2023 年 118 巻 1 号 論文ID: 221224a
2023 年 118 巻 1 号 論文ID: 221224a
H2O-CO2-dominated fluids play a crucial role in most geological phenomena involving fluid-mineral-melt interactions. The equation of state is an essential tool for understanding the phenomena because it predicts the thermodynamic properties of the fluids. The modified Lee-Kesler equation of state for H2O-CO2 mixture fluid developed by Duan and Zhang (2006) is the most accurate at present and is applicable to a wide pressure-temperature range (∼ 2573 K and ∼ 10 GPa). Because of its high accuracy and wide applicable range, the equation has been used for constructing solubility laws in silicate melts. In this paper I review the Duan and Zhang (2006) equation of state and present the calculation procedure. Because the equation for calculating the partial fugacity coefficient is erroneously presented in the original paper, the correct equation is provided here. A C-language code and a Windows executable program for computing thermodynamic properties are provided for the convenience of users. The influence of the nonideal behaviour of the H2O-CO2 mixture fluid on some geological situations is discussed.
The H2O-CO2 fluid is one of the important constituents in the crust. The fluid plays a crucial role in various geological phenomena including magma generation and differentiation, metamorphism, metasomatism, ore formation, and volcanic eruption, because the fluid changes phase relations and acts as a medium for effective transport of the thermal energy and chemical components (e.g., Ferry and Baumgartner, 1987; Holloway, 1987). To understand the phenomena, quantification of the thermodynamic properties of the fluid is essential.
An equation of state is a powerful tool to estimate the pressure-temperature-volume relations and thermodynamic properties of the fluid (e.g., Chapter 13 of Anderson, 2005; Gottschalk, 2007). The equation of state can be classified into two types: the Redlich-Kwong-type equation and the virial-type equation (e.g., Chapter 1 of Walas, 1985; Chapter 2 of Nordstrom and Munoz, 1994; Shibue, 1995; Chapter 13 of Anderson, 2005). The former is an extension of the van der Waals equation and has a limited number of parameters. Therefore the calculation is relatively easily performed and thus the Redlich-Kwong-type equation is favoured in many studies. For the H2O-CO2 fluid, the modified Redlich-Kwong (MRK) equation has been developed extensively for use in geological studies. The MRK equations of Holloway (1977) and Kerrick and Jacobs (1981) have been most widely used in petrological studies. The Holloway (1977) equation was then corrected by Flowers (1979). These equations were calibrated with experimental pressure-temperature-volume data, and the applicable pressure-temperature range is 450-1800 °C and 0.5-40 kbar for the equation by Holloway (1977) and ∼ 1000 °C and ∼ 30 kbar for the equation by Kerrick and Jacobs (1981).
The virial-type equation provides the compressibility factor (compression factor) as a polynomial equation of the volume (or density) terms. The number of parameters for the virial-type equation is much larger than those used in the Redlich-Kwong-type equations, and the calculation requires tasks. The virial-type equation was originally developed as a purely empirical equation in the earlier studies, but later physical meanings of the parameters were provided based on statistical mechanical analysis (Chapter 1 of Walas, 1985). Because the infinite series of the virial expansion is impractical for use, the polynomial equation is usually truncated at the several-order term. Some equations have an additional exponential term that acts as a catch-all for the missing terms. A modern successful equation of this type includes the Lee-Kesler equation (Lee and Kesler, 1975; See APPENDIX for detail), which has been mainly used for hydrocarbons in industry. For the H2O-CO2 fluid, Duan and coworkers in the 1990s began to develop a highly accurate equation by extending the Lee-Kesler equation. First, Duan et al. (1992a) developed an equation of state for pure H2O, CO2, and CH4 at 0-1000 °C and 0-800 MPa. Duan et al. (1992b) then introduced mixing rules into the equation of state and extended its applicability to the mixture of H2O-CO2-CH4 at 50-1000 °C and 0-100 MPa. Duan et al. (1992c, 1995) performed a molecular dynamics (MD) simulation to estimate the volume at high-pressure conditions because experimental determination for pressure-temperature-volume data at higher pressures is difficult and no more experiments are expected to be done. Using the MD simulation results, Duan et al. (1992c, 1996) developed a widely applicable equation for CO2, N2, CO, H2, O2, Cl2 (Duan et al., 1992c) and H2O-CO2-CH4-N2-CO-H2-O2-H2S-Ar systems (Duan et al., 1996). Finally, Duan and Zhang (2006) carried out ab initio simulation and obtained 690 points of pressure-temperature-volume data, through which the equation of state for H2O-CO2 mixtures applicable up to ∼ 10 GPa and 673.15-2573.15 K was developed. At present, this equation of state seems to be the most accurate for H2O-CO2 fluids, and it is applied to the H2O-CO2 solubility model for silicate melts (Duan, 2014; Ghiorso and Gualda, 2015), as reviewed in Part 2 (Yoshimura, 2023).
The present study (Part 1) reviews the equation of state for the H2O-CO2 mixture fluid developed by Duan and Zhang (2006). The following items are reviewed in this order: 1) the general mathematical form of the equation of state, 2) explicit equations for fugacity coefficient for pure and mixed fluids, 3) general characteristics of the molar volume and activity coefficient, and the comparison with the equation of state by Kerrick and Jacobs (1981). In addition, 4) the importance of nonideal mixing in geological situations where two fluids mix is discussed. It is cautioned that equations for the partial fugacity coefficient are erroneously presented in Duan’s papers. The correct equation is therefore presented in this paper. An example computer code written in the C-language and a Windows executable program are also provided as Supplementary Material (available online from https://doi.org/10.2465/jmps.221224a) so that readers can easily reproduce the correct calculations for the thermodynamic properties of the fluid.
Duan and Zhang (2006) used the modified version of the Lee-Kesler equation (Lee and Kesler, 1975). The original Lee-Kesler equation is briefly reviewed in the APPENDIX A. The modified Lee-Kesler equation for geological fluids was first formulated by Duan et al. (1992a, 1992b), and the parameters used therein were updated by Duan and Zhang (2006) so that the equation can be applicable to wider pressure-temperature conditions.
