2023 年 118 巻 1 号 論文ID: 221219d
2023 年 118 巻 1 号 論文ID: 221219d
Needle-shaped rutile inclusions occur in garnet within the quartz-eclogite at Mt. Gongen in the Sanbagawa belt, central Shikoku. They are approximately 5-25 µm along the long axis and are typically oriented along three directions, each intersecting at 120°. This indicates that the needle-shaped rutile is a lamella exsolved from the garnet. Garnet with needle-rutile inclusions is restricted to the quartz-poor domain of the quartz eclogite sample, which consist of quartz, garnet, omphacite, phengite, epidote, kyanite, and hornblende. Garnet grains with rutile lamellae show a composition of the almandine-pyrope series with 14-21 mol% grossular content. Rutile exsolution lamellae were concentrated in the range of 27-34 mol% pyrope of garnet crystals. The garnet host with rutile lamellae has a higher TiO2 content (TiO2 = 0.06-0.19 wt%) than those in rutile-free areas. These chemical compositional characteristics suggest that Ti was incorporated into the crystal structure during garnet growth and subsequently partially exsolved as rutile lamellae during the retrograde stage. Rutile lamellae in garnet have generally been regarded as indicators of ultrahigh-pressure metamorphism, but the present report from quartz-eclogite of the Sanbagawa belt, where no coesite has been found, provides evidence in a natural sample that the appearance of rutile exsolution lamellae is not necessarily under ultrahigh-pressure conditions.
The solubility of Ti in garnet has been experimentally proven to increase under high-temperature and high-pressure conditions (e.g., Zhang et al., 2003; Kawasaki and Motoyoshi, 2016). Therefore, Ti incorporated into the crystal structure of garnet becomes unstable with decreasing pressure and temperature, and is exsolved as lamellae of Ti minerals, such as rutile, during retrograde metamorphism (Zhang and Liou, 1999; Mposkos and Kostopoulos, 2001; Zhang et al., 2003). Rutile exsolution lamellae in natural garnet have been reported in many cases from ultra-high temperature (UHT) and/or ultra-high pressure (UHP) metamorphic or mantle sources, such as eclogite from Sulu, China (e.g., Zhang et al., 1994), majorite garnet from the Appalachian orogenic belt (Keller and Ague, 2020), garnet crystals from the Garnet Ridge in Utah, USA (e.g., Sato and Ogasawara, 2013; Sakamaki et al., 2016), kimberlite from Yakutia, Russia (Alifirova et al., 2012), and osumilite-bearing gneiss from East Antarctica (Kawasaki et al., 2011). Several studies have described rutile exsolution lamellae as indicators of UHP metamorphism (Attoh and Nude, 2008; Glassley et al., 2014). However, some recent studies have reported that the crystallographic characteristics of needle rutile in Idaho star garnet in schist/phyllite with no evidence of UHP are consistent with those of rutile needles in the garnet within the UHP rocks, thus claiming that the occurrence of rutile lamellae is not evidence of UHP (e.g., Hwang et al., 2015; Hwang et al., 2019).
In this report, we describe needle rutile inclusions in garnet within a quartz-eclogite from the Sanbagawa metamorphic belt in central Shikoku. Many previous petrological studies showed that the eclogite in the Sanbagawa metamorphic belt in central Shikoku had not reached UHP conditions (e.g., Miyamoto et al., 2007; Tomioka et al., 2022). The occurrence of rutile exsolution lamellae along with the chemical composition of garnet that have not experienced UHP/UHT conditions reported in this study will improve our understanding of Ti-in-garnet behavior during high-grade metamorphism.
The Sanbagawa metamorphic belt is a high-pressure (HP) type metamorphic belt and is considered to have developed in the Cretaceous along the eastern Asian convergent margin (Isozaki and Itaya, 1990; Wallis and Okudaira, 2016). The highest-grade parts of the Sanbagawa metamorphic belt are distributed extensively in the Besshi region, central Shikoku. Eclogite assemblages sporadically occur in this region, where metamorphic conditions are generally estimated to be 1.5-2.5 GPa and 500-750 °C (e.g., Wallis and Okudaira, 2016).
