2024 年 31 巻 8 号 p. 1113-1128
2024 年 31 巻 8 号 p. 1113-1128
In 2022, the Japan Atherosclerosis Society (JAS) updated its prevention guidelines, the “Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2022” (JAS2022GL), expanding its scope from coronary artery disease (CAD) to atherosclerotic cardiovascular diseases (ASCVDs), including atherothrombotic stroke. The following year, the Japanese Circulation Society (JCS) updated its guidelines for primary prevention entitled “JCS 2023 Guideline on the Primary Prevention of Coronary Artery Disease” (JCS2023GL). Since those publications, scientific advancements in relevant fields have continued. This review article outlines the current recommendations provided by the guidelines, provides background information supporting these recommendations, introduces scientific findings subsequent to prior publications, and discusses future directions on select topics for the primary prevention of CVD. The topics covered in this review are traditional risk factors, including dyslipidemia and hypertension, the application of comprehensive risk stratification or risk scoring systems, patient-specific topics, salt and alcohol, and environmental factors. These topics were deliberate and selected by the authors, who were involved in the compilation of either or both JAS2022GL and JCS2023GL. This review not only emphasizes the pivotal role of continuously updated guidelines in shaping clinical practice but also stresses the urgent need for ongoing research to bridge existing knowledge and practice gaps.
In 2022, the Japan Atherosclerosis Society (JAS) updated its prevention guidelines, the “Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2022” (JAS2022GL)1), expanding its scope from coronary artery disease (CAD) to atherosclerotic cardiovascular diseases (ASCVDs), including atherothrombotic stroke. In 2023, the Japanese Circulation Society (JCS) updated its guidelines for primary prevention entitled “JCS 2023 Guideline on the Primary Prevention of Coronary Artery Disease” (JCS2023GL)2).
In this review, we succinctly outline the current recommendations provided by these guidelines, provide background information supporting these recommendations, introduce scientific findings subsequent to prior publications, and discuss future directions on selected topics for the primary prevention of CVD. The topics covered in the articles were traditional risk factors, the application of comprehensive risk stratification or risk scoring systems, patient-specific topics (hormone replacement therapy for women and older individuals), salt and alcohol, and environmental factors (heat and air pollution). The selection of the topics was deliberate and made by the authors (AH, SK, AF), who were involved in the compilation of either or both JAS2022GL and JCS2023GL. As dyslipidemia is a key risk factor for CAD, we devoted much space to it.
It is important to note that this article was not intended to serve as a comprehensive summary of either JAS2022GL or JCS2023GL but rather as a complementary piece. Its purpose is to help readers gain an in-depth understanding and updated evidence on the selected topics relevant to the primary prevention of CVD.
The epidemiology of traditional CAD risk factors in Japan is delineated in the initial section of JCS2023GL. Of the numerous risk factors examined, high cholesterol is a well-documented and significant factor contributing to CAD. TC serves as an appropriate metric for evaluating long-term temporal trends in lipid profiles at the population level in Japan.
Since 1980, TC has been measured using a standardized method in Japanese national surveys. A series of national surveys showed a significant increase in mean serum TC levels from 1980 to 1990 in the Japanese population, which then plateaued for both sexes after 1990 3) (Fig.1).

(a) Men. (b) Women. Modified from Int J Epidemiol. 2015; 44: 1614-1624.
However, these statistics did not account for medication use. Given the increased use of efficacious cholesterol-lowering agents, such as statins, since 1989, it is plausible that medications have masked the increase in mean TC levels since the era of statin use3). In contrast, some developed countries have experienced a consistent decline in TC levels during the same period4) (Fig.1). According to a study by Sekikawa et al., the mean TC levels in individuals 50–69 years old were higher in Japan than in the USA for both sexes. Furthermore, TC levels 20 years earlier (i.e. in individuals 30–49 years old in 1990) were similar between Japan and the USA for both sexes4).
•Discussion of the ‘Japanese Paradox’The temporal trends of traditional risk factors, except for cholesterol levels, were broadly similar in both direction and magnitude between Japan and Western countries. Accordingly, age-adjusted CAD mortality declined in both regions between 1980–83 and 2004–07. This prompts the question of why the age-adjusted CAD mortality declined despite an increase in the TC level in the Japanese population, a puzzling observation sometimes called “the Japanese paradox”5). A possible reason could be the advancement of medical and surgical treatments for CAD over the years, coupled with improvements in risk factors other than TC within the population5). A modeling study of the Japanese general population 35–84 years old by Ogata et al. showed a 61% decrease in age-adjusted CAD mortality rates from 1980 to 2012 6). Approximately 56% of this decrease was attributable to medical and surgical treatments due to universal health coverage, and 35% was attributable to changes in risk factors, including decreased systolic blood pressure (BP; 8.87 mmHg) accounting for 25%, and decreased smoking prevalence (14.0%) contributing 11%. The study also showed that increased TC levels (11 mg/dL) attenuated the decrease in mortality rate by 2%. Based on these results, Ogata et al. suggested that Japan should continue policies promoting the control of BP and tobacco use while developing strategies to manage cholesterol levels to prevent CAD deaths6).