The modified Lee-Kesler equation is given as:
| \begin{align} Z &= \frac{PV_{\text{m}}}{RT} = 1 + \frac{BV_{\text{c}}}{V_{\text{m}}} + \frac{CV_{\text{c}}^{2}}{V_{\text{m}}^{2}} + \frac{DV_{\text{c}}^{4}}{V_{\text{m}}^{4}} \\&\quad+ \frac{EV_{\text{c}}^{5}}{V_{\text{m}}^{5}} + \frac{FV_{\text{c}}^{2}}{V_{\text{m}}^{2}}\left(\beta + \frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}}\right) \exp\left(-\frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}}\right) \end{align} | (1), |
where Z is the compressibility factor (a non-dimensional value), P is the pressure (bar), Vm is the molar volume (cm3 mol−1), R is the gas constant (83.14467 bar cm3 K−1 mol−1), and T is the temperature (K). The parameters B, C, D, E, and F are functions of temperature and are given as:
| \begin{equation} B = a_{1} + a_{2}\left(\frac{T_{\text{c}}}{T}\right)^{2} {}+ a_{3}\left(\frac{T_{\text{c}}}{T}\right)^{3} \end{equation} | (2a), |
| \begin{equation} C = a_{4} + a_{5}\left(\frac{T_{\text{c}}}{T}\right)^{2} {}+ a_{6}\left(\frac{T_{\text{c}}}{T}\right)^{3} \end{equation} | (2b), |
| \begin{equation} D = a_{7} + a_{8}\left(\frac{T_{\text{c}}}{T}\right)^{2} {}+ a_{9}\left(\frac{T_{\text{c}}}{T}\right)^{3} \end{equation} | (2c), |
| \begin{equation} E = a_{10} + a_{11}\left(\frac{T_{\text{c}}}{T}\right)^{2} {}+ a_{12}\left(\frac{T_{\text{c}}}{T}\right)^{3} \end{equation} | (2d), |
and
| \begin{equation} F = \alpha\left(\frac{T_{\text{c}}}{T}\right)^{3} \end{equation} | (2e). |
The parameters β and γ in Eq. (1) and a1, a2, …, a12, and α in Eq. (2a)-(2e) are constant values dependent on volatile species (H2O or CO2) and pressure conditions (Table 1): when the pressure is 0-0.2 GPa, the low-pressure values are used, whereas when the pressure is 0.2-10 GPa, the high-pressure values are used. Tc is the critical temperature (647.25 K for H2O and 304.1282 K for CO2). Vc in Eq. (1) is given as:
| \begin{equation} V_{\text{c}} = \frac{RT_{\text{c}}}{P_{\text{c}}} \end{equation} | (3), |
where Pc is the critical pressure (221.19 bar for H2O and 73.773 bar for CO2). At a given temperature and pressure, Vm is obtained by solving Eq. (1) numerically based on some techniques such as the Newton-Raphson method (e.g., Chapter 3 of Albarède, 1995).
| Parameters | H2O | CO2 | ||
| 0-0.2 GPa | 0.2-10 GPa | 0-0.2 GPa | 0.2-10 GPa | |
| a1 | 4.38269941 × 10−2 | 4.68071541 × 10−2 | 1.14400435 × 10−1 | 5.72573440 × 10−3 |
| a2 | −1.68244362 × 10−1 | −2.81275941 × 10−1 | −9.38526684 × 10−1 | 7.94836769 |
| a3 | −2.36923373 × 10−1 | −2.43926365 × 10−1 | 7.21857006 × 10−1 | −3.84236281 × 101 |
| a4 | 1.13027462 × 10−2 | 1.10016958 × 10−2 | 8.81072902 × 10−3 | 3.71600369 × 10−2 |
| a5 | −7.67764181 × 10−2 | −3.86603525 × 10−2 | 6.36473911 × 10−2 | −1.92888994 |
| a6 | 9.71820593 × 10−2 | 9.30095461 × 10−2 | −7.70822213 × 10−2 | 6.64254770 |
| a7 | 6.62674916 × 10−5 | −1.15747171 × 10−5 | 9.01506064 × 10−4 | −7.02203950 × 10−6 |
| a8 | 1.06637349 × 10−3 | 4.19873848 × 10−4 | −6.81834166 × 10−3 | 1.77093234 × 10−2 |
| a9 | −1.23265258 × 10−3 | −5.82739501 × 10−4 | 7.32364258 × 10−3 | −4.81892026 × 10−2 |
| a10 | −8.93953948 × 10−6 | 1.00936000 × 10−6 | −1.10288237 × 10−4 | 3.88344869 × 10−6 |
| a11 | −3.88124606 × 10−5 | −1.01713593 × 10−5 | 1.26524193 × 10−3 | −5.54833167 × 10−4 |
| a12 | 5.61510206 × 10−5 | 1.63934213 × 10−5 | −1.49730823 × 10−3 | 1.70489748 × 10−3 |
| α | 7.51274488 × 10−3 | −4.49505919 × 10−2 | 7.81940730 × 10−3 | −4.13039220 × 10−1 |
| β | 2.51598931 | −3.15028174 × 10−1 | −4.22918013 | −8.47988634 |
| γ | 3.94000000 × 10−2 | 1.25000000 × 10−2 | 1.58500000 × 10−1 | 2.80000000 × 10−2 |
Reproduced from Table 4 of Duan and Zhang (2006).
To apply Eq. (1) to H2O-CO2 binary mixture fluid, mixing rules are necessary. Duan et al. (1992b), after trial and error, found that the mixing rules that have the virial-expansion form similar to those of Anderko and Pitzer (1991) well reproduce the experimental data. The same rules are also employed in the equation of state by Duan and Zhang (2006).