On the southwestern slope of Mt. Gongen in the Besshi region, quartz-eclogite occurs between the ultramafic and mafic eclogitic bodies. The constituent minerals of quartz-eclogite are quartz, garnet, omphacite, phengite, epidote, kyanite, hornblende, rutile, and apatite (e.g., Ota et al., 2004; Miyamoto et al., 2007; Enami et al., 2018). The peak metamorphic conditions in this region are estimated to be 2.3-2.4 GPa and 675-750 °C (Miyamoto et al., 2007). Although there are inclusions of SiO2 in garnet, coesite, an indicator of UHP metamorphic rocks, was not found in this area. The quartz-eclogites from Gongen Mountain are divided into three domains defined by Enami et al. (2018): a quartz-rich domain, a quartz-poor domain, and a quartz-free mafic clot. The quartz-rich domain is mainly composed of quartz, garnet, and omphacite. The quartz-poor domain is mainly composed of quartz, garnet, omphacite, phengite, epidote, kyanite, and hornblende. The quartz-free mafic clot is mainly composed of hornblende, garnet, omphacite, and phengite. In this study, samples were collected near the summit (near a small stone shrine), on the mountain path (the maintenance track of the transmission tower), and at Tokonabe Creek. These samples showed the same three domains and compositions of minerals as those described in Enami et al. (2018).
The chemical compositions of the garnet grains were analyzed using an electron microprobe (JEOL JXA-8800R) with wavelength-dispersive X-ray spectrometers (WDS) at the Rock and Mineral Laboratory of Nagoya University. The accelerating voltage and current values for point quantification analysis are 12 nA at 15 kV, and 100 nA at 20 kV for areal analysis. The garnet samples used in this study contained a large number of fine rutile grains in some locations, and it was necessary to avoid rutile grains in the point analysis. Therefore, before EPMA analysis, we observed the surface and interior of the garnet using an optical microscope and analyzed points that were more than ∼ 10 µm away from the rutile needles to the spot. The analysis time was set to 20 s for the peak and background. The relative standard deviation (RSD) for determining Ti was approximately 42% (1σ) and the relative error in determining Ti was within 10% (2σ; TiO2:132-320 ppm). The detection limit at the confidence level was less than 150 ppm (3σ). In the future, it is essential to increase the analysis measurement time and counts to improve the accuracy of the quantitative analysis of Ti in garnet grains. In this study, we focus only on the relative concentration of titanium in garnet and do not discuss the details of the quantitative values.
Rutile and other mineral inclusions were identified using a laser Raman spectrometer (Nicolet Almega XR, Thermo Fisher Scientific) at Nagoya University, with gratings of 2400 lines/mm, 532 nm Nd-YAG laser at 25 mW, and a spot size of 1 µm.
Rutile occurs as microscopic crystals within several garnet grains in a thin section. The host garnet grains containing fine rutile inclusions are subhedral and relatively coarse-grained crystals (approximately 1-4 mm in diameter). Three forms of rutile were observed in the garnet: needle-like, rod-like, and spherical (Fig. 1a). Needle-shaped rutile particles are found in garnet grains in quartz-poor domains and not in quartz-rich domains or mafic clots.
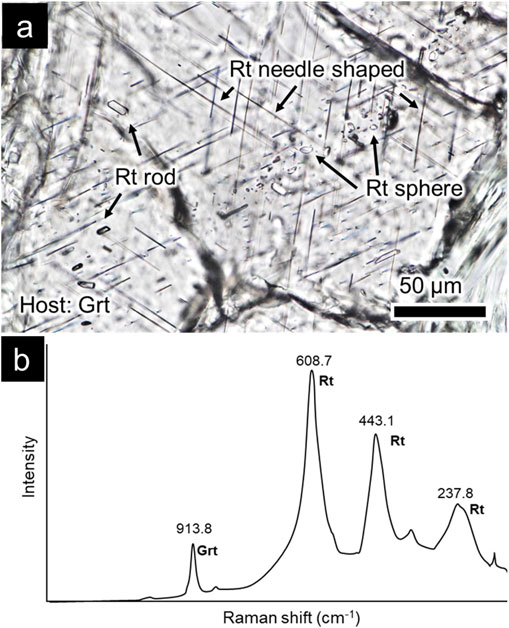
The needle rutile was approximately 5-25 µm long and 1 µm wide. The needle-like crystals showed three orientations, each intersecting at 120° (Fig. 1a). The rutile needles included in the garnet grains showed the strongest Raman bands at approximately 608, 443, and 237 cm−1 (Fig. 1b). The Raman spectra of rutile showed mixed peaks of the host garnet in some cases, but no other mineral peaks were identified. Raman peaks of rod and spherical forms also showed similar wavenumbers.
