2023 年 118 巻 1 号 論文ID: 220630b
2023 年 118 巻 1 号 論文ID: 220630b
Serpentinization is a widely observed hydration process in the earth’s crust. In this study, hydrothermal experiments were performed at different temperatures, times, and pH values to explore the geochemical processes of serpentinization in forsterite. The results indicated that forsterite converts to serpentine after reacting with SiO2 at 200 °C and a pH of 13 for 20 days in a hydrothermal system. X-ray diffraction patterns showed that the serpentine is lizardite, with lamellar and leaf-like micro-morphology. Furthermore, hydroxyl absorption peaks, which represent the formation of lizardite, were observed in the Fourier-infrared spectroscopy spectra. The newly formed serpentine showed high crystallinity and a relatively perfect crystal form. High-resolution electron microscopy indicated that the serpentine crystal layer only appeared on the forsterite surface. The transformation was a process of the coupling of dissolution and precipitation where the forsterite was replaced from the surface; [SiO4]4− and [MgO6]10− units were attached in situ to the forsterite surface, reacted, and formed a serpentine structure. These results are of fundamental significance for exploring the transformation and controllable synthesis between olivine group and serpentine group minerals as well as understanding the genesis, geochemical behavior, and geological environment of ultrabafic rock alteration deposits.
Serpentinization of olivine is widely observed in different geochemical environments that can be transformed into different serpentine minerals through geological processes, including magmatic activity and hydrothermal alteration in subduction zones, mid-ocean ridges and deep ocean bottoms (Mével, 2003; Paulick et al., 2006; Fryer, 2012). Understanding olivine serpentinization is helpful to grasping the phyllosilicate crystal chemistry and elucidating geochemical processes (Cuadros and Altaner, 1998; Ji et al., 2018; Li et al., 2019).
For the past several decades, in order to reproduce serpentinization and explain the growth of serpentine in hydrothermal environment, a large number of experimental studies have been carried out. Lafay et al. (2012) found olivine grains were replaced by chrysotile and brucite under high alkaline conditions. They believed that the coupled dissolution-precipitation caused the alteration of initial olivine particles. The extent of serpentinization depends on the crystal size of olivine. And beyond that, McCollom et al. (2020) conducted experiments to investigate the effect of pH value on the reaction pathways and rates during serpentinization. They found that, using olivine as reactant at 200 and 230 °C, higher pH resulted in more rapid serpentinization of the olivine and generation of larger amounts of H2 for comparable reaction times under strongly alkaline conditions. Nakatani and Nakamura (2016) and Sieber et al. (2022) performed a series of hydration experiments of peridotites in fore-arc mantle conditions at temperatures of 400-700 °C and pressures of 1-2.5 GPa, the serpentine varieties generated include lizardite, Al-lizardite and antigorite. Among them, antigorite is deemed to be a serpentine phase stable at high temperature and high pressure. Lamadrid et al. (2021) used synthetic fluid inclusions in olivine as reactors to monitor the effects of temperature, fluid composition, and total salinity on serpentinization rates of olivine under closed system conditions. The results show that serpentinization rate between 200 and 300 °C is faster than that between 100 and 200 °C or between 300 and 350 °C on geologic timescales when rocks with large reactive surface areas interact with a seawater-like aqueous solution. So far, the hydrothermal simulation of serpentinization of ultramafic rocks has focused on the effects of temperature, pressure, fluid chemistry and reaction time on reaction products, reaction kinetics and thermodynamics (Martin and Fyfe, 1970; Wegner and Ernst, 1983; O’Hanley and Dyar, 1993; Inoue et al., 2009; Okamoto et al., 2011; Malvoisin et al., 2012; McCollom et al., 2016; Lamadrid et al., 2017; Syverson et al., 2017; Tutolo et al., 2018;).
The serpentinization of ultramafic or mafic rocks is a hydrothermal alteration process under low-temperature and low-pressure conditions (Mével, 2003; Evans et al., 2013). When the original rocks are subjected to different hydrothermal processes, olivine and pyroxene are often transformed into serpentine minerals with different structures in the chemical system of fluid existence (Hyndman and Peacock, 2003; Sonzogni et al., 2017). This is crucial for revealing the evolution of crystal structure (Cuadros, 2012), transformation mechanism (Ji et al., 2018), and behavior of elemental migration during mineral transformation. It is also useful for exploring the physical and chemical properties of the lithosphere, analyzing the geochemical cycle of elements, and exploring the evolution of early life (Evans, 2010; McCollom et al., 2022) as well as mineral exploration.
Previous experimental studies have reported important information about the effects of pH, temperature, fluid chemistry, etc. on the serpentinization reaction rate of olivine. However, few experiments have been conducted on the changes and relations between the crystal structure, crystal chemistry and spectral parameters between primary minerals and secondary minerals during serpentinization of forsterite. In this study, a hydrothermal system was established in the laboratory to simulate the geological environment of alteration and evolution of forsterite serpentinization. The influencing factors and covariance laws of forsterite serpentinization were examined to obtain the optimal transformation conditions by controlling the temperature (Li, 1993; Syverson et al., 2017), time, and pH value. X-ray diffraction (XRD), X-ray fluorescence spectroscopy (XRF), Fourier-infrared spectroscopy (FTIR), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), thermogravimetric (TG), and differential scanning calorimetry (DSC) were used to determine the chemical composition, spectroscopy parameters, micro-morphology, and crystal structure of the products during the transformation, so as to reveal the processes involved in forsterite serpentinization.