For the H2O-CO2 binary mixture fluid with the H2O mole fraction of y1 and CO2 mole fraction of y2 (i.e., y1 + y2 = 1) (hereafter subscripts 1 and 2 attached to parameters represent those of H2O and CO2, respectively), the mixing rules are given as follows:
| \begin{equation} BV_{\text{c}} = \sum_{i=1}^{2}\sum_{j=1}^{2}y_{i}y_{j}B_{ij}{V_{\text{c}}}_{\,ij} \end{equation} | (4a), |
| \begin{equation} CV_{\text{c}}^{2} = \sum_{i=1}^{2}\sum_{j=1}^{2}\sum_{k=1}^{2}y_{i}y_{j}y_{k}C_{ijk}{V_{\text{c}}^{2}}_{ijk} \end{equation} | (4b), |
| \begin{equation} DV_{\text{c}}^{4} = \sum_{i=1}^{2}\sum_{j=1}^{2}\sum_{k=1}^{2}\sum_{l=1}^{2}\sum_{m=1}^{2}y_{i}y_{j}y_{k}y_{l}y_{m}D_{ijklm}{V_{\text{c}}^{4}}_{ijklm} \end{equation} | (4c), |
| \begin{equation} {EV_{\text{c}}^{5} = \sum_{i=1}^{2}\sum_{j=1}^{2}\sum_{k=1}^{2}\sum_{l=1}^{2}\sum_{m=1}^{2}\sum_{n=1}^{2}y_{i}y_{j}y_{k}y_{l}y_{m}y_{n}E_{ijklmn}{V_{\text{c}}^{5}}_{ijklmn}} \end{equation} | (4d), |
| \begin{equation} FV_{\text{c}} = \sum_{i=1}^{2}\sum_{j=1}^{2}y_{i}y_{j}F_{ij}{V_{\text{c}}}_{\,ij} \end{equation} | (4e), |
| \begin{equation} \beta = \sum_{i=1}^{2}y_{i}\beta_{i} \end{equation} | (4f), |
and
| \begin{equation} \gamma V_{\text{c}}^{2} = \sum_{i=1}^{2}\sum_{j=1}^{2}\sum_{k=1}^{2}y_{i}y_{j}y_{k}\gamma_{ijk}{V_{\text{c}}^{2}}_{ijk} \end{equation} | (4g), |
wherein the parameters for the mixture are given as follows:
| \begin{equation} B_{ij} = \left(\frac{\root 3 \of {B_{i}} + \root 3 \of {B_{j}}}{2}\right)^{3}k_{1,ij} \end{equation} | (5a), |
| \begin{equation} {V_{\text{c}}}_{\,ij} = \left(\frac{\root 3 \of {{V_{\text{c}}}_{\,i}} + \root 3 \of {{V_{\text{c}}}_{\,j}}}{2}\right)^{3} \end{equation} | (5b), |
| \begin{equation} C_{ijk} = \left(\frac{\root 3 \of {C_{i}} + \root 3 \of {C_{j}} + \root 3 \of {C_{k}}}{3}\right)^{3}k_{2,ijk} \end{equation} | (5c), |
| \begin{equation} {V_{\text{c}}}_{\,ijk} = \left(\frac{\root 3 \of {{V_{\text{c}}}_{\,i}}+ \root 3 \of {{V_{\text{c}}}_{\,j}}+\root 3 \of {{V_{\text{c}}}_{\,k}}}{3}\right)^{3} \end{equation} | (5d), |
| \begin{equation} D_{ijklm} = \left(\frac{\root 3 \of {D_{i}} + \root 3 \of {D_{j}} + \root 3 \of {D_{k}} + \root 3 \of {D_{l}} + \root 3 \of {D_{m}}}{5}\right)^{3} \end{equation} | (5e), |
| \begin{equation} {V_{\text{c}}}_{\,ijklm} = \left(\frac{\root 3 \of {{V_{\text{c}}}_{\,i}} + \root 3 \of {{V_{\text{c}}}_{\,j}} + \root 3 \of {{V_{\text{c}}}_{\,k}} + \root 3 \of {{V_{\text{c}}}_{\,l}} + \root 3 \of {{V_{\text{c}}}_{\,m}}}{5}\right)^{3} \end{equation} | (5f), |
| \begin{equation} E_{ijklmn} = \left(\frac{\root 3 \of {E_{i}} + \root 3 \of {E_{j}} + \root 3 \of {E_{k}} + \root 3 \of {E_{l}} + \root 3 \of {E_{m}} + \root 3 \of {E_{n}}}{6}\right)^{3} \end{equation} | (5g), |
| \begin{align} &{{V_{\text{c}}}_{\,ijklmn}} \\&\quad = {\left(\frac{\root 3 \of {{V_{\text{c}}}_{\,i}} + \root 3 \of {{V_{\text{c}}}_{\,j}} + \root 3 \of {{V_{\text{c}}}_{\,k}} + \root 3 \of {{V_{\text{c}}}_{\,l}} + \root 3 \of {{V_{\text{c}}}_{\,m}} + \root 3 \of {{V_{\text{c}}}_{\,n}}}{6}\right)^{3}} \end{align} | (5h), |
| \begin{equation} F_{ij} = \left(\frac{\root 3 \of {F_{i}} + \root 3 \of {F_{j}}}{2}\right)^{3} \end{equation} | (5i), |
and
| \begin{equation} \gamma_{ijk} = \left(\frac{\root 3 \of {\gamma_{i}} + \root 3 \of {\gamma_{j}} + \root 3 \of {\gamma_{k}}}{3}\right)^{3}k_{3,ijk} \end{equation} | (5j). |
The mixing calculation is performed as follows. First, values for parameters B, C, D, E, and F as well as β and γ for the pure H2O and CO2 fluid are obtained based on Eqs. (2a)-(2e) and Table 1. Vc and Vm for the pure H2O and pure CO2 are also calculated with Eq. (3) and (1), respectively. Subscripts 1 and 2 are attached to the parameters for these values (for example, B for pure H2O is set as B1 and B for pure CO2 is set as B2). Next, parameters for the mixture are calculated according to Eq. (5a)-(5j). For example, B12 (= B21) is obtained with B1, B2, and Eq. (5a). Similarly, C112 (= C121 = C211) and C122 (= C212 = C221) are calculated according to Eq. (5c). Other mixture parameters are also calculated in the same manner. In the case that all subscripts are identical (i = j; i = j = k), the parameter represents that for the pure fluid. For example, B11 is identical to B1 and B22 is identical to B2. Similarly, C111 is identical to C1 and C222 is identical to C2. Then, the terms BVc, $CV_{\text{c}}^{2}$, … for the mixture are calculated with Eq. (4a)-(4g). Note that the terms BVc, $CV_{\text{c}}^{2}$, … are not simple products of two parameters: for example, BVc is not a product of B and Vc, but is calculated as $BV_{\text{c}} = y_{1}^{2}B_{1}V_{\text{c}\,1} + 2y_{1}y_{2}B_{12}V_{\text{c}\,12} + y_{2}^{2}B_{2}V_{\text{c}\,2}$ according to Eq. (4a). Finally, putting all these parameters in Eq. (1), Z and Vm for the mixture are calculated. A numerical technique such as the Newton-Raphson method is again used for this calculation.