•Future PerspectiveCAD mortality can be influenced by not only risk factors but also CAD treatment. Therefore, from the primary prevention perspective, CAD incidence data are crucial for assessing each risk factor’s contribution on a national scale. Establishing a public framework is needed to collect medical information on CAD (including its incidence), as highlighted in the Japanese National Plan for Promotion of Measures Against Cerebrovascular and Cardiovascular Disease7).
Finally, there is a gap between guideline recommendations and real-world practices. The Ministry of Health, Labour and Welfare published recent self-reported treatment rates with “cholesterol-lowering medication” according to serum LDL-C levels8) using the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB) in 2018. The NDB includes data for annual specific health checkups for more than 20 million Japanese individuals/year 40-74 years old9). Fig.2 shows the treatment rate (defined as the proportion of those who reported use of “cholesterol-lowering medication” in those with LDL-C ≥ 180 mg/dL) by age group and sex. The JAS Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2022 (JAS2022GL) recommend considering cholesterol-lowering medication for individuals with LDL-C ≥ 180 mg/dL, regardless of their estimated risk for atherosclerotic diseases1). Despite this recommendation, the observed treatment rate for NDB was <10% in both sexes among those 40-69 years old. This raises concerns about significant undertreatment for high LDL-C levels as well as insufficient awareness of the latest clinical guidelines in Japan. It is also important not to overtreat individuals classified as at a low risk. The methods used to address this issue are outlined in the next section.
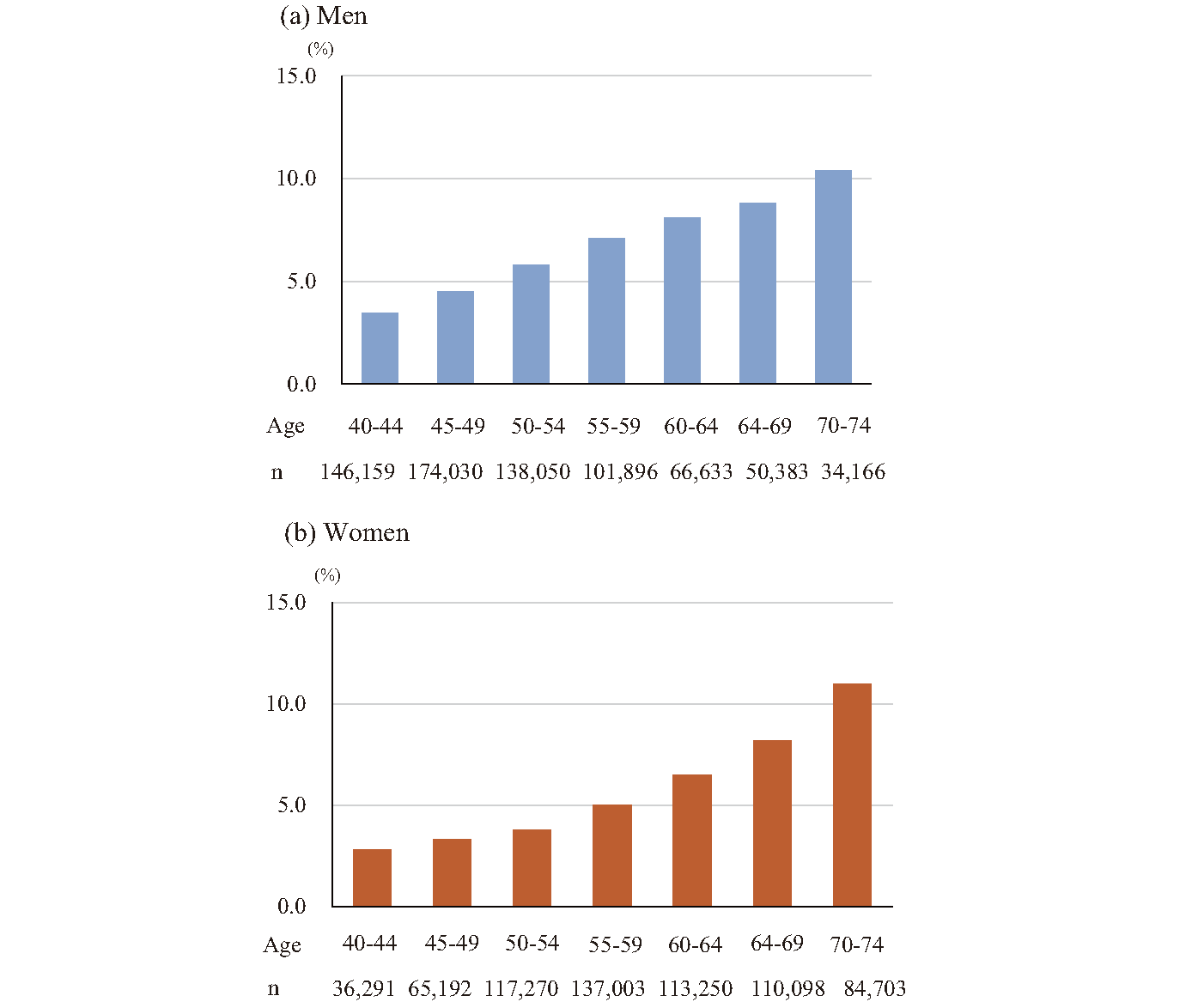
(a) Men. (b) Women. Source: Prepared from the Ministry of Health, Labour and Welfare. https://www.mhlw.go.jp/stf/newpage_26980.html
JCS2023GL emphasizes the crucial role of the comprehensive risk assessment in CAD prevention, which involves estimating the probability (absolute risk) of CAD incidence or death. Similar strategies have been recommended in recent international guidelines and consensus documents on CVD prevention. Subsequently, personalized management goals were set according to the estimated risk. In general, a higher estimated risk corresponds to a more stringent treatment goal for risk factors (e.g. serum LDL-C concentration in cholesterol management). However, risk prediction models constructed based on populations in Europe or the USA tend to overestimate the risk of CAD in cohort studies in Japan10). In the NIPPON DATA 80 cohort, for example, a low risk, as assessed by the European SCORE model, showed poor calibration, particularly in men11). Thus, the selection of an appropriate risk-prediction model for a target population is imperative.