Forsterite crystals (Fig. 1) were purchased from Niushan Clover Crystal Firm (Donghai County, China). After grinding and sieving, the forsterite samples under the 300 mesh sieve (grain size ≤48 µm) were prepared for experiments. The chemical composition measured by XRF is given in Table 1.

| Sample | SiO2 | MgO | Fe2O3T* | NiO | CaO | Al2O3 | SO3 | MnO | LOI |
| M2S | 40.13 | 47.85 | 8.43 | 0.26 | 0.59 | 0.4 | 0.06 | 0.16 | 0.26 |
* Fe2O3T (FeO and Fe2O3) refers to total iron.
Amorphous SiO2 nanoparticles (analytical reagent) were purchased from Dezhou Runxin Experimental Instrument Co., Ltd. (China). NaOH (analytical reagent) was purchased from Mianyang Xinjie Trading Co., Ltd. (China). The DI water used in the hydrothermal experiment was prepared by an ultrapure water system (Chengdu Ultrapure Technology Co., Ltd., China) with a resistivity >18.2 MΩ·cm. The experimental were conducted on seven 150 mL stainless steel hydrothermal reactors (Mianyang Xinjie Trading Co., Ltd., China).
Experimental procedureExperimental scheme. Forsterite was used as the precursor mineral and active SiO2 (amorphous SiO2 nanoparticles) as the external silicon source simulate the SiO2-rich fluid environment in the process of alteration. Experiments were performed at T = 100 and 200 °C; pH = 12 and 13; t = 5, 10, 15, and 20 d. The design of the experimental scheme is shown in Table 2.
| Sample ID | Temperature/°C | pH | Time/d | Pressure/Mpa |
| FS-t-01 | 200 | 12 | 5 | 3 |
| FS-t-02 | 200 | 12 | 10 | 3 |
| FS-t-03 | 200 | 12 | 15 | 3 |
| FS-t-04 | 200 | 12 | 20 | 3 |
| FS-pH-01 | 200 | 13 | 20 | 3 |
| FS-T-01 | 100 | 12 | 10 | 1.3 |
| FS-T-02 | 100 | 12 | 20 | 1.3 |
Experimental steps. A 0.5 mol/L NaOH solution was prepared in a 500 mL volumetric flask to adjust the pH of the hydrothermal system. Based on the chemical transformation process of forsterite to serpentine, 4.2 g of 300 mesh forsterite samples and 0.6 g of amorphous SiO2 nanoparticles were weighed and then placed into seven hydrothermal reactors. 100 mL DI water was added, and the mixture was evenly stirred using a magnetic stirrer. NaOH solution was then added in drops, and the pH value of the supernatant in the reaction vessel was monitored to adjust the pH of the system to the required value. The volume of the solution was maintained at 2/3 of the total volume of the hydrothermal reactor, sealed and placed in an oven. After reacting at a constant preset temperature for a specified duration, the hydrothermal reactor was removed and cooled naturally. The suspension was filtered and washed, and the solution was stored in a 10 mL centrifuge tube. The residue (sample after the hydrothermal reaction) was dried in an oven at 100 °C for 14 h.
Sample characterization. The chemical composition of the forsterite sample was analyzed using an XRF spectrometer with a ceramic X-ray tube (Rh target) (PANalytical Axios, wavelength dispersive type, PANalytical B.V., Eindhoven, The Netherlands). The phase composition of the forsterite sample and product of the hydrothermal reaction were analyzed using X-ray diffraction spectrometer (PANalytical X’pert PRO, PANalytical B.V., Eindhoven, The Netherlands) with Cu target (λ = 0.154178 nm, 40 kV and 40 mA). The molecular vibrational spectrum of the forsterite sample and hydrothermal reaction product were characterized using the infrared spectrometer (Perkins-Elmer 2000, Waltham, Massachusetts, USA), The sample prepared by KBr tablet method, and the scanning range was 400-4000 cm−1. The micro-morphology of the forsterite sample and hydrothermal reaction product were characterized using field emission electron scanning microscopy (Zeiss Ultra 55, Carl Zeiss NTS GmbH, Oberkochen, Germany). The micro-structure of the forsterite sample and hydrothermal reaction product were characterized using HRTEM (FEI Talos F200 S, FEI company, USA). Finally, Changes in the thermogravimetric (TG) and differential scanning calorimetry (DSC) features of the hydrothermal reaction product were characterized using the thermal analyzer (SDT Q160, TA Instruments, New Castle, Delaware, USA).
Figure 2 illustrates the XRD patterns of forsterite samples. As can be observed, only the diffraction peak of forsterite appeared. The main diffraction peaks were d200 = 5.113 Å, d101 = 4.318 Å, d210 = 3.890 Å, and d011 = 3.729 Å, indicating that the forsterite samples had high purity.