In Eqs. (5a), (5c), and (5j), the terms k1,ij, k2,ijk, and k3,ijk denote binary interaction parameters dependent on the temperature and pressure range and interacting components (Table 2). For example, for k1,ij, when i ≠ j (i.e., k1,12 = k1,21), k1,ij is 3.131 − 5.6024 × 10−3T + 1.8641 × 10−6T2 − 31.409/T where T represents temperature (K) if the pressure is 0-0.2 GPa, whereas it is 9.034 − 7.9212 × 10−3T + 2.3285 × 10−6T2 − 2.4221 × 103/T if the pressure is 0.2-10 GPa. When i = j (k1,11 = k1,22), k1,ij is 1 irrespective of temperature and pressure range. Similar rules apply to k2,ijk and k3,ijk.
| Pressure range | Parameters | Conditions for i, j, and k | Equation(1) |
| 0-0.2 GPa | k1,ij | i ≠ j | 3.131 − 5.0624 × 10−3 × T + 1.8641 × 10−6 × T2 − 31.409 × T−1 |
| i = j | 1 | ||
| k2,ijk | i ≠ j ≠ k, i = j ≠ k, j = k ≠ i | −46.646 + 4.2877 × 10−2 × T − 1.0892 × 10−5 × T2 + 1.5782 × 104 × T−1 | |
| i = j = k | 1 | ||
| k3,ijk | i ≠ j ≠ k, i = j ≠ k, j = k ≠ i | 0.9 | |
| i = j = k | 1 | ||
| 0.2-10 GPa | k1,ij | i ≠ j | 9.034 − 7.9212 × 10−3 × T + 2.3285 × 10−6 × T2 − 2.4221 × 103 × T−1 |
| i = j | 1 | ||
| k2,ijk | i ≠ j ≠ k, i = j ≠ k, j = k ≠ i | −1.068 + 1.8756 × 10−3 × T − 4.9371 × 10−7 × T2 + 6.6180 × 102 × T−1 | |
| i = j = k | 1 | ||
| k3,ijk | i ≠ j ≠ k, i = j ≠ k, j = k ≠ i | 1 | |
| i = j = k | 1 | ||
Reproduced from Table 6 of Duan and Zhang (2006).
(1)T represents the temperature (K) and its range is 673.15-2573.15 K.
Calculation of the fugacity coefficient is essential to evaluate phase equilibrium because it is directly related to the chemical potential. For a pure fluid (H2O or CO2), the fugacity coefficient ϕ is defined as:
| \begin{equation} \phi = \frac{f}{P} \end{equation} | (6), |
where f is the fugacity. The fugacity coefficient is obtained from the equation of state as (Chapter 3 of Walas, 1985):
| \begin{equation} \ln \phi = Z - 1 - \ln Z - \frac{1}{RT}\int_{\infty}^{V}\left(P - \frac{RT}{V}\right)dV \end{equation} | (7), |
where V represents the volume of the fluid. Using Eq. (1) and (7) and assuming 1 mol of fluid (V → Vm), the following equation is obtained:
| \begin{align} \ln \phi &= Z - 1 - \ln Z + \frac{BV_{\text{c}}}{V_{\text{m}}} + \frac{CV_{\text{c}}^{2}}{2V_{\text{m}}^{2}} + \frac{DV_{\text{c}}^{4}}{4V_{\text{m}}^{4}} + \frac{EV_{\text{c}}^{5}}{5V_{\text{m}}^{5}} \\ & \quad + \frac{F}{2\gamma}\left[\beta + 1 - \left(\beta + 1 + \frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}}\right)\exp\left(-\frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}}\right)\right] \end{align} | (8). |
Note that this equation is not shown in Duan and Zhang (2006) but is provided in Duan et al. (1992a) and the Appendix of Duan (2014).
