2023 年 118 巻 1 号 論文ID: 221116
2023 年 118 巻 1 号 論文ID: 221116
Microbial organic molecules are widely involved in the formation of calcium carbonate (CaCO3) minerals in various environments on the earth’s surface including hot springs. To evaluate the effect of acidic microbial polysaccharides on the CaCO3 polymorph formation in hot water environments, batch experiments on the CaCO3 formation were performed in the systems containing alginic acid (A system) or gellan gum (G system) at 40, 60, and 80 °C for 24 h. Each system contained 5.0 mmol/L Ca2+ and 20.0 mmol/L total carbonate ions with 0.0, 1.0, 5.0, 10, 50, and 100 mg/L alginic acid or gellan gum. Results showed that needle- or rod-like shaped aragonite crystals were formed as the predominant polymorph in the absence of polysaccharides at 40-80 °C. In the presence of polysaccharides, alginic acid greatly inhibited the aragonite formation and favored the rhombohedral calcite formation as the predominant polymorph with increasing alginic acid concentration at 40 °C. The effect of alginic acid on the polymorphs slightly decreased as temperature increased but was maintained up to 80 °C. Gellan gum also exhibited a similar effect to a lesser extent than alginic acid at the same concentrations and temperatures. Thus, acidic microbial polysaccharides exhibited a significant inhibitory effect on the aragonite formation and favored the calcite formation depending on their molecular structures in hot water environments. This effect of polysaccharides on the CaCO3 polymorphs is attributed to the high adsorption affinity of the polymer molecules for the aragonite surface.
Calcium carbonate (CaCO3) minerals are widely formed in various earth environments, ranging from the soils that cover the terrestrial surface to the deep interior of the crust. They are formed under varying physical and chemical conditions, from near-zero temperatures to hot springs and hydrothermal environments. In these environments, CaCO3 minerals occur as five different polymorphs, calcite, aragonite, vaterite, calcium carbonate monohydrate, and calcium carbonate hexahydrate by its formed environments. In hot temperature environments like hot springs, varying proportions of calcite and aragonite are commonly formed as the major polymorphic products that are mainly controlled by water temperature, concentrations of Mg2+, Sr2+, other cationic and anionic species, and various microbial involvements (Folk, 1994; Fouke et al., 2000; Pentecost, 2005; Fouke, 2011; Jones, 2017). Organic substances consisting of monomeric to polymeric molecules released from microbes also affect the precipitation rates of the CaCO3 minerals and their stable polymorphs through the interaction of these organic molecules with the mineral surfaces. The monomeric molecules, such as low-molecular-weight organic acids, are capable of adsorbing on the CaCO3 surfaces by binding of deprotonated carboxyl groups of the molecular structures to the specific sites on the CaCO3 surfaces. Furthermore, the adsorption affinity of the carboxylic acid is more significant for the aragonite surface than that for the calcite surface. Thus, these molecules significantly inhibit the aragonite formation, leading to the formation of calcite as a stable polymorph (Westin and Rasmuson, 2005; Kawano and Tokonami, 2014; Miyashita et al., 2018). For the microbial polymeric molecules, polysaccharides are the main constituents of extracellular polymeric substances (EPS) of bacteria including cyanobacteria, which widely inhabit hot springs (Whitton and Potts, 2000). These polysaccharides are known to be significantly involved in the formation process of CaCO3 as catalysts that affect the precipitation rate and the stable polymorphs formation (Wada et. al., 1993; Kawaguchi and Decho, 2002; Butler et al., 2006; Olderøy et al., 2009; Tourney and Ngwenya, 2009; Nielsen et al., 2012; Song et al., 2021). However, the magnitude of the effect and its reaction processes are highly dependent on the reaction temperature, polysaccharide molecular structure, and solution chemistry. In particular, the effect of polysaccharides in hot water environments like hot springs is poorly understood due to the lack of experimental verifications.
Therefore, in this study, experiments on the CaCO3 formation were performed in the presence of alginic acid and gellan gum at 40-80 °C to evaluate the effect of these polysaccharides on the CaCO3 polymorphs in hot water environments. The polysaccharide concentrations were varied from 0 to 100 mg/L, and inorganic ions such as Mg2+, Sr2+, and other cationic and anionic species that affect CaCO3 formation were not added to these systems. The results of this study provide important insight into the effect of microbial polysaccharides on the CaCO3 polymorph formation in natural hot sprigs.