Figure 3 illustrates the XRD patterns of forsterite and reaction products with amorphous SiO2 nanoparticles under hydrothermal conditions. The XRD patterns of FS-t-02 show that the diffraction peak intensities of (200), (210), and (301) planes for forsterite samples were weakened after 10 days under hydrothermal conditions with pH = 12. A diffraction peak (d001 = 7.34 Å) of serpentine at 2θ = 12.133° was observed (Whittaker and Zussman, 1956), indicating that serpentine was formed as a product. According to the XRD patterns of FS-t-04, it can be observed that the intensities of d001 = 7.34 Å, d200 = 5.11 Å, d101 = 4.31 Å, and d200 = 5.11 Å as well as intensities of other serpentine diffraction peaks increased and with sharp peaks when the reaction time was extended to 20 days. This indicates that the serpentine formed by the transformation had crystal form with high crystallinity and a high layer-stacking order. XRD patterns of FS-pH-01 indicate that after forsterite samples reacted for 20 days under hydrothermal conditions with pH = 13, and the characteristic diffraction peak intensity reduced considerably as the pH value of the system increased. The number of characteristic diffraction peaks of serpentine increased, and the intensity increased substantially. The resulting diffraction peaks of d001 = 7.28 Å, d110 = 4.59 Å, d-111 = 3.88 Å, d002 = 3.66 Å, d102 = 3.62 Å, and d-112 = 2.85 Å were consistent with the diffraction data belonging to lizardite (96-900-1640). The crystal structure of lizardite is flat. Among serpentine minerals, the structural deformation is the smallest and the symmetry is the highest. On the X-ray diffraction spectrum, the reflection intensity of the bottom surface is relatively weak and the peak shape is sharp (Yi and Wan, 1990). Brucite was also observed in a product of the hydrothermal reaction, and the main diffraction peaks were d001 = 4.77 Å, d010 = 2.72 Å, d002 = 2.38 Å, and d011 = 2.36 Å. By comparing XRD patterns of FS-t-04 and FS-T-01, the intensity of forsterite diffraction peak is weaker, but the intensity of serpentine diffraction peak does not increase. This observation indicates that a reduction of the temperature affects the formation of serpentine when other conditions are kept constant, that is, a temperature that is too low is unsuitable for forsterite serpentinization over the brief timescales of our experiments.
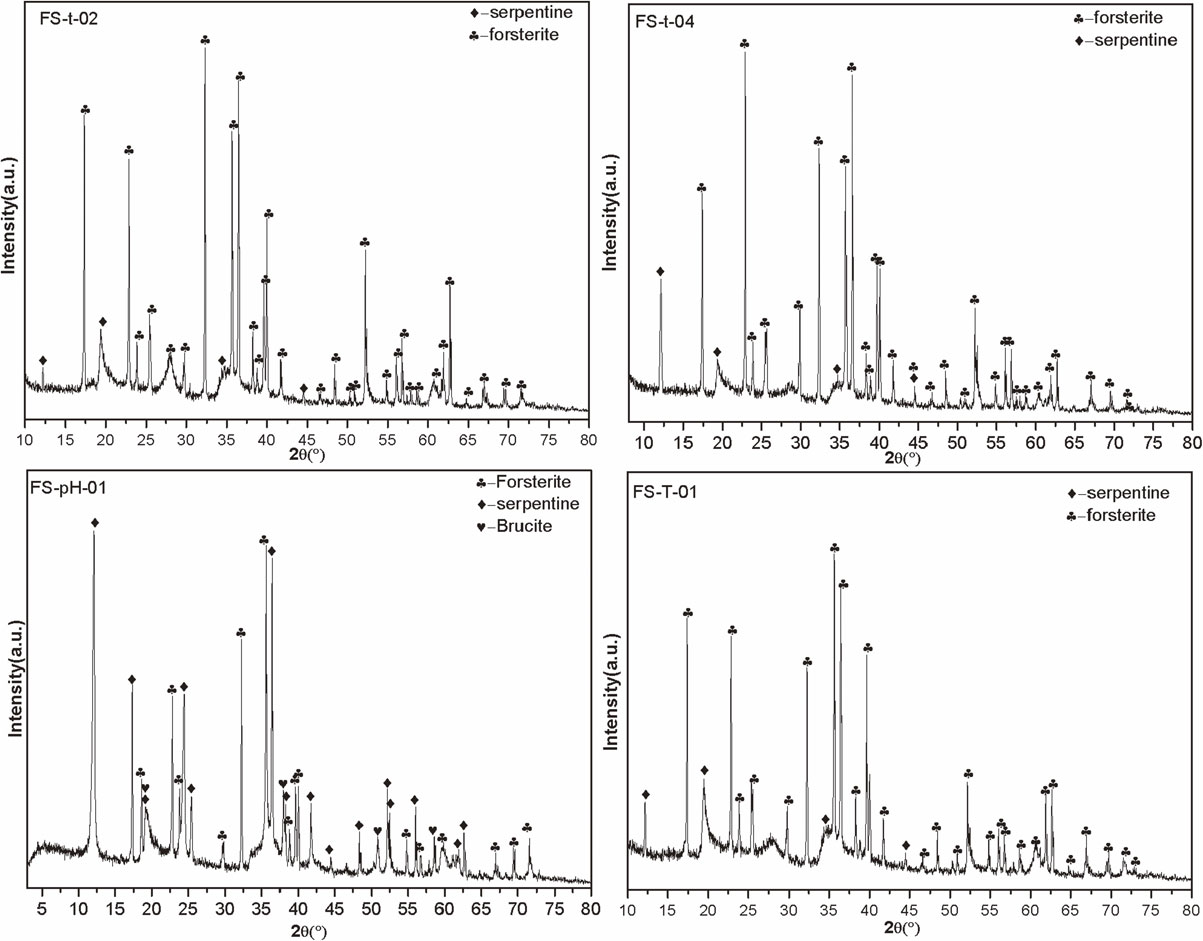
The XRD patterns of FS-t-04 and FS-pH-01 show that increasing the pH value of the hydrothermal system at a constant temperature and reaction time favored the transformation of forsterite to serpentine. This is because the increase in pH is conducive to increase the solubility and concentration of amorphous SiO2, thereby promoting the polymerization of [SiO4]4− in solution and [MgO6]10− on the surface of forsterite, and increasing the rate of serpentinization. As the reaction progressed, brucite appears in sample FS-pH-01, but it was not detected in sample FS-t-02. This is is mainly due to the serpentine gradually formed on the surface of the forsterite crystal, which hindered the internal forsterite serpentinization. The remaining Mg2+ from the serpentine crystallization further formed serpentine by reacting with [SiO4]4− while forming brucite by reacting with the OH−, which has a higher migration activity in the solution in the inner layer (Ren et al., 2004).