The partial fugacity coefficient of a component i in a mixture fluid, ϕi, is defined as:
| \begin{equation} \phi_{i} = \frac{f_{i}}{y_{i}P} \end{equation} | (9), |
where yiP represents the partial pressure and fi represents the partial fugacity of component i. The partial fugacity coefficient is given as (Chapter 3 of Walas, 1985; Chapter 3 of Prausnitz et al., 1999):
| \begin{equation} \ln \phi_{i} = \frac{1}{RT}\int_{0}^{P}\left(\bar{V_{i}} - \frac{RT}{P}\right)dP \end{equation} | (10), |
where $\bar{V_{i}}$ represents the partial molar volume of component i in the mixture fluid. Eq. (10) can be transformed into the following form (Chapter 3 of Walas, 1985; Chapter 3 of Prausnitz et al., 1999):
| \begin{equation} \ln \phi_{i} = \frac{1}{RT}\int_{\infty}^{V}\left[\left(\frac{\partial P}{\partial n_{i}}\right)_{T,V,n_{j}} {}- \frac{RT}{V} \right]dV - \ln Z \end{equation} | (11), |
where ni and nj represent the amount of substance for H2O or CO2. Applying Eq. (11) to Eq. (1), ϕi is obtained as:
| \begin{align} &\ln \phi_{i} \\ & \quad= -\ln Z + \frac{(BV_{\text{c}})_{i}{'}}{V_{\text{m}}} + \frac{(CV_{\text{c}}^{2})_{i}{'}}{2V_{\text{m}}^{2}} + \frac{(DV_{\text{c}}^{4})_{i}{'}}{4V_{\text{m}}^{4}} + \frac{(EV_{\text{c}}^{5})_{i}{'}}{5V_{\text{m}}^{5}} \\ & \qquad+ \frac{(FV_{\text{c}}^{2})_{i}{'}\beta + \beta_{i}{'}FV_{\text{c}}^{2}}{2\gamma V_{\text{c}}^{2}}\left[1 - \exp\left(-\frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}}\right)\right] \\ & \qquad + {\frac{(FV_{\text{c}}^{2})_{i}{'}\gamma V_{\text{c}}^{2} + (\gamma V_{\text{c}}^{2})_{i}{'}FV_{\text{c}}^{2} - FV_{\text{c}}^{2}\beta [(\gamma V_{\text{c}}^{2})_{i}{'} - \gamma V_{\text{c}}^{2}]}{2(\gamma V_{\text{c}}^{2})^{2}}}\\ & \qquad\times \left[1 - \left(\frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}} + 1\right)\exp\left(-\frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}}\right)\right] \\ & \qquad - \frac{[(\gamma V_{\text{c}}^{2})_{i}{'} - \gamma V_{\text{c}}^{2} ]FV_{\text{c}}^{2}}{2(\gamma V_{\text{c}}^{2})^{2}}\\ & \qquad \times\left[2 - \left(\frac{(\gamma V_{\text{c}}^{2})^{2}}{V_{\text{m}}^{4}} + \frac{2\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}} + 2\right)\exp \left(-\frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}}\right)\right] \end{align} | (12), |
where $(BV_{\text{c}})_{i}{}'$, $(CV_{\text{c}}^{2})_{i}{}'$, … represent the following functions:
| \begin{equation} (BV_{\text{c}})_{i}{'} = 2\sum_{j=1}^{2}y_{j}B_{ij}{V_{\text{c}}}_{\,ij} \end{equation} | (13a), |
| \begin{equation} (CV_{\text{c}}^{2})_{i}{'} = 3\sum_{j=1}^{2}\sum_{k=1}^{2}y_{j}y_{k}C_{ijk}{V_{\text{c}}^{2}}_{ijk} \end{equation} | (13b), |
| \begin{equation} (DV_{\text{c}}^{4})_{i}{'} = 5\sum_{j=1}^{2}\sum_{k=1}^{2}\sum_{l=1}^{2}\sum_{m=1}^{2}y_{j}y_{k}y_{l}y_{m}D_{ijklm}{V_{\text{c}}^{4}}_{ijklm} \end{equation} | (13c), |
| \begin{equation} {(EV_{\text{c}}^{5})_{i}{'} = 6\sum_{j=1}^{2}\sum_{k=1}^{2}\sum_{l=1}^{2}\sum_{m=1}^{2}\sum_{n=1}^{2}y_{j}y_{k}y_{l}y_{m}y_{n}E_{ijklmn}{V_{\text{c}}^{5}}_{ijklmn}} \end{equation} | (13d), |
| \begin{equation} (FV_{\text{c}}^{2})_{i}{'} = 2\sum_{j=1}^{2}y_{j}F_{ij}{V_{\text{c}}}_{\,ij} \end{equation} | (13e), |
| \begin{equation} \beta_{i}{'} = \beta_{i} \end{equation} | (13f), |
and
| \begin{equation} (\gamma V_{\text{c}}^{2})_{i}{'} = 3\sum_{j=1}^{2}\sum_{k=1}^{2}y_{j}y_{k}\gamma_{ijk}{V_{\text{c}}^{2}}_{ijk} \end{equation} | (13g). |
When the fluid is pure (y1 = 1 or y2 = 1), Eq. (12) is simplified to Eq. (8). The mathematical derivation of Eq. (12) is found in the Electronic Supplementary Material of Ghiorso and Gualda (2015). An important caution is that Eq. (12) has been erroneously presented in a series of Duan’s papers: For example, Eq. (11) of Duan and Zhang (2006) contains undefined variable G and thus calculation is impossible; Eq. (9) of Duan et al. (1992b) has an error on the exponent of the $\gamma V_{\text{c}}^{2}$ term. Nieva and Barragán (2003), which presented software to calculate thermodynamic properties of the H2O-CO2-CH4 fluid based on equations of state by Duan et al. (1992b), has the same error and also omitted a minus sign in an exponential term. Eq. (B13) of Duan (2014) has identical errors to those in Nieva and Barragán (2003). These errors seem to be typographical errors because their calculation results are consistent with those calculated using the correct equation.
To calculate ϕi, further procedures are required. When P = 0-0.2 GPa, Eq. (12) is simply used to calculate ϕi with the low-pressure set of variables listed in Table 1. When P > 0.2 GPa, ϕi is calculated as:
| \begin{equation} \ln \phi_{i} = \ln \phi_{i}^{\text{high},P} - \ln \phi_{i}^{\text{high},0.2\,\text{GPa}} + \ln \phi_{i}^{\text{low},0.2\,\text{GPa}} \end{equation} | (14), |
where $\phi_{i}^{\text{high},P}$ is the partial fugacity coefficient calculated from Eq. (12) with the high-pressure variables and the given pressure (P), $\phi_{i}^{\text{low}, 0.2\,\text{GPa}}$ is the partial fugacity coefficient calculated from Eq. (12) with the low-pressure variables and pressure of 0.2 GPa, and $\phi_{i}^{\text{high}, 0.2\,\text{GPa}}$ is the partial fugacity coefficient calculated from Eq. (12) with the high-pressure variables and pressure of 0.2 GPa. This procedure is necessary because parameters are switched from low-pressure variables to high-pressure variables at 0.2 GPa. Equation (14) is the sum of the integral of Eq. (10) from 0 to 0.2 GPa with low-pressure variables and the integral from 0.2 GPa to P with high-pressure variables. The same calculation procedure needs to be applied to Eq. (8) when the fluid is pure.