Incorporating these insights, JCS2023GL and JAS2022GL recommend personalized risk assessments using a Japanese population-based risk score for individual risk prediction1, 2). The details of this approach are shown below:
•Risk Stratification Algorithm (Fig.3)The JAS currently offers a free online estimator for 10-year risk and a downloadable “App” (in Japanese) for personal smartphones or PCs to aid clinical practice (available at https://www.j-athero.org/jp/general/ge_tool/). The initial step is to identify individuals requiring secondary prevention (e.g. those with a history of ASCVD) (upper half of Fig.3). Subsequently, individuals targeted for primary prevention undergo stratification based on their estimated 10-year ASCVD risk, categorized as low (<2%), moderate (2% to <10%), or high risk (≥ 10%). Individuals are deemed at a high risk if they have diabetes mellitus (DM), chronic kidney disease (CKD), or peripheral arterial disease (PAD). The remaining individuals undergo a comprehensive risk assessment using the Hisayama risk prediction model, a validated model that has been shown to predict the 10-year risk of ASCVD. Consequently, the individuals are stratified into three categories (Fig.3). A lipid management goal is determined based on the respective estimated risk categories, where higher estimated risks entail more stringent goals (Table 1), with most rigorous goals assigned to diabetic patients with PAD or diabetic microangiopathy (retinopathy, nephropathy, or neuropathy) or those actively engaged in smoking.

Footnote: *JAS2022GL encompasses not only CAD but also atherothrombotic stroke within its scope of prevention, collectively termed atherosclerotic cardiovascular diseases (ASCVD). Adapted from J Atheroscler Thromb, 2023; 30: 000-000. http://doi.org/10.5551/jat.GL2022
| Lipid management targets (mg/mL) | LDL-C | Non-HDL-C |
|---|---|---|
| Low-Lisk (<2%)* | <160 | <190 |
| Medium-risk (2-9%) | <140 | <170 |
| High-risk (>9%) | <120 | <150 |
| Diabetes** complications | <100 | <130 |
*The percentages shown are the expected 10-year risks of atherosclerotic disease.
**Diabetes with peripheral artery disease, microangiopathy (retinopathy, nephropathy, or neuropathy), or diabetic individuals who actively smoke.
The primary treatment target was the LDL-C level. Once the LDL-C goal was met, achieving a non-HDL-C goal was recommended. Adapted from Circ J. 2024 Mar 13. doi: 10.1253/circj.CJ-23-0285. Online ahead of print.
Although the rationale behind the comprehensive approach seems logical, there is limited evidence supporting its effectiveness in improving clinically important outcomes, such as reduction in incidence or mortality from ASCVD. When compiling JCS2023GL, the authors conducted a systematic review to assess the effectiveness of using a prediction model for managing risk factors in reducing CVD mortality in the primary prevention setting. Of the nine identified randomized controlled trials (RCTs), only one demonstrated a reduction in mortality rate. Owing to the overall low quality and high heterogeneity among the studies, no meta-analysis was conducted. Consequently, JCS2023GL concluded that the efficacy of using a prediction model to reduce CVD mortality remains uncertain, necessitating further studies, ideally RCTs, to validate the effectiveness of this approach.
Finally, how commonly this comprehensive risk assessment is used in clinical practice across Japan remains unknown. Recognizing the disparity between recommendations and real-world practices is crucial. Understanding the existing gap and its underlying reasons will be a pivotal first step towards improving real-world clinical practice.
(3) Clinical Management of LDL-COf the various antilipid medications, statins remain the recommended first-line drugs for lowering LDL-C levels. However, statin intolerance is a concern, with 15%-20% of patients discontinuing statins due to “side effects”, such as myalgia and elevated creatine kinase levels, as per the Statin Intolerance Clinical Guide 2018 by the Japan Atherosclerosis Society Statin Intolerance Clinical Guide Working Group. Notably, a detailed review suggests that “true” statin intolerance, necessitating complete discontinuation, is estimated to occur in only 0.5% of cases. The recent Self-Assessment Method for Statin Side-effects Or Nocebo (SAMSON) trial showed that 90% of adverse symptoms related to statins were also induced by a placebo, showcasing the powerful nocebo effect12). Crucially, 50% of patients successfully restarted statin therapy. Overall, although statin intolerance may seem common, it can often be managed by setting expectations and adjusting the doses and/or dosing regimens.