The analytical grade polysaccharides, alginic acid and gellan gum, were purchased from Nacalai Tesque Inc., Japan. Alginic acid is a main component of extracellular polymeric substances of various microbes, which consists of two residues of D-mannuronic acid and L-guluronic acid with typical molecular weight of 200 kDa (Donati and Paoletti, 2009). Gellan gum is a microbial biopolymer produced mainly by Sphingomonas strains (Pollock, 1993), which consists of a tetrasaccharide repeating unit composed of D-Glucose, D-Glucuronic acid, D-Glucose, and L-Rhamnose, with molecular weight of approximately ∼ 500 kDa (Nussinovitch, 1997).
The experiments on the CaCO3 precipitation were conducted by the batch method using 100 ml of glass flasks at 40, 60, and 80 °C for 24 h. Before the experiments, stock solutions of 100 mmol/L CaCl2 (Nacalai tesque, Inc.) and NaHCO3 (Nacalai tesque, Inc.) were prepared using guaranteed grade reagents. The stock solutions of 1000 mg/L alginic acid and gellan gum were prepared by mixing deionized water and then heating at 100 °C to dissolve entirely in water. Using these stock solutions, three sets of 100 mL solutions containing 0.0, 1.0, 5.0, 10, 50, and 100 mg/L alginic acid (A system: A0-A100), where each solution contained 5.0 mmol/L Ca2+ and 20 mmol/L total carbonate ions were prepared in glass flasks. Similarly, three sets of 100 mL solutions containing 5.0 mmol/L Ca2+ and 20 mmol/L total carbonate ions with 0.0, 1.0, 5.0, 10, 50, and 100 mg/L gellan gum (G system: G0-G100) were prepared (Table 1). The pH of each solution was maintained below 7.5 throughout the solution preparation using 1.0 M HCl, and was finally adjusted to 7.0 as the initial pH for the precipitation. The glass flasks of both systems were sealed with aerated caps to release degassing CO2, and subsequently incubated in a water bath at three different temperatures of 40, 60, and 80 °C for 24 h.
| Run | Ca2+ (mmol/L) |
Total carbonate (mmol/L) |
Alginic acid (mg/L) |
Gellan gum (mg/L) |
Initial pH | Reaction temp. (°C) |
| A system | ||||||
| A0 | 5.0 | 20.0 | 0.0 | - | 7.0 | 40, 60, 80 |
| A1 | 5.0 | 20.0 | 1.0 | - | 7.0 | 40, 60, 80 |
| A5 | 5.0 | 20.0 | 5.0 | - | 7.0 | 40, 60, 80 |
| A10 | 5.0 | 20.0 | 10.0 | - | 7.0 | 40, 60, 80 |
| A50 | 5.0 | 20.0 | 50.0 | - | 7.0 | 40, 60, 80 |
| A100 | 5.0 | 20.0 | 100.0 | - | 7.0 | 40, 60, 80 |
| G system | ||||||
| G0 | 5.0 | 20.0 | - | 0.0 | 7.0 | 40, 60, 80 |
| G1 | 5.0 | 20.0 | - | 1.0 | 7.0 | 40, 60, 80 |
| G5 | 5.0 | 20.0 | - | 5.0 | 7.0 | 40, 60, 80 |
| G10 | 5.0 | 20.0 | - | 10.0 | 7.0 | 40, 60, 80 |
| G50 | 5.0 | 20.0 | - | 50.0 | 7.0 | 40, 60, 80 |
| G100 | 5.0 | 20.0 | - | 100.0 | 7.0 | 40, 60, 80 |
After the incubation, the solutions were cooled to 25 °C and their pH was measured using a glass electrode. Subsequently, the concentrations of Ca2+ and total carbonate ions in each solution were measured by high performance liquid chromatography (HPLC) with a Hitachi instrument (LaChrom Elite) using a TSKgel IC-Cation I/II HR column for Ca2+ and a TSKgel OApak-P column for total carbonate ions. Using these data, saturation indices [SI = log(IAP/K)] with respect to calcite and aragonite were calculated by the geochemical code of PHREEQC with a database of minteq.dat (Parkhurst and Appelo, 1999). The precipitates in each flask were rinsed with distilled water, and small amounts of the precipitates were placed on a glass slide for optical microscopy. The remaining precipitates were washed with ethanol and then collected by centrifugation. This sample was transferred to an agate mortar, pulverized by adding ethanol, and dried on a slide glass for X-ray powder diffraction (XRD) analysis, which was performed with a Rigaku RINT2000 diffractometer using CuKα radiation generated at 40 kV and 30 mA with a scanning speed of 2° 2θ/min. Optical microscopic observation was conducted with a digital microscope (DN-107T, WanTong Precision Instruments Co. Ltd) using a 10× objective lens and a 10× eyepiece.