In this study, we focus on three characteristic garnet grains containing needle-shaped rutile inclusions. An example of a representative composition of garnet with rutile inclusions in quartz-eclogite is presented in Table 1. The garnet of 0601g01 (Grt-0601g01) had a concentration of rutile inclusions in the core (Fig. 2a). Pyrope value of the core with needle rutile inclusions is 32-34 mol% (MgO = 8.7-9.2 wt%), the rutile-free mantle is 35-37 mol% (MgO = 9.2-10.0 wt%), and with a further edge rim is 32-35 mol% (MgO = 8.4-9.3 wt%). The Grossular content of Grt-0601g01 decreases from the core (16-18 mol%; CaO ∼ 7 wt%) to the mantle (12-16 mol%; CaO ∼ 5 wt%) and increases at the rim (15-16 mol%; CaO ∼ 6 wt%). Grt-2205g01 appears to combine the two garnet crystals based on the compositional zoning structure, especially for grossular content (Fig. 2b). In this garnet, needle rutile inclusions were concentrated in the region corresponding to the grossular-poor core (Grs14-15; CaO = ∼ 5.1 wt%). The pyrope content of the garnet in the region containing rutile needles is approximately 32 mol% (MgO = ∼ 8.5 wt%). In the rim part, CaO slightly increases by about 0.4 wt% (Grs15; CaO ∼ 5.5 wt%). Grt-0803g01 contains rutile needles in the rim part (Fig. 2c). The compositional zoning structure of this garnet is also characteristic that the map of pyrope content shows the coalescence of multiple garnet nuclei. The nuclei of those garnet crystals do not contain rutile, and the rims, which grew at the same stage after coalescence, contain needle-shaped rutile. The rutile-free garnet core is about Prp25-28 (MgO = 5.8-7.2 wt%) and the rim containing rutile needles is Prp28-34 (MgO = 7.5-9.1 wt%). The core of Grt-0803g01 is about Grs18-21 (CaO = 6.7-7.7 wt%) and about Grs19-21 (CaO = 7.1-7.8 wt%) in the rim containing rutile needles. Near the edge of this garnet, Grs is lower than the core value below 18 mol% (CaO >7.0 wt%), and MgO content reaches the highest value (Prp35; MgO = 9.43 wt%).
| Grt No. | 0601g01 | 0601g01 | 0601g01 | 2205g01 | 2205g01 | 0803g01 | 0803g01 | 0803g01 |
| Rt | with Rt | No Rt | No Rt | with Rt | No Rt | No Rt | with Rt | No Rt |
| Core | Mantle | Rim | Core | Rim | Core | Rim | Rim (edge) | |
| SiO2 | 39.12 | 39.52 | 39.34 | 38.14 | 38.40 | 38.13 | 39.14 | 39.22 |
| TiO2 | 0.11 | 0.00 | 0.00 | 0.11 | 0.01 | 0.05 | 0.19 | 0.06 |
| Al2O3 | 21.08 | 21.85 | 21.70 | 21.79 | 21.72 | 21.23 | 21.41 | 21.74 |
| Cr2O3 | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 | 0.02 | 0.03 | 0.03 |
| FeO* | 23.58 | 23.63 | 23.05 | 24.75 | 24.99 | 25.09 | 23.61 | 22.45 |
| MnO | 0.59 | 0.56 | 0.51 | 0.79 | 0.48 | 1.63 | 0.50 | 0.37 |
| MgO | 8.86 | 9.97 | 9.33 | 8.51 | 8.41 | 6.54 | 7.59 | 9.43 |
| CaO | 6.63 | 4.64 | 5.96 | 5.11 | 5.47 | 6.67 | 7.40 | 6.37 |
| Total | 99.96 | 100.18 | 99.89 | 99.20 | 99.47 | 99.36 | 99.87 | 99.67 |
| Si | 3.01 | 3.01 | 3.01 | 2.96 | 2.97 | 2.99 | 3.01 | 3.00 |
| Ti | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 |
| Al | 1.91 | 1.96 | 1.95 | 1.99 | 1.98 | 1.96 | 1.94 | 1.96 |
| Cr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fe | 1.51 | 1.50 | 1.47 | 1.61 | 1.62 | 1.64 | 1.52 | 1.44 |
| Mn | 0.04 | 0.04 | 0.03 | 0.05 | 0.03 | 0.11 | 0.03 | 0.02 |
| Mg | 1.01 | 1.13 | 1.06 | 0.98 | 0.97 | 0.76 | 0.87 | 1.07 |
| Ca | 0.55 | 0.38 | 0.49 | 0.43 | 0.45 | 0.56 | 0.61 | 0.52 |
| Total | 8.03 | 8.01 | 8.02 | 8.03 | 8.03 | 8.03 | 8.00 | 8.02 |
| Prp | 33 | 37 | 35 | 32 | 32 | 25 | 29 | 35 |
| Alm | 49 | 49 | 48 | 52 | 53 | 53 | 50 | 47 |
| Grs | 18 | 12 | 16 | 14 | 15 | 18 | 20 | 17 |
| Sps | 1 | 1 | 1 | 2 | 1 | 4 | 1 | 1 |
*Total ions as FeO, Abbreviation of (Grt) and (Rt) are garnet and rutile, respectively.