Table 3 presents the lattice parameters of forsterite and serpentine calculated using MDI Jade6.5 (Materials Data Ltd., USA) fitting for different samples. The axial length of the forsterite unit cell showed varying degrees of fluctuation after reacting for a certain time under different conditions when compared with natural forsterite, but the overall trend decreases progressively. This is because the radius of Fe2+ is larger than Mg2+, while Fe3+ smaller than Mg2+. During serpentinization, Fe2+ precipitates from forsterite and is oxidized to Fe3+, which leads to the reduction of cell axis length of forsterite crystal. The unit cell axis length of serpentine minerals formed by forsterite serpentinization is smaller (except FS-t-04) when compared with natural serpentine. This phenomenon is related to Fe2+ or Fe3+ entering the serpentine and displacing Mg2+, and a lower reaction temperature or shorter reaction time will not conducive to Fe2+ entering serpentine structure.
| Sample ID | a0/nm | b0/nm | c0/nm | β(°) | |
| M2S | 1.0227 | 0.5995 | 0.4764 | 90 | |
| FS-t-02 | Forsterite | 1.0213 | 0.5965 | 0.4731 | 90 |
| Serpentine | 0.5302 | 0.9200 | 0.7302 | 90 | |
| FS-t-04 | Forsterite | 1.0210 | 0.5945 | 0.4712 | 90 |
| Serpentine | 0.5386 | 0.9268 | 0.7366 | 90 | |
| FS-pH-01 | Forsterite | 1.0205 | 0.5964 | 0.4732 | 90 |
| Serpentine | 0.5306 | 0.9186 | 0.7289 | 90 | |
| FS-T-02 | Forsterite | 1.0202 | 0.5946 | 0.4727 | 90 |
| Serpentine | 0.5274 | 0.9185 | 0.7286 | 90 | |
| Natural serpentine | Serpentine | 0.5316 | 0.9210 | 0.7310 | 90 |
Figure 4 shows the SEM images of forsterite and serpentine samples generated at different reaction temperatures, reaction times, and pH values. Figure 4a shows a SEM image of forsterite particles (M2S), where the surface is flat and smooth.
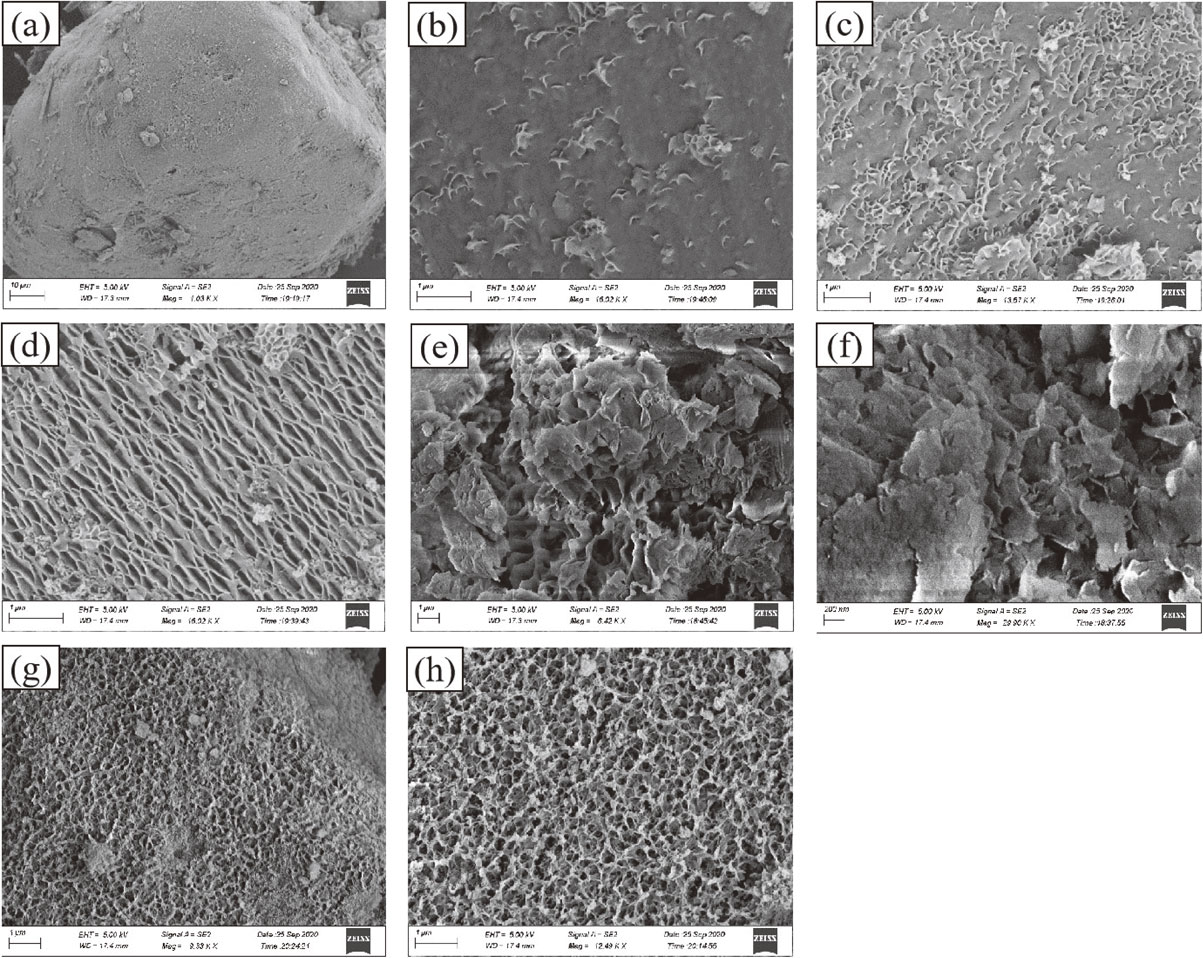
As shown in Figures 4b-4e, the originally flat forsterite particles formed a thin layer of clear, flaky, leaf-like products. The contours of the flaky crystals in the samples became clearer as the reaction time was prolonged. Further, the flaky crystals gradually increased in size and became intertwined. Overall, the flaky crystals demonstrated a regularity in growth perpendicular to the forsterite particles (base) (Figs. 4d-4h). The complete sheet-like and leaf-like