The activity of component i (ai) is defined as:
| \begin{equation} a_{i} = \frac{f_{i}}{f_{i}^{\text{pure}}} \end{equation} | (15), |
where $f_{i}^{\text{pure}}$ is the fugacity of pure component i. Figure 1 shows the activity values calculated at 1073.15 K and 0.6 and 1.4 GPa through the presented equations. The results are identical to those shown in Figure 10 of Duan and Zhang (2006) at the same temperature and pressure, confirming that the calculation procedure and equations presented here are correct.

Example calculations for the compressibility factor (Z), molar volume (Vm), and activity coefficient (ai) were performed. Figure 2 represents Z of pure H2O and CO2 as a function of pressure at various temperatures. For H2O, with increasing pressure, Z first decreased and then turned to increase. Such a trend is common for nonideal fluids where Z has a minimum near the critical point (e.g., Atkins and de Paula, 2006). For CO2, Z only increased with increasing pressure because the critical temperature of CO2 is much lower than the temperature range investigated here. Z is larger for CO2 than for H2O at identical pressure and temperature, indicating that CO2 is less compressible than H2O. Note that a small discontinuity is seen for Z at the pressure of 0.2 GPa (Fig. 2) because the parameter values used are switched at this pressure (Table 1). The discontinuity for Z seemingly does not become a serious problem in practical use because it is small (<1.0% for pure H2O and <1.5% for pure CO2).
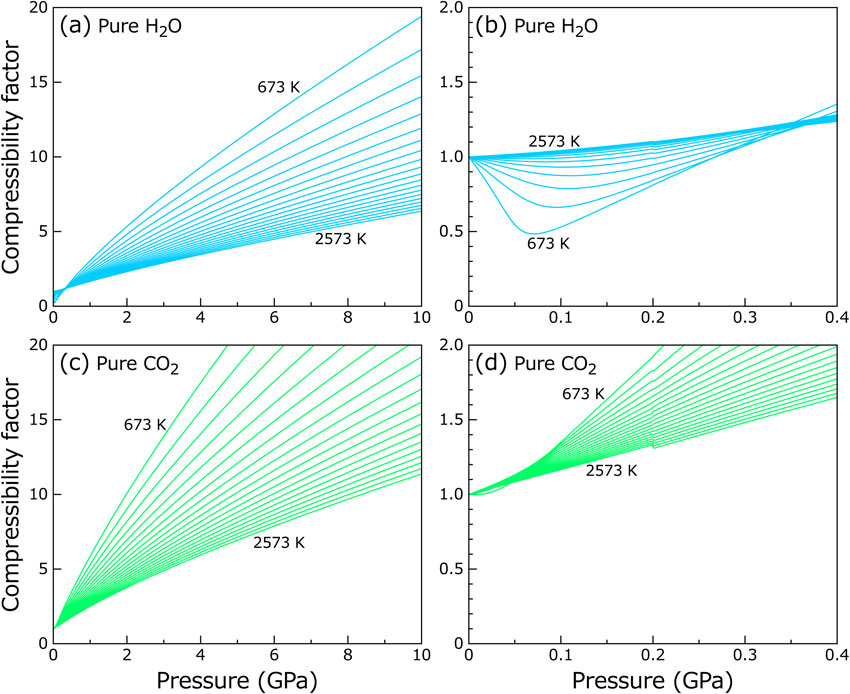
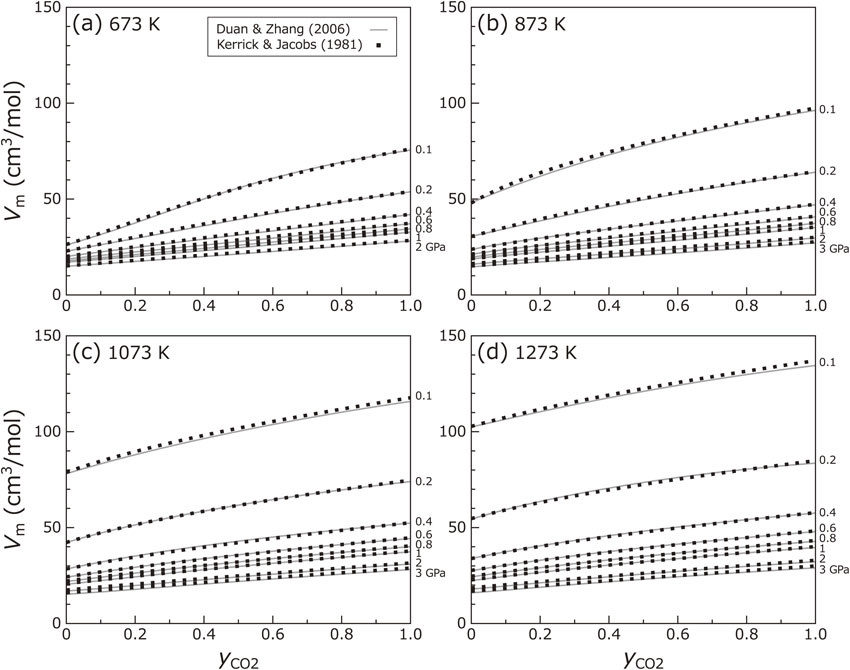
Figure 3 shows the molar volume (Vm) of the fluid as a function of fluid composition ($y_{\text{CO}_{2}}$). The molar volume is positively proportional to $y_{\text{CO}_{2}}$ because CO2 is less compressible than H2O. The Vm shows a convex upwards curve as a function of $y_{\text{CO}_{2}}$, indicating that the fluid mixing generates an excess of volume. The Vm values are consistent with those calculated from the equation of state by Kerrick and Jacobs (1981) within ∼ 5%.