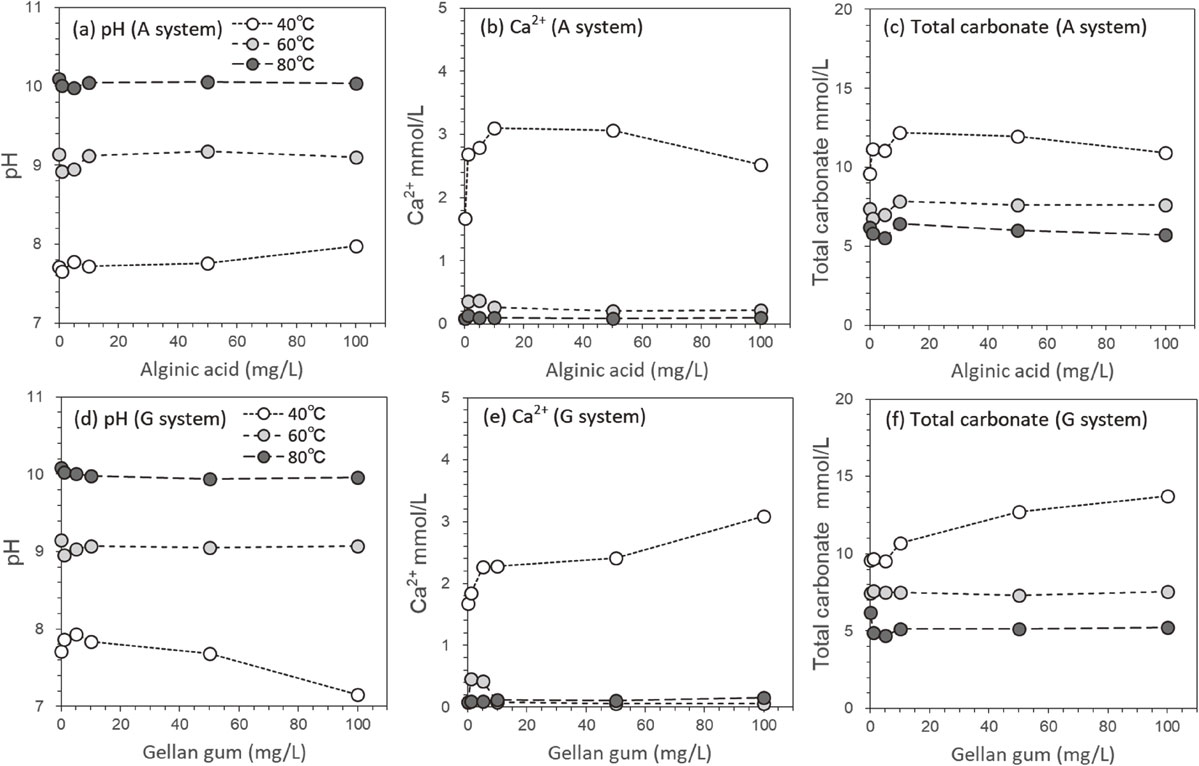
In both A (alginic acid) and G (gellan gum) systems, fine precipitates began to form slowly within several hours of incubation depending on the polysaccharide concentration and temperature. The precipitate formation was slightly delayed with increasing polysaccharide concentration and rapidly accelerated as the temperature increased. After 24 h of incubation, the solution pH of the A system increased up to approximately 7.9, 9.1, and 10.0 at 40, 60, and 80 °C, respectively (Fig. 1a). The concentrations of Ca2+ decreased to 1.7-3.0 mmol/L at 40 °C and <0.4 mmol/L at 60 and 80 °C (Fig. 1b). The total carbonate concentrations also decreased to 10-12 mmol/L at 40 °C, and to 7-8 and 5-6 mmol/L at 60 and 80 °C, respectively (Fig. 1c). For the G system, the solution pH and concentrations of Ca2+ and total carbonate showed relatively similar changes to those of the A system (Figs. 1d-1f). The saturation indices of both systems were SIcalcite = 0.8-1.4 and SIaragonite = 0.5-1.2 regardless of the polysaccharide concentration and incubation temperature, indicating that these systems remained supersaturated with respect to calcite and aragonite throughout the incubation. However, the concentrations of Ca2+ suggested that the precipitation proceeded only halfway at 40 °C and almost completed at 60 and 80 °C. The CaCO3 formation in weakly alkaline solutions around pH < 10 proceeds mainly as follows,