serpentines were observed after 20 days of reaction (Figs. 4e and 4f). It can be inferred that the product is lizardite according to the morphology (Li et al., 2017). As shown in Figure 4f, the serpentine crystal form was more perfect, and the flake diameter was larger after 20 days of reaction at pH = 13 and T = 200 °C. Figures 4g and 4h show the micro-morphology of the reaction product at a low temperature. Only a layer of curved flakes was formed on the serpentine surface. The flakes had small diameters and were intertwined to form a mesh-like structure. More and denser flake serpentine was formed with an increase in reaction time, but the formation had little effect on the overall morphology and structure.
Compared with Figure 4e (T = 200 °C, pH = 12), the serpentinization product formed as shown in Figure 4f (temperature = 200 °C, pH = 13) had better crystallinity, larger flaky crystals, and a clearer outline. Figures 4g and 4h illustrate that the serpentine formed at low temperature and low alkalinity had a porous network structure. Therefore, the increase in the reaction temperature and pH of the system facilitated the transformation of forsterite to serpentine. In order to fully conver forsterite into serpentine, sufficient transformation temperature, pressure, fluid conditions and reaction time must be provided.
Variations of molecular vibrational spectroscopyFigure 5 illustrates the FTIR spectra of the forsterite samples. As can be observed from the figure, 839 cm−1 (Si-O-Si symmetric stretching vibration), 882 cm−1, and 983 cm−1 (Si-O-Si anti-symmetric stretching vibration) and 605 cm−1 (Si-O bending vibration) appeared in the wavenumber region of 1000-600 cm−1 belonged to the characteristic absorption peak formed by the vibration of the silicon-oxygen tetrahedron in forsterite (Zhu et al., 2019). The absorption peaks of Mg-O bending vibration were at 503 and 415 cm−1. The absorption peak of OH vibration was at 3430 cm−1 (Ai et al., 2013).

Figure 6 illustrates the FTIR spectra of the forsterite samples after keeping it for 20 days under hydrothermal conditions (FS-pH-01). FTIR absorption peaks are mainly observed in 3700-3400, 1000-800, and 650-400 cm−1. The infrared bands of serpentinization products were assigned after comparison with those of previous studies (Table 4).