Figures 4 and 5 show the activity-composition relation. At pressures of <1 GPa (Fig. 4), the activity-composition curves moderately positively deviate from the ideal mixing line. This trend is typical for most nonideal fluids and is consistent with previous studies (Flowers, 1979; Kerrick and Jacobs, 1981). However, at higher pressures (>3 GPa; Fig. 5), several curves show a complex, sometimes unusual shape, especially at high-pressure and low-temperature conditions. For example, at 873 K and pressure of >3 GPa, the activity curves do not show a simple monotonic increase or decrease but have maxima at $y_{\text{CO}_{2}}$ ∼ 0.05 (CO2) and $y_{\text{CO}_{2}}$ ∼ 0.2-0.4 (H2O). Similar peaks are observed at high pressures when the temperature is <1473 K. In addition, when the pressure is >5 GPa, curves for H2O activity often deviate below the ideal mixing line at $y_{\text{CO}_{2}}$ ∼ 0.1-0.3. Whether such a trend is real has not been examined experimentally. These apparently unrealistic curves may be the result of inadequate calibration of parameters: although the number of simulation data is large (690 points), three fluid compositions ($y_{\text{CO}_{2}}$ = 0.25, 0.50, and 0.75) are only examined at each temperature and pressure (the interval of temperature and pressure was 50-100 K and 1 GPa, respectively). Therefore, users need to exercise caution when calculating the activity (and the partial fugacity coefficient) if the pressure is extremely high or if the temperature is low. Nevertheless, the equation of state seems to be valid enough for practical use if the pressure and temperature are appropriate for geologically realistic conditions. The equation of state seems to be safely used at most temperatures if the pressure is <3 GPa. For higher pressure conditions, the equation seems valid if the pressure-temperature conditions are on the typical geotherm.


It is desirable that a calculation code is provided so that readers can reproduce identical results without suffering from unnecessary errors. Such an effort has been made by, for example, Jacobs and Kerrick (1981), who provided Fortran and APL codes for the equation of state by Kerrick and Jacobs (1981). Here, a C-language code for the calculation of molar volume, compressibility factor, fugacity coefficient, and activity of the H2O-CO2 fluid is provided as Supplementary Material. A Windows executable program, which was produced by compiling the same code with MS Visual C++ .NET 2003 on Windows XP, is also provided for convenience. Readers may freely download these items from https://doi.org/10.2465/jmps.221224a and modify them for their purpose.
The presented calculation showed that the mixing of H2O and CO2 occurs highly nonideally at crustal conditions: the molar volume of the mixed fluid is greater than the molar volume expected from ideal mixing (Fig. 3). If the volume is instead fixed, the pressure increases. This excess volume or pressure may exert a direct influence on geological phenomena such as CO2 fluxing, in which a CO2-rich fluid originated from deep-derived magma fluxes in an H2O-rich shallow-stored magma (e.g., Yoshimura and Nakamura, 2011; van Hinsberg et al., 2016; Caricchi et al., 2018) and carbonic fluid metamorphism (metasomatism), in which a CO2-rich fluid, possibly discharged from a magma, infiltrates into an H2O-rich crustal rock (e.g., Frost and Frost, 1987; Newton, 1989, 1992). How the nonideal effects influence the phenomena is discussed here.
Figure 6 shows the molar volume of the H2O-CO2 fluid at 1123 K and 200 and 800 MPa. The pressure of 200 MPa is a typical condition for silicic magmas stored in the upper crust (e.g., Huber et al., 2019). If identical amounts of pure CO2 fluid and pure H2O fluid mix to produce the mixture fluid with $y_{\text{CO}_{2}}$ = 0.5 at this pressure, the molar volume of the mixed fluid is calculated to be 64.7 cm3/mol, which is ∼ 6.2% higher than the volume expected from the ideal mixing (60.9 cm3/mol). Therefore, if a fluid mixing phenomenon occurs in the crustal magma, a similar range of the volume increase is expected. This excess volume may exert a strong influence on the eruption dynamics because the increase in the fluid volume leads to an increase in magma buoyancy and may activate the convection of the magma chamber (e.g., Sparks et al., 1977). If the volume is instead fixed upon fluid mixing, then the fluid pressure increases to 221 MPa, which is ∼ 10% higher than before mixing. The pressure increase also influences the eruption dynamics, because the overpressure may act as a trigger for the eruption (e.g., Tait et al., 1989).
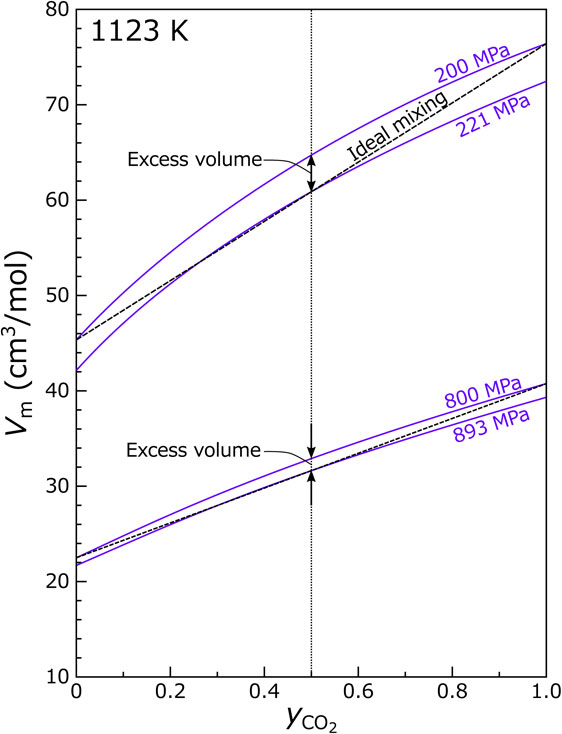
Figure 6 also shows the volume of the H2O-CO2 fluid at 800 MPa and 1123 K, a condition typical of the lower crust. If the fluid mixing associated with the carbonic fluid metamorphism occurs at this condition, the nonideal effect is still significant: if identical amounts of pure CO2 fluid and pure H2O mixes to produce the fluid of $y_{\text{CO}_{2}}$ = 0.5 at 800 MPa, an excess volume of ∼ 3.3% is expected (31.6 cm3/mol for the ideal mixing versus 32.9 cm3/mol for the nonideal mixing). If the volume is instead fixed, the excess pressure is ∼ 11% (the pressure after mixing is 892 MPa). These effects may promote metamorphism because the porosity increase enlarges the fluid pathways, while the pressure increase drives further fluid infiltration.