| \begin{equation} \text{Ca}^{2+} + \text{HCO}_{3}^{-} \rightarrow \text{CaCO}_{3} + \text{H}^{+} \end{equation} | (1) |
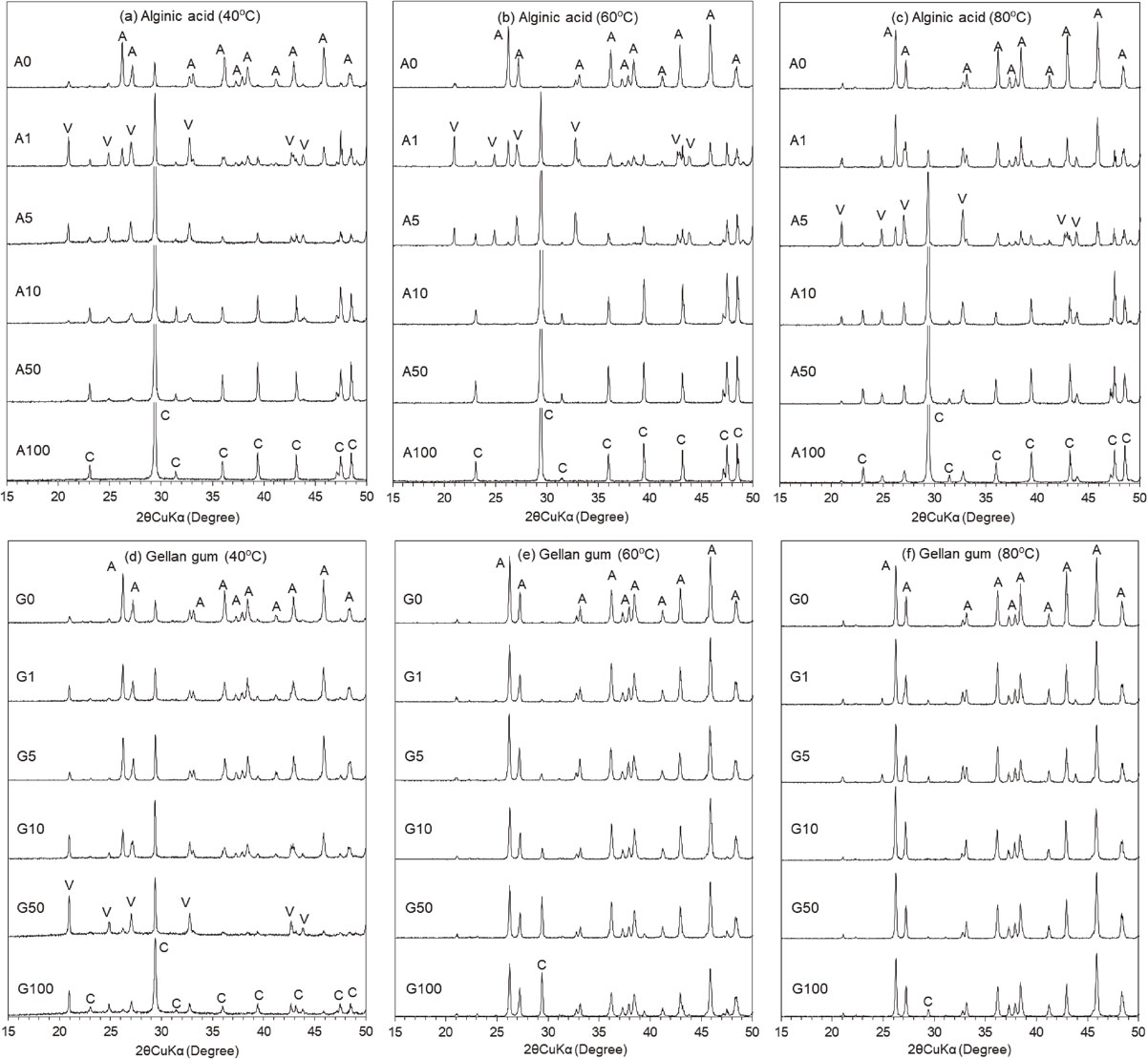
XRD confirmed that aragonite was formed as a predominant polymorph with minor amounts of calcite and vaterite in the A system without alginic acid at 40 °C (Fig. 2a; A0). However, the aragonite formation decreased rapidly at a low alginic acid concentration of 1.0 mg/L, and the calcite formation increased with increasing alginic acid concentrations (Fig. 2a; A1-A100). Additionally, significant amounts of vaterite appeared as a transition phase between aragonite and calcite at alginic acid concentration of 1.0 mg/L, which decreased in amounts as alginic acid concentrations increased. At higher temperatures, aragonite was more predominant and calcite almost disappeared in the absence of alginic acid at 60 and 80 °C (Figs. 2b and 2c; A0). Similar polymorphic changes to those at 40 °C were observed with increasing alginic acid concentrations at 60 and 80 °C (Figs. 2b and 2c), while the changes required slightly higher alginic acid concentrations than those at 40 °C. In the G system, aragonite polymorph was also predominant in the absence of gellan gum at 40-60 °C as observed in the A system (Figs. 