We are grateful for the valuable help of the nursing staff of the catheterisation laboratory and all cardiologists at Showa University Hospital’s Department of Cardiology. We are also grateful for the technical assistance provided by Motoko Ohta, Yasuki Itoh, Kei Iwadate, and Maki Namatame. We would like to thank Editage (www.editage.com) for English language editing.
Supplemental Methods
OCT Analysis
The proximal and distal references were defined as the sites with the largest lumen diameter within 10 mm proximally and distally to the regions with the smallest lumen area and before any side branch1). The minimum lumen area (MLA) was measured at the site with the smallest lumen area in the culprit lesion, whereas the reference lumen area was measured at the reference cross-section. Area stenosis was calculated as follows: [(mean reference lumen area−minimum lumen area)÷mean reference lumen area] ×100. Lesion length was defined as the region around the MLA where the lumen area was <50% of the largest reference lumen area2). Plaque rupture was defined as a plaque with intimal tearing, disruption, or dissection of the cap3). TCFA was defined as a lipid-rich plaque (>90°) overlaid with a thin fibrous cap (<65 µm)4). An intracoronary thrombus was defined as a mass (diameter ≥ 250 µm) attached to the luminal surface or floating within the lumen with high backscattering and high attenuation ([red blood cell-rich] thrombus) or that had lower backscattering and low attenuation and was homogeneous (white [platelet-rich] thrombus)5). The thrombus volume was calculated by multiplying the thrombus areas in each frame by the number of frames (0.2 mm)6). The lipidic plaque was defined as a diffusely bordered, signal-poor region lipid pools7). The lipid arc was measured at 0.2-mm intervals throughout the length of each lesion, and the values were averaged. The length of the lipid was measured longitudinally. The lipid index was defined as the mean lipid arc multiplied by the lipid length8). Calcified plaque was identified as an area with a low backscattering signal and a sharp border3). The calcification arc was measured at 0.2-mm intervals throughout the length of each lesion, and the values were averaged. The length of the calcification was also measured longitudinally. As with lipid index, the calcification index was defined as the mean calcification arc multiplied by the calcification length. Cholesterol crystal was defined as a thin, linear region of high-intensity3, 9). Macrophage accumulation was defined as a high-intensity signal-rich linear region with sharp attenuation. Macrophage signals were semi-quantitatively graded according to previous reports4, 7): grade 0, no macrophages; grade 1, localized macrophage accumulation; grade 2, clustered accumulation in <1 quadrant; grade 3, clustered accumulation in >1 quadrant but in <3 quadrants; and grade 4, clustered accumulation in >3 quadrants. To distinguish between grades 1 and 2, the degree of macrophage extension was defined as 30°. Microchannels were defined as small vesicular or tubular structures with diameters of 50–300 µm and differentiated from any other branch10).
References
1)Nagasawa Y, Shinke T, Toh R, Ishida T, Otake H, Takaya T, Sugiyama D, Toba T, Kuroda M, Takahashi H, Terashita D, Tahara N, Shinkura Y, Uzu K, Kashiwagi D, Kuroda K, Nagano Y, Yamamoto H, Yanaka K, Tsukiyama Y and Hirata KI: The impact of serum trans fatty acids concentration on plaque vulnerability in patients with coronary artery disease: Assessment via optical coherence tomography. Atherosclerosis, 2017; 265: 312-317
2)Gonzalo N, Escaned J, Alfonso F, Nolte C, Rodriguez V, Jimenez-Quevedo P, Banuelos C, Fernandez-Ortiz A, Garcia E, Hernandez-Antolin R and Macaya C: Morphometric assessment of coronary stenosis relevance with optical coherence tomography: a comparison with fractional flow reserve and intravascular ultrasound. J Am Coll Cardiol, 2012; 59: 1080-1089