The present study reviewed the equation of state for H2O-CO2 binary mixture fluid developed by Duan and Zhang (2006). The calculation procesure for the equation was presented. Also, a correct equation for the partial fugacity coefficient of the mixture fluid [Eq. (12)], which has been erroneously presented in previous studies, was provided. For most geologically realistic pressure-temperature conditions, molar volume, fugacity, and activity are well calculated with the equation. However, when the pressure is high and the temperature is low, caution should be used because the activity (the partial fugacity coefficient) seems to be unrealistic. Application to geological situations suggested that the excess volume generation or the pressure increase, which occur when a H2O-rich fluid and a CO2-rich fluid mix nonideally, may play an important role in volcanic eruption and metamorphism.
I thank two anonymous reviewers and Editor Tetsu Kogiso for constructive comments. This study was supported by JSPS KAKENHI Grant Number JP20H01989.
Supplementary code and executable file are available online from https://doi.org/10.2465/jmps.221224a.
The original Lee-Kesler equation (Lee and Kesler, 1975) is briefly reviewed here. The Lee-Kesler equation was developed based on the formulation of the BWR equation (Benedict et al., 1940) and the concept of acentric factor by Pitzer and Curl (1957). The equation is the corresponding-state equation and applies to many fluids if their critical pressure, critical temperature, and acentric factor are identified. The acentric factor is the measure of deviation of the intermolecular potential from that of a ‘simple’ fluid, which is composed of a spherical, nonpolar molecule such as methane, argon, and krypton (Pitzer and Curl, 1957). In the Lee-Kesler formulation the compressibility factor of the fluid (Z) is given as:
| \begin{equation} Z = Z^{(0)} + \frac{\omega}{\omega^{(r)}}(Z^{(r)} - Z^{(0)}) \end{equation} | (A1), |
where Z(0) is the compressibility factor of a simple fluid and Z(r) is the compressibility factor for a ‘reference’ fluid, the representative of which is n-octane, the heaviest hydrocarbon for which there are extensive data. ω is the acentric factor for the fluid of interest and ω(r) is the acentric factor for the reference fluid (ω(r) is fixed to 0.3978, the value for n-octane). Eq. (A1) therefore means that Z of the fluid is provided as the sum of Z of the simple fluid and the deviation from the simple fluid. Z(0) and Z(r) are calculated separately with the following equation (so-called Lee-Kesler equation):
| \begin{align} Z &= \frac{PV_{\text{m}}}{RT} = 1 + \frac{BV_{\text{c}}}{V_{\text{m}}} + \frac{CV_{\text{c}}^{2}}{V_{\text{m}}^{2}} + \frac{EV_{\text{c}}^{5}}{V_{\text{m}}^{5}} + \frac{FV_{\text{c}}^{2}}{V_{\text{m}}^{2}}\left(\beta + \frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}}\right)\exp\left(-\frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}}\right) \end{align} | (A2), |
where parameters are provided as:
| \begin{equation} B = b_{1} - b_{2}\left(\frac{T_{\text{c}}}{T}\right) - b_{3}\left(\frac{T_{\text{c}}}{T}\right)^{2} {}- b_{4}\left(\frac{T_{\text{c}}}{T}\right)^{3} \end{equation} | (A3), |
| \begin{equation} C = c_{1} - c_{2}\left(\frac{T_{\text{c}}}{T}\right) + c_{3}\left(\frac{T_{\text{c}}}{T}\right)^{3} \end{equation} | (A4), |
| \begin{equation} E = e_{1} + e_{2}\left(\frac{T_{\text{c}}}{T}\right) \end{equation} | (A5), |
and
| \begin{equation} F = c_{4}\left(\frac{T_{\text{c}}}{T}\right)^{3} \end{equation} | (A6). |
Here, parameter values used in Eq. (A2)-(A6) are listed in Table A1. Equation (A2) is similar to Eq. (1) of the main text, but the fourth-order term is not used here.
The acentric factor in Eq. (A1) is defined as:
| \begin{equation} \omega = -\log_{10}\left(\frac{P_{T_{\text{r}} = 0.7}^{\text{sat}}}{P_{\text{c}}}\right) - 1 \end{equation} | (A7), |
where $P_{T_{\text{r}} = 0.7}^{\text{sat}}$ represents the saturation vapour pressure of the volatile at the reduced temperature $(T_{\text{r}} = \frac{T}{T_{\text{c}}})$ of 0.7. The value of ω is dependent on the substance, and it is ∼ 0 when the molecule is spherical (simple fluid), while it is a greater value when the molecule is nonspherical and polarized. The ω values are listed in literature such as Appendix of Poling et al. (2001).
The calculation is performed as follows: First, calculate Z(0) and Z(r) separately according to Eq. (A2)-(A6), values in Table A1, and Pc and Tc values of the fluid of interest. Second, find ω of the fluid from the literature or calculate ω based on experimental data and Eq. (A7). Finally, input all these values in Eq. (A1) to obtain Z of the fluid.
| Parameters | Simple fluids | Reference fluids |
| b1 | 0.1181193 | 0.2026579 |
| b2 | 0.265728 | 0.331511 |
| b3 | 0.154790 | 0.027655 |
| b4 | 0.030323 | 0.203488 |
| c1 | 0.0236744 | 0.0313385 |
| c2 | 0.0186984 | 0.0503618 |
| c3 | 0.0 | 0.016901 |
| c4 | 0.042724 | 0.041577 |
| e1 | 1.55488 × 10−5 | 4.8736 × 10−5 |
| e2 | 6.23689 × 10−5 | 7.40336 × 10−6 |
| β | 0.65392 | 1.226 |
| γ | 0.060167 | 0.03754 |
Reproduced from Lee and Kesler (1975).