2d-2f). As gellan gum concentrations increased, polymorphic changes from aragonite to calcite proceeded gradually at 40 °C, with the appearance of vaterite as a transition phase at gellan gum concentrations of 10-50 mg/L (Fig. 2d). The polymorphic changes with gellan gum concentration were pronounced at 40 °C and remarkably decreased at 60 and 80 °C (Figs. 2e and 2f). These results indicated that alginic acid and gellan gum inhibited the aragonite formation and favored the calcite formation as the predominant polymorph depending on their concentration and temperature. Moreover, alginic acid exhibited a greater effect on the polymorphic changes than gellan gum at the same polysaccharide concentrations and temperatures.
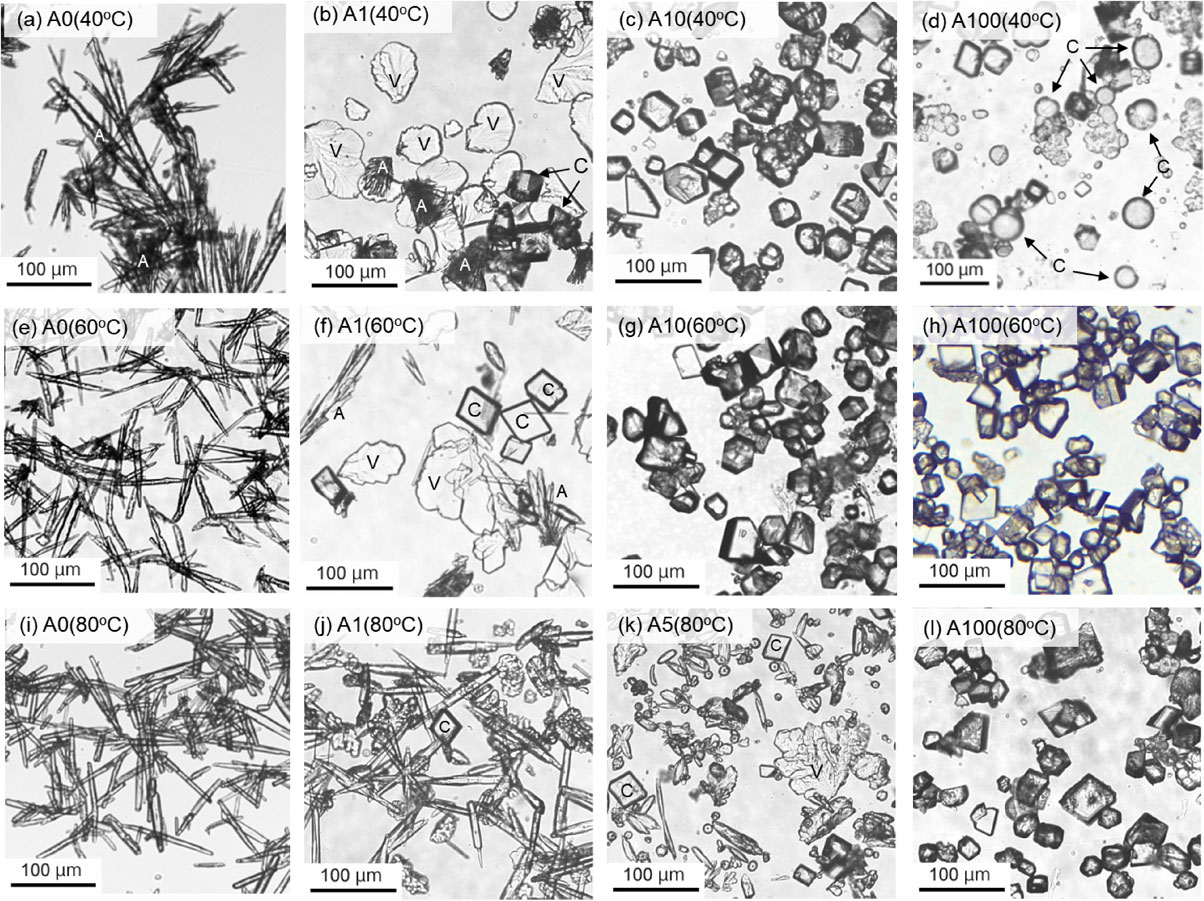
These CaCO3 products formed in both systems exhibited characteristic morphologies depending on the structure and concentration of polysaccharides and the reaction temperature. For the A system, the aragonite crystals formed in the absence of polysaccharides showed needle- or rod-like habits with <200 µm in length and <20 µm in width independent of temperature (Figs. 3a, 3e, and 3i). Vaterite particles exhibiting leaf-like habit up to approximately 200 µm in size were abundantly observed with small amounts of rhombohedral calcite crystals with <50 µm in size in the A1 at 40 °C (Fig. 3b). With increasing alginic acid, vaterite particles disappeared and rhombohedral calcite extensively increased in the A10 (Fig. 3c), and subsequently spherical calcite crystals with <50 µm in diameter coexisted with the rhombohedral calcite particles in the A100 at 40 °C (Fig. 3d). Similar leaf-like vaterite and rhombohedral calcite were observed in the A1 to A100 at 60 (Figs. 3f-3h) and 80 °C (Figs. 3j-3l), while no spherical calcite particles were observed in the A100 at both temperatures (Figs. 3h and 3l). The formation mechanism of spherical calcite is not clear. However, there are some reports of spherical calcite formation in the presence of inorganic ions such as Mg2+ and SO42− and organic molecules such as malic and citric acids (Tracy et al., 1998; Meldrum and Hyde, 2001), as well as the involvement of microorganisms (Zhang et al., 2017). These reports indicated that the precipitation rate of calcite slowed down due to the interaction with these coexisting substances, and therefore the formation of spherical calcite proceeded in the slow crystal growth process. In this study, spherical calcite was formed in the A100 at 40 °C, which contained the highest concentration of alginic acid (100 mg/L). Therefore, the rate of crystal growth was decreased, which possibly favored the formation of spherical calcite as indicated in the reports above.