•Future PerspectivesIn the current era of aggressive lipid management for high-risk or very high-risk individuals, various agents are available, including statins, ezetimibe, PCSK9 inhibitors, and benzoic acid. However, only a small fraction of patients in primary or secondary prevention programs were treated with combination therapy involving ezetimibe (9%) and/or PCSK9 inhibitors (1%)13). There appear to be significant gaps between guideline recommendations and real-world practices for managing dyslipidemia. Recently, inclisiran, an siRNA capable of blocking PCSK9 mRNA with twice-yearly injections, was developed. RCTs of inclisiran have shown a 50% reduction in LDL-C levels14). To bridge the gaps in cholesterol management, especially in high-risk patients, the deployment of novel agents, coupled with an appropriate risk stratification strategy, is necessary.
Based on epidemiological studies conducted in Japan, the updated JCS2023GL set the diagnostic criteria for hypertension as office BP ≥ 140/90 mmHg and home BP ≥ 135/85 mmHg. However, EPOCH-JAPAN, an individual participant data meta-analysis of 10 cohort studies that included approximately 70,000 Japanese men and women, showed that the association between the systolic BP (SBP)/ diastolic BP (DBP) [please define abbreviation] and CVD mortality was almost logarithmically linear and that the risk was lowest for individuals with BP levels < 120/80 mmHg in middle-aged (40–64 years old) and elderly (65–74 years old) age groups15). Furthermore, the Systolic Blood Pressure Intervention Trial (SPRINT), an RCT that enrolled 9,361 patients ≥ 50 years old with high-risk hypertension and no history of DM or stroke, endorsed an aggressive BP reduction strategy. This approach led to a 25% reduction in composite cardiovascular (CV) endpoints (myocardial infarction, other acute coronary syndromes, stroke, heart failure, and CV death) compared with the standard antihypertensive treatment group16). Incorporating the above findings, most current international guidelines advocate a risk-based approach, setting general targets for the overall population while recommending stricter targets for individuals at high risk of CVD or those with chronic kidney disease. For these high-risk groups, a lower target (<130/80 mmHg) is advised17-19).
•More Recent FindingsMore recently, the ESPRIT, conducted at 116 sites across China (11,255 patients with a mean age of 65 years old and an average BP of 147/83 mmHg at baseline), supported the SPRINT findings by including an exclusively Asian population and patients with type 2 DM (T2DM) or a history of stroke (such patients were excluded from the earlier trial)20). The trial demonstrated that, for every 1,000 patients treated with the intensive versus conventional systolic BP goal for 3 years, there would be 14 major vascular events and 8 deaths avoided at the cost of 3 additional serious adverse events involving syncope (rates of all safety outcomes were lower in ESPRIT than in SPRINT). The ESPRIT provides “reassuring” information on the safety of aggressive BP management.
•Future PerspectiveAlthough encouraging results of aggressive BP-lowering efforts have been reported, BP control in real-world practice, even when following conventional goals, remains suboptimal. According to a population-based study across 90 countries between 2000 and 2010, a significant proportion of individuals with hypertension worldwide failed to achieve optimal BP control21). Insufficient BP control is especially pronounced in lower- and middle-income countries. This population-based study also[which study?] showed a decline in the proportion of controlled hypertension in these regions, with control rates falling from 8.4% to 7.7%. Furthermore, there was a noticeable decrease in hypertension awareness (from 37.9% to 32.3%) and in the rates of hypertension treatment (from 29.0% to 24.9%)21). The primary challenge lies in identifying patients at risk for future CV events. To better translate information from clinical trials into real-world practice, various modalities, including risk-stratification charts and algorithms, have been introduced with varying success rates.
The presence of DM was considered equivalent to two of the three established CAD risk factors. Studies in Western countries have indicated that individuals with DM are at risk of developing their first myocardial infarction (MI), which is similar to the risk of recurrent MI in non-diabetic individuals. Patient characteristics and outcomes in Asians with T2DM differ notably from those in Western countries. Studies from the Asia-Pacific Cohort Studies Collaboration, pooling retrospective studies from the region, confirmed a two-fold increase in the absolute risk of CVD in Asians with T2DM compared with those without, with higher hazard ratios in younger individuals22). Furthermore, large-scale CAD registries in Japan and the USA reported significantly lower long-term mortality in Japanese patients with T2DM than in American patients with T2DM, although T2DM was more prevalent in Japanese patients23).
The traditional approach to diabetes management emphasizes the importance of glycemic control: high HbA1c levels are considered a key risk factor, and the primary goal for treatment is to maintain the patient’s HbA1c within a certain range. However, recent evidence from large-scale RCTs has significantly changed the treatment perspective from controlling glucose levels to reducing CV risk. SGLT2 inhibitors have been shown to improve outcomes in patients with diabetes more rapidly than observed in traditional trials, in which benefits (of glycemic control) are typically observed after several years of stringent glycemic control24). Similarly, results from trials of GLP-1 agonists followed this trend, markedly changing our approach to diabetes management25). Consequently, updated clinical practice guidelines have emphasized reducing the CV risk (‘risk’ management) and recommending these agents for individuals at high risk.