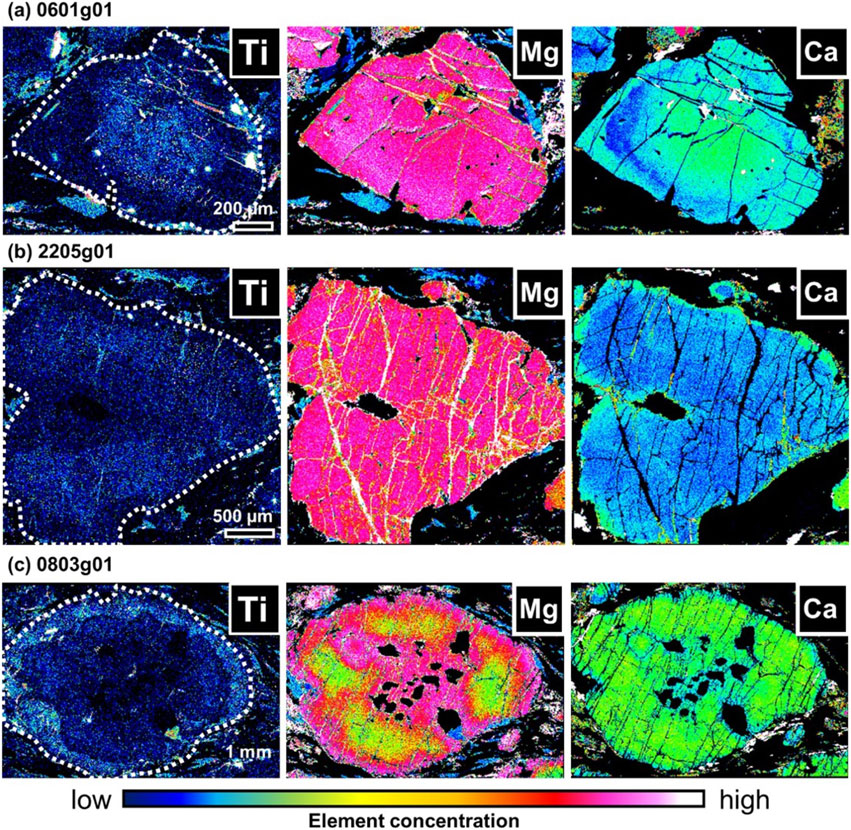
TiO2 is detected in the areas where needle rutile grains are distributed, with TiO2 = 0.06-0.19 wt% in the included areas whereas TiO2 = 0.00-0.06 wt% in the excluded areas (Fig. 2).
Three types of rutile inclusions (needle, rod, and spherical) were observed in this study, and the needle-shaped rutile inclusions can be determined as exsolution lamellae in the following situations: (1) fine-grained less than 25 µm, (2) needle-like shape, and (3) crossed and preferred oriented at an angle of 120° in the garnet (Fig. 1a). The rod-like and spherical rutile grains are interpreted from its shapes as solid inclusion minerals incorporated during the garnet growth.