3)Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S, Costa MA, de Silva R, Dijkstra J, Di Mario C, Dudek D, Falk E, Feldman MD, Fitzgerald P, Garcia-Garcia HM, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CC, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel MA, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Raber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PW, Shimada K, Shinke T, Shite J, Siegel E, Sonoda S, Suter M, Takarada S, Tanaka A, Terashima M, Thim T, Uemura S, Ughi GJ, van Beusekom HM, van der Steen AF, van Es GA, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G and International Working Group for Intravascular Optical Coherence T: Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol, 2012; 59: 1058-1072
4)Kume T, Okura H, Yamada R, Kawamoto T, Watanabe N, Neishi Y, Sadahira Y, Akasaka T and Yoshida K: Frequency and spatial distribution of thin-cap fibroatheroma assessed by 3-vessel intravascular ultrasound and optical coherence tomography: an ex vivo validation and an initial in vivo feasibility study. Circ J, 2009; 73: 1086-1091
5)Kume T, Akasaka T, Kawamoto T, Ogasawara Y, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y and Yoshida K: Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol, 2006; 97: 1713-1717
6)Higuma T, Soeda T, Yamada M, Yokota T, Yokoyama H, Izumiyama K, Nishizaki F, Minami Y, Xing L, Yamamoto E, Lee H, Okumura K and Jang IK: Does Residual Thrombus After Aspiration Thrombectomy Affect the Outcome of Primary PCI in Patients With ST-Segment Elevation Myocardial Infarction?: An Optical Coherence Tomography Study. JACC Cardiovasc Interv, 2016; 9: 2002-2011
7)Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Kang DH, Halpern EF and Tearney GJ: Characterization of human atherosclerosis by optical coherence tomography. Circulation, 2002; 106: 1640-1645
8)Kato K, Yonetsu T, Kim SJ, Xing L, Lee H, McNulty I, Yeh RW, Sakhuja R, Zhang S, Uemura S, Yu B, Mizuno K and Jang IK: Comparison of nonculprit coronary plaque characteristics between patients with and without diabetes: a 3-vessel optical coherence tomography study. JACC Cardiovasc Interv, 2012; 5: 1150-1158
9)Janoudi A, Shamoun FE, Kalavakunta JK and Abela GS: Cholesterol crystal induced arterial inflammation and destabilization of atherosclerotic plaque. Eur Heart J, 2016; 37: 1959-1967
10)Uemura S, Ishigami K, Soeda T, Okayama S, Sung JH, Nakagawa H, Somekawa S, Takeda Y, Kawata H, Horii M and Saito Y: Thin-cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur Heart J, 2012; 33: 78-85
References
- 1) Maseri A, Severi S, Nes MD, L’Abbate A, Chierchia S, Marzilli M, Ballestra AM, Parodi O, Biagini A and Distante A: “Variant” angina: one aspect of a continuous spectrum of vasospastic myocardial ischemia. Pathogenetic mechanisms, estimated incidence and clinical and coronary arteriographic findings in 138 patients. Am J Cardiol, 1978; 42: 1019-1035
- 2) Yasue H, Nakagawa H, Itoh T, Harada E and Mizuno Y: Coronary artery spasm--clinical features, diagnosis, pathogenesis, and treatment. J Cardiol, 2008; 51: 2-17
- 3) Virmani R, Kolodgie FD, Burke AP, Farb A and Schwartz SM: Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol, 2000; 20: 1262-1275
- 4) Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, Choi KB, Shishkov M, Schlendorf K, Pomerantsev E, Houser SL, Aretz HT and Tearney GJ: Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol, 2002; 39: 604-609
- 5) Tanaka A, Shimada K, Tearney GJ, Kitabata H, Taguchi H, Fukuda S, Kashiwagi M, Kubo T, Takarada S, Hirata K, Mizukoshi M, Yoshikawa J, Bouma BE and Akasaka T: Conformational change in coronary artery structure assessed by optical coherence tomography in patients with vasospastic angina. J Am Coll Cardiol, 2011; 58: 1608-1613
- 6) Shin ES, Ann SH, Singh GB, Lim KH, Yoon HJ, Hur SH, Her AY, Koo BK and Akasaka T: OCT-Defined Morphological Characteristics of Coronary Artery Spasm Sites in Vasospastic Angina. JACC Cardiovasc Imaging, 2015; 8: 1059-1067