•Recent FindingsAs discussed above, the recent clinical goal of diabetes management has shifted from controlling glucose levels to reducing CV risk. However, due to the economic cost burden associated with medications, a majority of international practice guidelines predominantly target patients at high risk of CAD. A community study in Japan found that approximately 10% of patients met criteria similar to those in the EMPA-REG trial, and 45% met criteria similar to those in the DECLARE trial, indicating that a substantial proportion of patients in the community met the criteria for high-risk CAD26). Of the 2,849 patients meeting either set of criteria, the estimated 5-year cumulative incidence decreased from 97.1 to 75.6 events with 75% adoption of SGLT2 inhibitors. Encouragingly, international collaborative database studies demonstrated that initiating treatment with an SGLT2 inhibitor was linked to a significantly lower CV risk than starting treatment with other glucose-lowering drugs, even in Asia-Pacific countries27).
JCS2023GL added a new section for women, introducing HRT. The guideline recommends HRT for symptomatic menopausal women with dyslipidemia, provided that HRT is not contraindicated or its administration requires careful consideration (Class IIa)2). In this section, background information on HRT for women is examined.
(1) Historical Background of HRTIn the 1960s, HRT gained widespread use for relieving peri-menopausal symptoms in Europe and the USA28). In addition, HRT was expected to prevent CAD in peri- and postmenopausal women because it ameliorates the physiological increase in serum LDL-C in this population. However, unopposed estrogen therapy is associated with an increased risk of endometrial cancer. By the early 1980s, adding progestogen to estrogen resulted in a reduction in endometrial cancer risk, establishing the concept of “combination estrogen-progestin therapy (EPT)” for women with an intact uterus28). Furthermore, a substantial reduction in incident CAD and CVD death in estrogen users has been reported in observational studies29).
(2) Timing HypothesisThe Women’s Health Initiative (WHI) study unexpectedly showed a significant increase in incident CAD in the HRT group compared with the placebo group30). Nevertheless, 10 years after the first publication of the WHI study, an age-stratified, longer follow-up analysis (median duration 13 years) showed that the effect of HRT differed by age at the start of HRT31, 32). The absolute risk of adverse events (CAD, breast cancer, pulmonary embolism) after HRT initiation was much lower in women 50-59 years old than in older women and those who started HRT within 10 years of menopause30). These results support “the timing hypothesis,” i.e. the notion that the beneficial CV effects of exogenous estrogen are dependent on the timing of HRT initiation and are limited to women who start therapy within several years of menopause28). This hypothesis was corroborated by a meta-analysis of 19 human RCTs. According to that meta-analysis, women who received HRT less than 10 years after menopause had a lower CVD risk (risk ratio: 0.52, 95% confidence interval [CI]: 0.29-0.96) than those who received HRT ≥ 10 years after menopause33). Notably, regardless of HRT timing, an increase in venous thromboembolism (VTE) events was observed28, 33). Furthermore, the Early vs. Late Intervention Trial with Estradiol (ELITE) study using carotid intima-media thickness (cIMT) progression as an outcome showed significantly slower progression of cIMT in women of recent menopause who were assigned HRT than those assigned placebo. This finding was consistent with the timing hypothesis.
In summary, estrogen may have adverse effects, including plaque destabilization in women with advanced atherosclerosis, but it provides appropriate benefits in ameliorating menopausal symptoms and CAD prevention when started in healthy women <60 years old or within 10 years of menopause onset28).
(3) HRT Route and the Risk of Adverse EventsThe risk of adverse events due to HRT differs according to the type, dose, administration route, and administration method of estrogen and progestin2).
A. Oral vs. Transdermal Estrogen for MI and VTEAccording to a recent review article, no study has compared the difference in the risk of MI between oral and transdermal HRT28). For VTE risk, no RCT data are available, and observational studies have reported that oral HRT increases the risk, whereas transdermal HRT has a neutral effect28).
B. Oral vs. Transdermal Estrogen for Lipid MetabolismPrevious studies (11 RCTs and 1 cohort study) showed that LDL-C reduction was greater with oral HRT than with transdermal HRT. However, studies also consistently reported that oral HRT increased triglyceride levels, whereas transdermal HRT decreased triglyceride levels28, 34).
C. Progesterone Component and Breast CancerAccording to the 2017 guidelines for HRT by the Japan Society of Obstetrics and Gynecology and Japan Society for Menopause and Women’s Health (2017JSOG/JMWHGL), the risk of breast cancer increases with a longer duration of combined estrogen progestin therapy (EPT)35). However, observational studies have suggested that the risk of breast cancer does not increase with natural progesterone36).