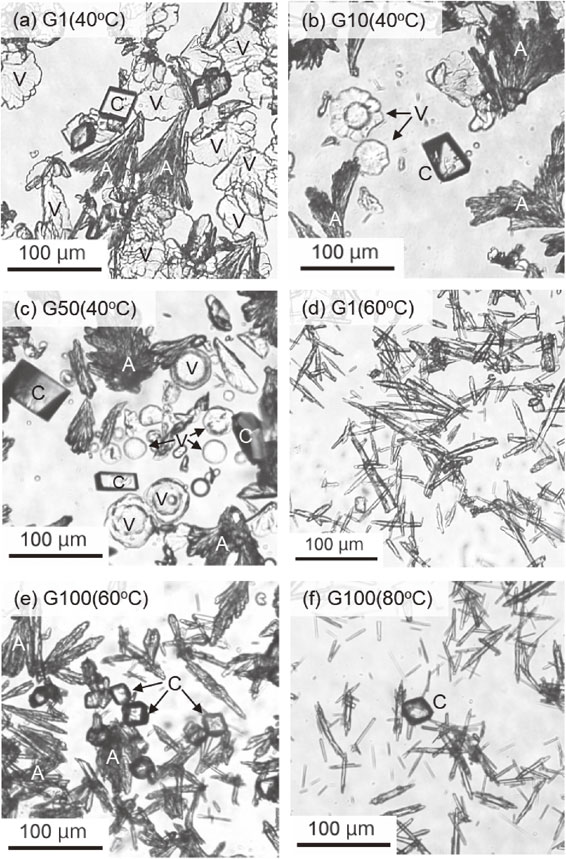
In the G system, needle-like or acicular aragonite crystals with <200 µm in length were formed, which were partially aggregated into a bouquet-like shape in the G0 to G100 at 40 °C (Figs. 4a-4c). Furthermore, rhombohedral calcite crystals with approximately <100 µm in size were present at gellan gam concentrations up to 100 mg/L. Vaterite exhibiting a leaf-like shape appeared at 1.0 mg/L gellan gum concentration (Fig. 4a), and changed to flower-like then to a disk-like morphology with increasing the concentrations (Figs. 4b and 4c). Such flower-like vaterite crystals were reported as synthetic products in systems with organic additives such as urea (Wang et al., 1999), 2-naphthaleneacetic acid and ethylene glycol (Guan et al., 2018). At 60 and 80 °C, needle-like aragonite with almost <100 µm in length formed, and retained morphology throughout the gellan gum concentrations at 1.0-100 mg/L (Figs. 4d-4f), in which rhombohedral calcite particles with <50 µm in size appeared as the gellan gum concentration increased.
CaCO3 products formed in the absence of polysaccharides were dominated by the aragonite polymorph at temperatures of 40-80 °C, with a significant amount of calcite only at 40 °C. Generally, calcite is the most stable polymorph at the ambient atmospheric temperature on the earth’s surface except for specific chemical environments such as high Mg2+/Ca2+ ratio (De Choudens-Sánchez and González, 2009; Lin and Singer, 2009) and high concentrations of Sr2+, Ba2+, and other divalent cations (Wray and Daniels, 1957; Buerger, 1971; McCauley and Roy, 1974; Mucci and Morse, 1983; Wada et al., 1995; Park et al., 2008). These cationic species inhibit the calcite formation and favor the aragonite formation by interacting with the calcite surface and/or incorporating into the calcite lattice (Berner, 1975; Mucci and Morse, 1985; Davis et a., 2000). A highly supersaturated environments also affect the CaCO3 polymorph formation (De Choudens-Sánchez and González, 2009). Namely, vaterite is formed first instead of calcite and aragonite at sufficiently high supersaturation conditions by the Ostwald repining mechanism (Ostwald, 1897). Such specific chemical conditions were not used in this study, thereby the predominant aragonite formation in the absence of polysaccharides was proceeded by the effect of temperature at 40-80 °C. As there are many reports, temperature has a great effect on the polymorph of CaCO3 minerals. Precisely, calcite is formed as the predominant polymorph at 25 °C, while aragonite predominates at least >30 °C with variable amounts of vaterite depending on the experimental conditions (Kitano, 1962; Burton and Walter, 1987; Ogino et al., 1987; Sawada, 1997).