| Number | Frequency/cm−1 | (Post and Borer 2000) | (Yariv and Heller-Kallai 1975) | Assignment |
| Lizardite | Lizardite | |||
| 1 | 3690 | 3690-3692 | -OH stretching vibration | |
| 6 | 1076 | 1071-1075 | 1072-1083 | Si-O stretching vibration |
| 7 | 806 | 760-800 | -OH bending vibration | |
| 8 | 618 | 610-617 | Si-O vibration | |
| 9 | 570 | 616-622 | 565-575 | -OH in-plane swing, Mg-O bending vibration |
| 10 | 503 | 500 | 485 | Si-O deformation vibration |
| 11 | 449 | 450-458 | Si-O bending vibration |
The FTIR spectra of samples after forsterite serpentinization, as shown in Figure 6, and those of the forsterite samples shown in Figure 5 had clear distinguishing features. The OH stretching vibration zone was in the region of 3750-3600 cm−1 and had a single absorption peak at 3690 cm−1 while an absorption shoulder appeared on the low-wavenumber side of the peak; this observation is consistent with the infrared spectrum characteristics of lizardite (Post and Borer, 2000). The natural chrysotile with a perfect structure had two absorption peaks at ~ 3670 and ~ 3650 cm−1. The two peaks were caused by the outer and inner hydroxyl groups with an absorption band intensity of approximately 3:1 (Ma, 2012). In the 1200-800 cm−1 zone, there is only one broad and strong Si-O stretching vibration absorption band located at 1076 cm−1, and it is typically serpentine has two absorption peaks near 1077-1082 and 955-967 cm−1 (Yariv and Heller-Kallai, 1975; Post and Borer, 2000). The samples contained a broad and strong absorption peak, which indicates the product’s multi-phase characteristics. In addition to the residual forsterite, it is also associated with the intermediate product of forsterite serpentinization and the imperfect degree of order of the formed lizardite structure. The -OH bending vibration caused the weak band at 806 cm−1. Two absorption peaks occurred near 618 and 570 cm−1 as well as between 700 and 540 cm−1, which were attributed to the Si-O vibration, -OH in-plane swing, and Mg-O bending vibration (Post and Borer, 2000). Absorption peaks near 449 and 503 cm−1 were attributed to the Si-O deformation vibration between 550 and 400 cm−1. The infrared sample spectrum showed that serpentine had a higher crystallinity and a more complete structure after transformation.
Notably, chrysotile often showed a split peak near 1016 cm−1, while a single degenerate peak appeared in FS-pH-01, which is inconsistent with the infrared spectral characteristics of the fiber serpentine. A comparison of the infrared spectra of the forsterite ore and the hydrothermal product under optimized conditions showed that the use of a hydrothermal device can convert forsterite into lizardite in the active SiO2 solution. The artificial samples had a relatively complete structure and high crystallinity at pH = 13 and T = 200 °C.
Variations of micro-structureTo reveal the micro-structure and changes of the products during the transformation process of forsterite to serpentine, the forsterite serpentinization product, which reacted with forsterite under hydrothermal conditions for 20 day, was analyzed by HRTEM (Figs. 7a-7f). Figure 7a shows the HRTEM image of forsterite, where the lattice stripes are continuous and clear, and dp001 = 0.47 nm. Figure 7b shows the forsterite serpentinization product formed after the hydrothermal treatment: lattice stripes with a periodicity of ~ 0.73 nm (Fig. 7c) and internally distributed lattice stripes with a periodicity of ~ 0.47 nm (Fig. 7d) appeared on the surface, corresponding to the (001) plane of serpentine (Uehara, 1998) and (001) plane of forsterite, respectively. As shown in Figure 7c, the lattice stripes (periodicity, ~ 0.73 nm) formed on the forsterite surface were parallel and had strong continuity. Although some ‘point’ defects were observed, no line or surface defects were found, and no wavy structure appeared; these observations are typical lizardite micro-structure characteristics. The micro-structure demonstrated that the forsterite surface layer was transformed into serpentine. The transformation indicates that in forsterite serpentinization, the lizardite layer formed by serpentinization only appears on the edge of the forsterite, and the grain still retains the forsterite structure of the core part (inner area).
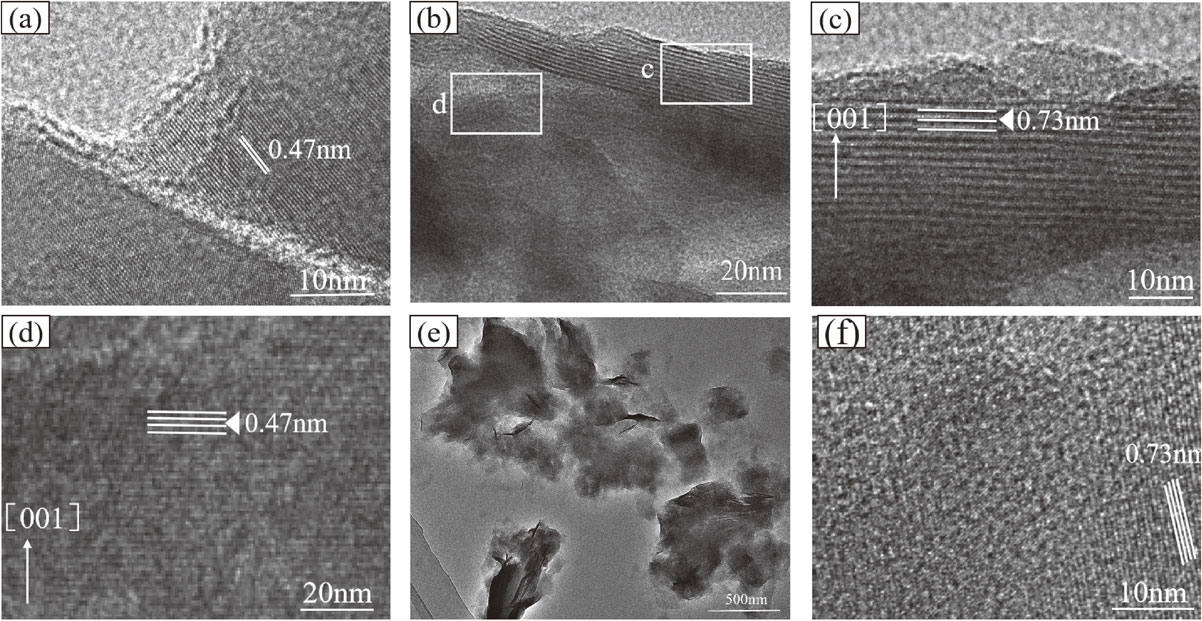
The morphology of the newly generated serpentine had irregular flakes (Fig. 7e), and the cross section of the curved flaky antigorite was visible. The thickness, uniformity, and integrity of the flakes were poor, indicating a lower layer-stacking order of serpentine structure (Amouric and Olives, 1998). The serpentine layer-stacking sequence grew to several 10 nm (Fig. 7f) as the reaction time was prolonged. The above analysis of micro-structure properties show that the elemental process of the serpentinization of forsterite is the coupling of forsterite dissolution and serpentine precipitation.