(4) Caution in the Japanese Guideline for HRTAlthough HRT has demonstrated efficacy and safety in alleviating postmenopausal symptoms, there are instances where caution is warranted or contraindications exist. According to the 2017 JSOG/JMWHGL, HRT is contraindicated in women with a history of myocardial infarction or coronary atherosclerotic lesions35). In addition, the guidelines advocate for heightened caution in starting HRT under the following conditions: obesity, age ≥ 60 years old, initiation of HRT ≥ 10 years after menopause, a history of coronary spasm and microvascular angina, severe hypertriglyceridemia, or uncontrolled DM or hypertension35).
•Future PerspectiveAlthough the present overview underscores the significance of timing and administration route for safer HRT, certain questions remain unanswered. For instance, the endpoint of the ELITE trial was subclinical atherosclerosis (i.e. cIMT) rather than clinical events. Consequently, there is a pressing need for a double-blind, placebo-controlled RCT to assess the timing hypothesis using clinically relevant outcomes, including incident CVD (encompassing CAD, stroke, and VTE). Furthermore, it is imperative to acknowledge the existing gap in evidence for non-White populations since most clinically important data have been derived from White populations. Addressing these gaps will help refine our understanding of HRT safety and efficacy across diverse demographic groups.
In JCS2023GL, the entire section was devoted to the management of older adults, acknowledging the increasing demand for an effective preventive strategy. The guideline proposed Class I recommendations for the following: 1) “non-pharmacological therapy should be promoted aggressively among older adults, and those who can visit outpatient clinics on their own should be proactively treated with drugs,” but the importance of individualized treatment is emphasized with respect to the quality of life (QOL); and 2) frailty, the cognitive function, activities of daily living (ADL), nutritional status, and medication adherence were described as factors that should be assessed prior to treating them2).
Under Class IIa recommendations, the guidelines suggest considering discontinuation of drug therapy as older adults approach the end of life2). Practical tools for assessing frailty, the cognitive function, and other conditions were introduced in the JCS2023GL. Specific precautions for managing coronary risk factors and conditions that require caution, such as polypharmacy, frailty, dementia, and end-of-life considerations, are outlined. For example, the revised Japanese Cardiovascular Health Study standards (J-CHS)37) have been suggested as a suitable tool for evaluating frailty in clinical practice2).
(1) Defining “Older Adult”Owing to differing definitions of older adults among European, US, and Japanese GLs, recommendations for each age group may vary by guideline. For example, the 2021 ESC guidelines (ESC2021GL) defined older persons as those ≥ 70 years old38), whereas the 2022 US Preventive Services Task Force Recommendation Statement (USPSTFR2022) divided management policies for LDL-C control between those ≤ 75 and ≥ 76 years old39). JCS2023GL affirmed clear evidence that reducing LDL-C levels with statin treatment prevents CAD and non-cardiogenic cerebral infarction in individuals <75 years old. However, the GL emphasized increased caution, at least for those 65-74 years old, by advocating an individualized approach based on the biological and/or functional status rather than relying solely on chronological age. All GLs expressed caution in adults >75 years old. For example, ESC2021GL suggests that a conventional LDL-C target of <100 mg/dL may be reasonable considering frailty, polypharmacy, and muscle symptoms. However, all GLs agreed that further research on the effectiveness of treatment for high LDL-C levels in patients >75 years old is needed. Notably, a study showed that discontinuation of statin medication increased the risk of hospitalization for CV events by 33% in a cohort study of individuals ≥ 75 years old40). Considering this result, JCS2023GL recommends continuation of treatment in patients ≥ 75 years old who are taking statins2).
A significant evidence gap exists regarding medications for the primary prevention of CAD in older adults >75 years old. Taking LDL-C control as an example again, the results of ongoing primary prevention trials, such as the STAtins in Reducing Events in the Elderly (STAREE) trial41), are needed to provide more insight into the efficacy and safety of interventions in this specific age group. Considering the differences in the absolute risk of CAD and the prevalence of CAD risk factors between Japanese and Western populations, there is a crucial need to accumulate evidence specifically in elderly Japanese individuals >75 years old.
Finally, the importance of individualized treatment for CAD prevention in older adults cannot be overstated. This approach should consider various factors such as the QOL, frailty, cognitive function, ADL, nutritional status, polypharmacy, medication adherence, and end-of-life considerations.
Observational studies have shown an association between an increased salt intake and elevated BP42, 43). Salt reduction trials have demonstrated that reducing salt intake leads to decreased BP in both hypertensive and normotensive individuals44). Lowering the BP is known to reduce the risk of CAD and stroke45, 46). Despite this evidence, establishing a direct link between a low salt intake and reduced risk of CAD and stroke has been difficult, until recently. This was due in part to long-term dietary intervention being difficult to conduct; thus, researchers have had to rely on findings from observational studies, which have inherent difficulties in controlling confounding. Nonetheless, recent high-quality evidence supports a positive association between sodium intake and the risk of CV incidence and/or death47, 48) in a dose-dependent manner, at least in the sodium (or its equivalent) intake range of 5 to 15 g per day47). It is noteworthy that studies reporting a U-shaped relationship between the salt intake and CVD risk often have flaws, such as insufficient control for confounding and the inaccurate assessment of dietary sodium intake49-52).