Effect of polysaccharideExperiments on the CaCO3 precipitation demonstrated that polysaccharides inhibited the aragonite formation depending on the structure and concentration of the polymer molecules. Notably, alginic acid showed a high inhibitory effect on the aragonite formation even at a low concentration of 1.0 mg/L, resulting in predominant calcite formation at alginic acid concentrations of >1.0 mg/L. This effect of alginic acid on the CaCO3 polymorph was pronounced at 40 °C and slightly decreased as temperature increased to 60 and 80 °C. Similarly, gellan gum inhibited the aragonite formation and promoted the calcite formation depending on the gellan gum concentration, while the effect on the polymorph was lower than that of alginic acid. For the structure of these polysaccharides, alginic acid is composed of disaccharide repeating units of D-mannuronic acid and L-guluronic acid, each of which has a carboxyl group in its molecular structure. On the other hand, gellan gum is composed of tetrasaccharide repeating units consisting of two residues of D-glucose and one each of residues of L-rhamnose and D-glucuronic acid, in which only D-glucuronic acid residue has a carboxyl group (Milas et al., 1990). Thus, the carboxyl group density of alginic acid is four times higher than that of gellan gum. The pK values of the carboxyl groups in the D-mannuronic acid and L-guluronic acid residues of alginic acid are 3.38 and 3.65, respectively (Donati and Paoletti, 2009), and that of the D-glucuronic acid in the gellan gum polymer is 3.06 (Milas et al., 1990), indicating that these carboxyl groups are fully deprotonated in the pH range of this study (pH7-10). Therefore, these polysaccharides can be interacted with the CaCO3 surface by binding the deprotonated carboxyl sites to the positively charged surface sites on the CaCO3 minerals. In fact, the adsorption affinity of monomeric to polymeric organic molecules for the CaCO3 surface increases with increasing the functional group density in the molecular structure (Kitano and Hood, 1965; Berner et al., 1978; Amjad et al., 1998; Hoch et al., 2000; Klepetsanis et al., 2000; Wada et al., 2001; Lin et al., 2005). Furthermore, many organic molecules having negatively charged surface sites such as the carboxyl group tend to adsorb significantly to the aragonite surface than to the calcite surface (Westin and Rasmuson, 2005; Kawano and Tokonami, 2014; Miyashita et al., 2018), because of the dominating terminated Ca2+ sites on the aragonite surface than the calcite surface (De Leeuw and Parker, 1998.). In the present study, polysaccharides were preferentially adsorbed on the aragonite surface than on the calcite surface depending on the carboxyl group density. Consequently, alginic acid exhibited a high effect on the CaCO3 polymorph formation than gellan gum at hot temperature conditions of 40-80 °C.
In natural hot spring systems, the formation of CaCO3 polymorphs is widely controlled by many physical and chemical factors including water temperature, the chemistry of inorganics and organic molecules, and various microbial involvement. This study provides important findings for investigating the effect of polysaccharides on the CaCO3 polymorph formation in natural hot spring systems.
The experiments on the CaCO3 formation in the A and G systems demonstrated that needle- or rod-like shaped aragonite crystals were formed as the predominant polymorph in the absence of polysaccharides at temperatures of 40-80 °C. In the presence of polysaccharides, alginic acid inhibited the aragonite formation and favored the rhombohedral calcite formation as the predominant polymorph at alginic acid concentrations of >1.0 mg/L at 40 °C. This effect of alginic acid on CaCO3 polymorph formation decreased slightly with increasing temperature up to 60 and 80 °C. Gellan gum also exhibited a similar effect to a lesser extent than alginic acid at the same concentrations and temperatures. The difference in the magnitude of the effect of polysaccharides is consistent with the carboxyl group density of their molecular structure. Therefore, the effect of polysaccharides on the CaCO3 polymorph formation at 40-80 °C is attributed to the preferential adsorption of the alginic acid on the aragonite surface.
The authors would like to thank Y. Koyama, Kagoshima University, for his cooperation in the experiments. We wish to express our appreciation to the anonymous reviewers for thier comments that helped to improve the manuscript.