Thermal analysis spectrum characteristics of transformation productsForsterite (Mg2[SiO4]) is an island-like structure silicate and without thermal effect in the temperature range from 0 to 1200 °C. Figure 8 illustrates the TG-DSC sample curve of forsterite ore. Within the temperature range of 500-800 °C, the TG curve showed a small decrease, and the mass loss rate was ~ 0.14, which was attributed to the loss of free water caused by the burst of vapor-liquid inclusions in forsterite. This change corresponds to the endothermic valley at 680.5 °C on the DSC curve. Lizardite [Mg6[Si4O10](OH)8] is a 1:1 type layered silicate formed by Si-O tetrahedral and Mg-(O, OH) octahedral sheets. Based on the ideal crystal chemical formula of Mg6[Si4O10](OH)8, the H2O+ content has 13 wt%. Figure 9 illustrates the TG-DSC sample curves after 20 days of hydrothermal reaction (FS-pH-01) at T = 200 °C and pH of 13.

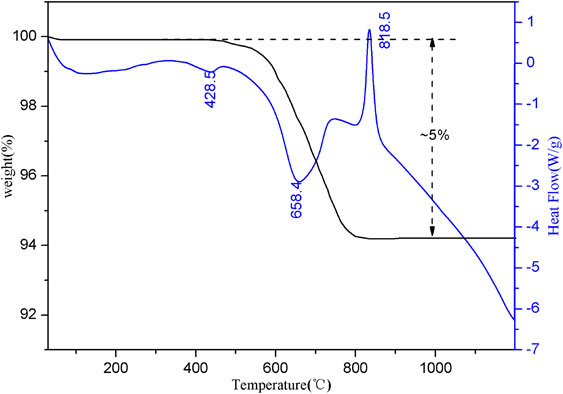
Figure 9 illustrates a small endothermic valley appeared near 428.5 °C on the DSC curve, which was caused by the destruction of the brucite structure in the product and the removal of structural water. The wide and large endothermic valley appearing near 658.4 °C was caused by removing the structural water after the lizardite structure was damaged in the product. A sharp exothermic peak appeared near 818.5 °C, indicating that as the temperature increased, serpentine underwent a phase change, the dehydroxylation process ended, and the structural transformation generated a new phase (forsterite). In the TG curves, no significant weight-loss step occurred when the brucite generated thermal effects, indicating that the brucite content formed during forsterite to serpentine transformation was low. The significant weight-loss step occurred between 500 and 820 °C, which was mainly caused by the loss of structural water with the destruction of the serpentine structure. The weight loss rate of the sample at this stage was ~ 5%, which was equivalent to ~ 38.5% of the serpentine content in the sample. McCollom et al. (2020) found that about 8.1 wt% of the mass loss in the experimental (reactants: olivine, 230 °C, pH = 12.5, 2709 h) was attributed to dehydration of chrysotile while 2.3 wt% was attributed to brucite through thermogravimetric analysis. By comparison, M2S requires a lower temperature and a shorter time, but the serpentine content has little difference.
The island-like structure silicate forsterite can be converted to 1:1 type layered silicate serpentine under hydrothermal conditions. In the transformation process, solid-state forsterite under the action of hot aqueous solution to produce serpentinization, and transformed into serpentine, the excess Mg2+ component is released and converted to brucite [reaction (1)]. The XRD image shows that a large number of brucite diffraction peaks appeared in the product after 20 days of reaction in Figure 3. (FS-pH-01), and this part of Mg2+ mainly came from the dissolution of forsterite in the system, which formed brucite in an alkaline environment. According to the molar ratio of reaction between forsterite and lizardite, the excess [MgO6]10− reacts on the surface of forsterite to form lizardite, which is mainly attached to the surface of forsterite, as shown in Figures 4b and 7b. If the hot aqueous solution contains sufficient [SiO3]2− (dissolved SiO2), all the forsterite components can be converted into lizardite [reaction (2)]. This part of lizardite is formed by the reaction of free [MgO6]10− in the solution after the dissolution of forsterite with dissolved active SiO2, and it does not adhere to the surface of forsterite, as shown in Figure 4f. Reaction (3) shows that the released Mg+ component during the serpentine formation from forsterite in reaction (1) and also reacts with silicic acid in the solution to form serpentine minerals. The amorphous SiO2 forms water-soluble Na2SiO3 under alkaline conditions and produces [SiO4]4− complex anions in the hot aqueous solution [reaction (4)]. Reactions (5) and (6) are further reaction processes of reactions (2) and (3). Alkaline conditions facilitate the reaction of forsterite and amorphous SiO2 to form serpentine as illustrated by reactions (4) and (5).