(2) Alcohol: Challenging the J-Shaped RelationshipThere is a consensus that heavy alcohol consumption, typically defined as ≥ 46-60 g of pure alcohol per day, is a significant risk factor for CAD and stroke. Conversely, light-to-moderate alcohol consumption (with no universal definitions, but generally considered to be within 10–40 g/day) has traditionally been believed to have a CV benefit (i.e. reduced risk), especially for CAD53). Nevertheless, accumulating evidence has challenged this conventional view of alcohol consumption. For instance, the Suita Study, an urban cohort study conducted in Japan, found that men who engaged in light-to-moderate drinking with serum gamma-glutamyltransferase (GGT) levels exceeding the median (>32 IU/L) had a higher risk of total stroke than men who never drank and had GGT levels below the median54). A systematic review of community-based studies failed to identify the “benefits” of light-to-moderate drinking on CVD incidence in individuals with multiple comorbidities55). Furthermore, a recent meta-analysis focusing solely on high-quality cohort studies showed that the purported “benefit” of low-dose alcohol consumption on all-cause mortality risk vanished, resulting in no significant difference from lifelong non-drinkers56). Furthermore, the expected risk reduction in CAD deaths was not observed in young individuals ≤ 55 years old57).
Most evidence concerning the long-term effects of alcohol consumption on health outcomes stems from observational studies owing to the ethical and feasibility challenges associated with conducting long-term interventional studies on alcohol consumption. Furthermore, most observational studies have relied on self-reported measures of usual alcohol consumption, potentially resulting in biased estimates57). An approach to mitigating this inherent limitation could involve employing a Mendelian randomization analysis to estimate alcohol consumption. A Mendelian randomization meta-analysis of a European population (N=261,991) suggested that even in light-to-moderate drinkers, a further reduction in alcohol consumption is beneficial for CV health58). Another Mendelian randomization analysis conducted in China and South Asia showed no clear dose-response relationship between alcohol consumption and CAD risk. However, it showed a monotonically increasing dose-response relationship (i.e. not a J-curve) with the risk of stroke59). Notably, this Mendelian randomization analysis accounted for two genetic variants: aldehyde dehydrogenase (ALDH2, rs671) and alcohol dehydrogenase (ADH1B, rs1229984). While the ALDH2 variant is uncommon in persons of European ancestry, both are common in persons of Asian ancestry. Therefore, this analysis is more pertinent to East Asians, including the Japanese population, than other populations.
The aforementioned studies cast doubt on the “CV benefit” of a small alcohol intake. Based on CVD outcomes, ethyl alcohol has been classified as a group 1 carcinogen, indicating that there is sufficient evidence in humans for its carcinogenicity according to the International Agency or Research on Cancer (IARC)60). Studies have consistently indicated a positive monotonic association between alcohol consumption and various types of cancer61). The Global Burden of Disease Study, encompassing 23 alcohol-related health outcomes including violence and injuries, concluded that “the level of alcohol consumption that minimized harm across health outcomes was 0”62). In alignment with this evidence, the World Health Organization (WHO) has stated that “no level of alcohol consumption is safe for our health”63). Considering the comprehensive body of evidence on alcohol consumption, we agree with the WHO statement. Therefore, we encourage healthcare professionals to impart current scientific evidence on the overall health effects of alcohol on their patients and clients, fostering mindful discussions that balance the risks and potential benefits (non-biological or social) of alcohol consumption.
Extensive evidence highlights the adverse effects of air pollution on cardiometabolic health. Cardiometabolic conditions affected by air pollution include CAD, heart failure, cardiac arrhythmia and arrest, cerebrovascular diseases, hypertension, insulin resistance, and venous thromboembolism64). Recognizing the accumulating evidence against CV health, the European Society of Cardiology65), the American College of Cardiology64), and the American Heart Association (AHA)66) have issued position papers or review articles on air pollution in recent years. In its Policy Statement, the AHA asserted that extensive research suggests that exposure to air pollutants, such as fine particulate matter (PM2.5), ozone, and traffic-related pollutants, is associated with adverse CV events. In light of this evidence, JCS2023GL recommends avoiding air pollution exposure for individuals at a high risk of CAD.
•Recent Evidence from Japan on Adverse CV Effects of Air PollutionThe adverse effects of air pollution have also been reported in Japan. For instance, a nationwide study using the All-Japan Utstein Registry showed that a 10-µg/m3 increase in PM2.5 at lag0-1 (i.e. difference in PM2.5 concentration between the index day and 1 d earlier) was associated with a significantly increased incidence of out-of-hospital cardiac arrests of cardiac origin across most of the 47 prefectures in Japan, without evidence of heterogeneity67). Another study conducted in Kumamoto Prefecture showed acute exposure to Asian dust in air-triggered acute MI68). Notably, Asian dust, which mixes with air pollutants during atmospheric transport, contains harmful chemical species, such as sulfur and nitrogen oxides, or by-products formed from combusted coal and other fossil fuels68).
•Biological MechanismAn attempt to understand the intricate mechanisms by which air pollution triggers adverse CV events is underway. Proposed initiating mechanisms include oxidative stress, direct translocation (of ultrafine particles) into the systemic circulation, and the effects of particles and secondary biological intermediators64). These processes initiate or contribute to multiple pathways, as follows: 1) endothelial barrier disruption; 2) inflammation involving immune components; 3) the prothrombotic pathway; 4) autonomic imbalance favoring sympathetic tone; 5) central nervous system effects on hypothalamic-pituitary-adrenal axis activation; and 6) epigenomic changes64).