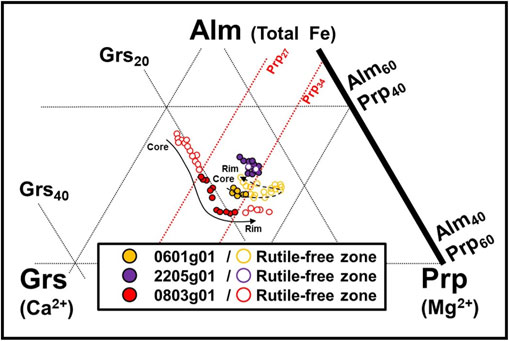
Grt-0803g01 shows a typical prograde zoning pattern, with the pyrope component increasing from core to rim, but the other two garnet grains show a complex pattern of varying grossular content with less variation in pyrope content (Fig. 2). The interpretation of the growth history of these garnet grains in quartz-eclogite has been discussed in previous studies. Enami et al. (2018) divide the garnet grains in quartz-eclogite into four types, from Type I to Type IV, based on the chemical zoning patterns. The most common garnet is Type I, in which the pyrope increases monotonically and the grossular decreases outward, and the second most common type is Type II, in which the grossular increases slightly at the outermost margin. The chemical zoning pattern of Grt-0803g01 with rutile lamellae at the rim showed that the core was pyrope-poor and increased toward the rim when focusing on one of the assembled nuclei (Fig. 2c). Grossular content decreased toward the rim. Based on these characteristics, Grt-0803g01 can be classified as Type I. In Grt-2205g01, focusing on one of the two merged garnet crystals, pyrope content is almost constant and grossular content is also homogeneous and increases slightly at the outermost rim (Fig. 2b). Grt-0601g01 is difficult to attribute, but a triangle diagram of Alm-Grs-Prp shows a composition similar to that of the rim of Grt-0803g01 (Type I; Fig. 3). Therefore, Grt-0601g01 can be interpreted as a garnet that started growing in the middle stage of Type I garnet growth. Enami et al. (2018) explained the differences in the chemical zoning structure of garnet to differences in mineral assemblage under different whole-rock compositions. The Type I core was formed with epidote and kyanite during the prograde eclogite facies stage. Type II cores crystallized under the eclogite facies stage with epidote-free and kyanite-bearing whole-rock composition. The rims of both Type I and Type II crystallized during the later stage of prograde eclogite facies metamorphism. Based on these interpretations, the three analyzed garnet grains can be divided into Type I with a grossular-rich core (Grt-0601g01 and Grt-0803g01) and Type II with a grossular-poor core (Grt-2205g01) which crystalized in the eclogite facies stage under different whole-rock chemical composition environments. Based on these interpretations, the analyzed three garnet grains can be divided into Type I with grossular-rich core (Grt-0601g01 and Grt-0803g01) and Type II with grossular-poor core (Grt-2205g01), and they have crystalized in eclogite facies stage under the different whole-rock chemical composition environments.
The regions of garnet containing rutile lamellae tended to be enriched in Ti (Table 1). This is thought to be the result of Ti being incorporated into the crystal structure during the garnet growth, and subsequently partially being exsolved as rutile lamellae during the retrograde stage. Ti-bearing calcic garnets generally occur as andradite garnets, such as schorlomite [Ca3Ti2(SiO4)(Fe3+O4)2], morimotoite [Ca3(Fe2+Ti)(SiO4)3], and Ti-bearing andradite (‘melanite’) [Ca3(Fe3+>Ti)2(SiO4)3; Huggins et al., 1977]. In contrast, pyralspite (almandine-pyrope series) garnet is less likely to contain a large amount of Ti in its crystal structure (Proyer et al., 2013). For example, almandine-rich garnet in ultra-high temperature granulite (>850 °C) contains a minor amount of Ti by generally supposed substitutions in the octahedral and tetrahedral sites VIAl + IVSi ⇄ VITi + IVAl and/or substitution in the octahedral site 2Al ⇄ (Fe2+, Mg) + Ti (e.g., Ague and Eckert, 2012; Kawasaki and Motoyoshi, 2016). Proyer et al. (2013) proposed the following reaction as one possible means of producing rutile exsolution lamellae.
| \begin{align*} &\text{M$_{3}$(MTi)Si$_{3}$O$_{12}$} + 3\text{M$_{3}$(TiAl)(AlSi$_{2}$)O$_{12}$} \\&\quad = 3\text{M$_{3}$Al$_{2}$Si$_{3}$O$_{12}$} + 4\text{TiO$_{2}$} + 4\text{MO} \end{align*} |
The Sanbagawa belt in Shikoku has been studied for more than half a century without discovering any coesite, and quartz-eclogite in this area has been reported to be below ultrahigh pressure (Miyamoto et al., 2007; Tomioka et al., 2022). Thus, the identification of rutile exsolution lamellae in garnet grown under eclogite facies conditions from the Sanbagawa belt provides important mineralogical evidence that the conditions for the occurrence of rutile exsolution lamellae in nature are satisfied under non-UHP/UHT conditions. Future research prospects include more detailed constraints on P-T conditions under which Ti substitution and exsolution into garnet and a discussion of which sites Ti can be incorporated in the garnet. These results will provide important clues in constraining the conditions for the formation of the needle rutile-containing garnet.
We are grateful to Dr. M. Enami, Dr. T. Kato and all members of the Rock and Mineral Laboratory at Nagoya University for their useful advice. We appreciate the constructive comments from the two anonymous reviewers. This study was supported by JSPS KAKENHI Grant Numbers JP20H02005, JP21H01188, and JP22H01333 to Y.K. and K.M.