| \begin{align} &2\text{Mg$_{2}$SiO}_{\text{4(forsterite)}} + 3\text{H$_{2}$O} \\&\quad= \text{Mg$_{3}$Si$_{2}$O$_{5}$(OH)}_{\text{4(lizardite)}} + \text{Mg(OH)}_{\text{2(brucite)}} \end{align} | (1) |
| \begin{align} &3\text{Mg$_{2}$SiO}_{\text{4(forsterite)}} + 4\text{H$_{2}$O} + \text{SiO}_{\text{2 (aq)}} \\&\quad= 2\text{Mg$_{3}$Si$_{2}$O$_{5}$(OH)}_{\text{4(lizardite)}} \end{align} | (2) |
| \begin{align} &3\text{Mg(OH)}_{\text{2(brucite)}} + 2\text{SiO}_{\text{2 (aq)}} \\&\quad= \text{Mg$_{3}$Si$_{2}$O$_{5}$(OH)}_{\text{4(lizardite)}} + \text{H$_{2}$O} \end{align} | (3) |
| \begin{align} &\text{SiO}_{\text{2(amorphous)}} + 2\text{NaOH}_{\text{ (aq)}} \\&\quad= \text{Na$_{2}$SiO$_{3}$} + \text{H$_{2}$O} \end{align} | (4) |
| \begin{align} &3\text{Mg$_{2}$SiO}_{\text{4(forsterite)}} + 5\text{H$_{2}$O} + \text{Na$_{2}$SiO$_{3}$} \\&\quad= 2\text{Mg$_{3}$Si$_{2}$O$_{5}$(OH)}_{\text{4(lizardite)}} + 2\text{NaOH} \end{align} | (5) |
| \begin{align} &3\text{Mg(OH)}_{2} + 3\text{Na$_{2}$SiO$_{3}$} + 4\text{H$_{2}$O} \\&\quad= 2\text{Mg$_{3}$Si$_{2}$O$_{5}$(OH)}_{\text{4(lizardite)}} + 6\text{NaOH} \end{align} | (6) |
Three possible paths exist for forsterite serpentinization based on the existence and formation of [SiO4]4− tetrahedron in the hydrothermal system. (1) In the alkaline hydrothermal system, [SiO4]4− in the forsterite structure polymerizes under the activation of water molecules to form sheet silicate framework [Si2O5]2−, and combines with Mg2+ in the structure to form serpentine in situ, while releasing the remaining Mg2+. (2) The [SiO4]4− in the forsterite structure and the [SiO4]4− in the solution polymerize and form a sheet silicate framework [Si2O5]2− under the activation of water molecules; the silicate framework combines with the Mg2+ in the structure to form serpentine in situ. (3) The [SiO4]4− in the liquid phase of the hydrothermal system polymerizes to form sheet silicate framework [Si2O5]2− and combines with the Mg2+ released in reaction (1) to ectopically form serpentine under liquid-phase conditions or grows with the in situ formed serpentine as crystalline nucleus. The three formation paths are illustrated in Figure 10.

Forsterite can produce slow serpentinization in alkaline hydrothermal systems. Forsterite serpentinization mechanism is the coupling of forsterite dissolution and serpentine precipitation. The serpentine formed under the experimental conditions was lizardite, without antigorite and chrysotile. The transformation rate of forsterite serpentinization was positively correlated with temperature, pH, and reaction time. The forward reaction was promoted while the forsterite to serpentine transformation rate was increased by increasing temperature and pH, and the increase in reaction time increased the degree of forsterite serpentinization in the system. Compared with the previously reported studies (Lafay et al., 2012; McCollom et al., 2016) on the transformation of olivine into chrysotile and lizardite. In the active silica environment system, the time required to synthesize the same content of lizardite is shorter, the temperature is lower, and the crystallinity is higher. During forsterite serpentinization, the [SiO4]4− in the forsterite structure or [SiO4]4− from the solution were polymerized by activating water molecules under hydrothermal conditions. The formed [Si2O5]2− was combined with Mg2+ to form serpentine in situ, or Mg2+ was released by forsterite serpentinization and combined with the [SiO4]4− in the solution to form ectopic serpentine, which can grow on the in situ serpentine nucleus. The formed serpentine was attached to the forsterite surface and extended to the core with an increase in the reaction time. The hydrothermal conditions in the presence of alkaline and active SiO2 were the key factors for transformation.
This work was supported by the National Natural Science Foundation of China (Grant No. 41972042, 42072048). We thank Haiyang Xian from Guangzhou Institute of Geochemistry, Chinese Academy of Sciences for performing high-resolution transmission electron microscopy imaging.