- 7) Shin ES, Her AY, Ann SH, Balbir Singh G, Cho H, Jung EC, Shim EB, Koo BK and Akasaka T: Thrombus and Plaque Erosion Characterized by Optical Coherence Tomography in Patients With Vasospastic Angina. Rev Esp Cardiol (Engl Ed), 2017; 70: 459-466
- 8) Takei Y, Mori H, Sone H, Tashiro K, Sasai M, Sato T and Suzuki H: Polygon-like luminal configuration captured by intracoronary optical frequency domain imaging of a spontaneous coronary spasm in an ST-elevation myocardial infarction patient. Coron Artery Dis, 2019; 30: 314-315
- 9) Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S, Costa MA, de Silva R, Dijkstra J, Di Mario C, Dudek D, Falk E, Feldman MD, Fitzgerald P, Garcia-Garcia HM, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CC, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel MA, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Raber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PW, Shimada K, Shinke T, Shite J, Siegel E, Sonoda S, Suter M, Takarada S, Tanaka A, Terashima M, Thim T, Uemura S, Ughi GJ, van Beusekom HM, van der Steen AF, van Es GA, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G and International Working Group for Intravascular Optical Coherence T: Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol, 2012; 59: 1058-1072
- 10) Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, Tian J, Lee H, Park SJ, Jang YS, Raffel OC, Mizuno K, Uemura S, Itoh T, Kakuta T, Choi SY, Dauerman HL, Prasad A, Toma C, McNulty I, Zhang S, Yu B, Fuster V, Narula J, Virmani R and Jang IK: In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol, 2013; 62: 1748-1758
- 11) Wakabayashi K, Suzuki H, Honda Y, Wakatsuki D, Kawachi K, Ota K, Koba S, Shimizu N, Asano F, Sato T and Takeyama Y: Provoked coronary spasm predicts adverse outcome in patients with acute myocardial infarction: a novel predictor of prognosis after acute myocardial infarction. J Am Coll Cardiol, 2008; 52: 518-522
- 12) Deyama J, Nakamura T, Saito Y, Obata JE, Fujioka D, Nakamura K, Watanabe K and Kugiyama K: Effect of coronary artery spasm on long-term outcomes in survivors of acute myocardial infarction. Int J Cardiol, 2018; 257: 7-11
- 13) Ishii M, Kaikita K, Sato K, Tanaka T, Sugamura K, Sakamoto K, Izumiya Y, Yamamoto E, Tsujita K, Yamamuro M, Kojima S, Soejima H, Hokimoto S, Matsui K and Ogawa H: Acetylcholine-Provoked Coronary Spasm at Site of Significant Organic Stenosis Predicts Poor Prognosis in Patients With Coronary Vasospastic Angina. J Am Coll Cardiol, 2015; 66: 1105-1115
- 14) Suzuki H, Kawai S, Aizawa T, Kato K, Sunayama S, Okada R and Yamaguchi H: Histological evaluation of coronary plaque in patients with variant angina: relationship between vasospasm and neointimal hyperplasia in primary coronary lesions. J Am Coll Cardiol, 1999; 33: 198-205
- 15) Brozovich FV, Nicholson CJ, Degen CV, Gao YZ, Aggarwal M and Morgan KG: Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol Rev, 2016; 68: 476-532
- 16) Hoshino M, Yonetsu T, Usui E, Kanaji Y, Ohya H, Sumino Y, Yamaguchi M, Hada M, Hamaya R, Kanno Y, Murai T, Lee T and Kakuta T: Clinical Significance of the Presence or Absence of Lipid-Rich Plaque Underneath Intact Fibrous Cap Plaque in Acute Coronary Syndrome. J Am Heart Assoc, 2019; 8: e011820
- 17) Sekimoto T, Mori H, Koba S, Arai T, Matsukawa N, Sakai R, Yokota Y, Sato S, Tanaka H, Masaki R, Oishi Y, Ogura K, Arai K, Nomura K, Sakai K, Tsujita H, Kondo S, Tsukamoto S, Suzuki H and Shinke T: Clinical features and lipid profiles of plaque erosion over lipid-rich plaque versus fibrous plaque in patients with acute coronary syndrome. Atherosclerosis, 2022; 360: 47-52
- 18) Li J, Chen R, Zhou J, Wang Y, Zhao X, Liu C, Zhou P, Chen Y, Song L, Yan S, Yan H and Zhao H: Lipid Content Distribution and its Clinical Implication in Patients with Acute Myocardial Infarction-Plaque Erosion: Results from the Prospective OCTAMI Study. J Atheroscler Thromb, 2024; 31: 23-35