Apart from CVD, it is noteworthy that the IARC has classified outdoor air pollution as carcinogenic to humans, causing lung cancer (group 1). The Agency separately evaluated particulate matter and categorized it as a group 1 carcinogen69).
•Future PerspectiveIn addition to unraveling detailed mechanisms, there is sufficient evidence linking air pollution to adverse CV outcomes. Importantly, the AHA stated that adverse effects on CVD “are observed even below the PM2.5, as required by the US Environmental Protection Agency (EPA): a long-term average of 12 µg/m3 and a short-term average of <25 µg/m3 66). In Japan, the Environmental Quality Standards for PM2.5, set by the Ministry of the Environment, are “the annual standard” (one-year average) of ≤ 15.0 µg/m3 and the “24-hour standard” (i.e. annual 98th percentile values at designated monitoring sites in an area) of ≤ 35 µg/m3 70, 71). Further confirmatory evidence from Asian populations, including Japan, and investigation of the potential effects of low-level exposure on CVD may heighten awareness of the importance of the air quality for clinicians and the public.
Although controlling the ambient air quality seems beyond individual control, meaningful reduction or avoidance of personal exposure is feasible. The development of monitoring technologies, personal exposure-reduction devices such as air filters, and behavioral choices (i.e. avoiding rush hour or crowded streets, closing windows) have been suggested, yielding proven benefits64) even in relatively young adults72) who are generally considered more resilient to such exposure than older individuals.
(2) Heat, Humidity, and CADAmbient temperatures at low (cold) and high (heat) levels, as well as fluctuations in temperature, increase the risk of CAD. Therefore, JCS2023GL recommends that individuals at high risk of CAD be instructed to avoid such exposures.
Although the adverse effects of low temperatures and rapid temperature changes on CVD are well recognized, the impact of high heat is often overlooked. Increased incidences and exacerbations of cardiopulmonary diseases, including ischemic heart disease, cardiac dysrhythmia, and ischemic stroke, have been observed during periods of elevated temperatures73), in addition to well-known heat-related illnesses such as heat stroke. A recent meta-analysis showed that a temperature 1℃ above the reference value (different in each study) was positively associated with an increase in CV-related mortality by 2.1% (95% CI 2.0%-2.3%)74). This risk is amplified in women, elderly persons (≥ 65 years old), individuals residing in tropical climates, and those in low-to-middle-income countries74). Notably, evidence from Japan showed an association between an acute MI onset and the maximum temperature recorded two days prior, based on regional registry data in Oita Prefecture, Japan75).
The potential mechanisms by which heat causes CV events encompass various factors, including dehydration leading to increased blood viscosity and vasoconstriction76). Because the CV system plays a key role in thermoregulation to dissipate excessive body heat, prolonged heat exposure can strain the CV system77). In addition, heat can increase cytokine release and promote thrombosis, thereby increasing the risk of MI and stroke78).
•Future Perspectives
The global rise in ambient temperature coupled with an aging population worldwide raises concerns regarding the increased risk of CV events, including CAD and stroke, due to extreme heat and increased humidity. A recently published study estimated that, in the years from 2008 to 2019, extreme heat was associated with 1651 (95% CI, 921–2381) excess CV deaths per year in the USA78). This number can be translated as 1 additional extreme heat day being associated with a 0.05% (95% CI, 0.02%–0.07%) increase in CV mortality in the USA during this period, and the amount is projected to increase in the future. Region-specific evidence is crucial to confirm these findings in Asian populations and ascertain potential differences between the USA and Western countries concerning the magnitude of the effects and the affected disease subtypes.
Incorporating humidity with temperature assessment, not just temperature alone, to evaluate the impact of heat on CV outcomes is relatively new, particularly in non-Western countries. Metrics considering temperature and humidity include the wet-bulb globe temperature (WBGT) and heat index (HI). Although both metrics are in use, establishing evidence-based recommendations regarding reasonable thresholds for each metric to alert the public or individuals at an elevated CVD risk is imperative.
In Japan, the expansion of the JAS2022GL’s scope to include all forms of ASCVD, beyond CAD, marked a significant step forward, a move further bolstered by the subsequent updates for primary CAD prevention in JCS2023GL. This review not only emphasizes the pivotal role of continuously updated guidelines in shaping clinical practice but also stresses the urgent need for ongoing research to bridge existing knowledge and practice gaps.
In certain sections of the earlier version of the manuscript, we used generative AI ( ChatGPT version 3.5) to enhance readability. Nonetheless, the authors assume complete responsibility for all content in the final version of the manuscript. In addition, the views and opinions expressed in this article are those of the authors and do not necessarily represent those of either the Japan Atherosclerosis Society or the Japanese Circulation Society.
None.
Dr. Kohsaka received grants from Bristol-Myers Squibb, Novartis, and AstraZeneca outside the submitted work. The other authors declare that they have no conflict of interest.