2023 年 118 巻 1 号 論文ID: 221224b
2023 年 118 巻 1 号 論文ID: 221224b
The solubility of H2O and CO2 in silicate melts is crucial in understanding the geological phenomena occurring in a fluid-melt system. Although several recent solubility models are used in petrological studies, the model of Duan (2014) has rarely been used and has been evaluated less thoroughly, despite its potentially high applicability, mainly because calculation software is unavailable. This paper reviews the Duan (2014) solubility model and its calculation procedure for H2O-CO2 solubility. It was found that the Duan (2014) model uses a special protocol to calculate the fluid partial fugacity coefficient, for which no explanation is given in the original paper, thereby limiting the general use of the model. Therefore, I present an appropriate calculation procedure and provide a C-language code for convenience. The predictability of the model was evaluated by comparison with experimental solubility data. It was shown that the Duan (2014) model reproduces well the data for rhyolitic melts, whereas predictability is somewhat weak for other melts, including dacitic, andesitic, and basaltic melts. Caution is thus required when applying the Duan (2014) model to SiO2-poor melts.
Volatiles dissolved in silicate melt control phase relations and physicochemical properties of the melt. When the volatile content exceeds the solubility limit, fluid exsolution occurs, causing dramatic changes in bulk density, bulk viscosity, and the elemental partitioning. Volatile solubility is therefore crucial in understanding the fluid-involved geological phenomena. Because H2O and CO2 are the major volatiles, many experimental studies have investigated the solubility of pure H2O and CO2 and H2O-CO2 mixture (see Holloway and Blank, 1994; Moore, 2008; Baker and Alletti, 2012, for a comprehensive review). Solubility models were constructed based on experimental data and thermodynamic or empirical equations. Models with high predictability and wide applicability are required to promote our understanding of geological phenomena.
For the practical use of a solubility model in petrological studies, accessibility and usability are also important factors. In this regard, the solubility model developed by Newman and Lowenstern (2002) has received great attention because the authors developed a user-friendly MS Excel program (VolatileCalc), through which the solubility of H2O and CO2 for both rhyolitic and basaltic melts could easily be computed. Papale (1997, 1999) and Papale et al. (2006) pioneered general thermodynamic models that predict H2O-CO2 solubility in silicate melts with arbitrary compositions, providing a new tool to study fluid-melt equilibrium in varieties of natural magmas. Ghiorso and Gualda (2015) then developed a solubility model applicable to various silicate compositions and a wide pressure-temperature range (MagmaSat). This model is compatible with Rhyolite-MELTS (Gualda et al., 2012) and is becoming increasingly popular because the calculation can be performed relatively easily through a web application or software package. More recently, Iacovino et al. (2021) and Wieser et al. (2022) developed a new type of solubility calculation package (VESIcal), through which H2O-CO2 fluid saturation conditions can be computed based on seven solubility models, namely Ghiorso and Gualda (2015) (MagmaSat), Dixon (1997), Moore et al. (1998), Liu et al. (2005), Iacono-Marziano et al. (2012), Shishkina et al. (2014), and Allison et al. (2019). VESIcal enables not only solubility calculation but also a comparison of the models, allowing a less biased interpretation of the fluid-melt equilibrium state.
One solubility model that has both wide applicability and clear equation sets, but has not been thoroughly examined, is the model of Duan (2014). This solubility model is constructed on a thermodynamic basis and is applicable to wide pressure-temperature conditions (up to 3 GPa and 933-2003 K) and varieties of melt compositions from ultramafic to rhyolitic (granitic) melts. However, despite its potentially high applicability, the Duan (2014) model has rarely been used, and it was also excluded from recent reviews because the software for the solubility calculation is not available (Iacovino et al., 2021; Wieser et al., 2022). Previously, a Windows executable file and a web application were provided on Duan’s personal website, but these are no longer available. In addition, calculation protocols for the partial fugacity coefficient, which is required for solubility calculation and is computed through the equation of state by Duan and Zhang (2006), seem to have been modified from those of the original paper without providing any clear explanation, limiting the general use of the model in the petrological community. This paper reviews the Duan (2014) solubility model and show the modified calculation protocol for the partial fugacity coefficient. A C-language code and an executable program for solubility calculation are also provided so that readers will be able to freely use or implement the model in their work. The suitability of the Duan (2014) model is evaluated by comparison with experimental data. The model is also compared with MagmaSat (Ghiorso and Gualda, 2015) to illustrate their differences in predictability.
Before focusing on the Duan (2014) solubility model, dissolution species of H2O and CO2 in silicate melts are briefly overviewed (see, e.g., Blank and Brooker, 1994; McMillan, 1994; Zhang, 1999; Zhang et al., 2007, for a comprehensive review). Infrared spectroscopy revealed that H2O dissolves in silicate melts as both hydroxyl groups (OH) and H2O molecules (H2Omol) through the fluid-melt reaction (1a) and the homogeneous dissociation reaction (1b):
| \begin{equation} \text{H$_{2}$O (fluid)} \leftrightarrow \text{H$_{2}$O$_{\text{mol}}$ (melt)} \end{equation} | (1a) |
and
| \begin{equation} \text{H$_{2}$O$_{\text{mol}}$ (melt)} + \text{O (melt)} \leftrightarrow \text{2OH (melt)} \end{equation} | (1b), |
where O (melt) is the bridging oxygen (and a small amount of free oxygen) (Zhang et al., 2007). For a given melt, the ratio of OH and H2Omol changes as a function of the total H2O (OH + H2Omol) content: as the total H2O content increases, the ratio of OH/H2Omol decreases (e.g., Zhang, 1999). Therefore, a water-poorer melt has a higher OH/H2Omol ratio. The OH/H2Omol ratio also increases with increasing temperature (e.g., Ihinger et al., 1999). In the present study, the term H2O content is used in a generic sense for the total H2O because the Duan (2014) solubility model only considers the total H2O content and does not take into account the speciation reaction.
CO2 dissolves in silicate melt as CO2 molecules (CO2mol) and/or carbonate ions (CO32−) through the following reaction:
| \begin{equation} \text{CO$_{2}$ (fluid)} \leftrightarrow \text{CO$_{2\,\text{mol}}$ (melt)} \end{equation} | (2a) |
and
| \begin{equation} \text{CO$_{2\,\text{mol}}$ (melt)} + \text{O$^{2-}$ (melt)} \leftrightarrow \text{CO$_{3}^{2-}$ (melt)} \end{equation} | (2b), |
where O2− denotes the non-bridging oxygen (Ni and Keppler, 2013). Therefore, which of the species (CO2mol or CO32−) is dominant or exclusive is a function of the degree of polymerization of the melt, which is strongly dependent on melt composition and water content (e.g., Konschak and Keppler, 2014). In general, rhyolitic melts contain CO2mol as the single species, basaltic melt only contains CO32−, and intermediate melts contain both species. Note however that the presence of CO32− in hydrous high-SiO2 rhyolitic melts was reported by recent studies (Moore et al., 2008; Yoshimura et al., 2017). Hereafter, the term CO2 content is used for the content of all CO2 species dissolved in the melt irrespective of the CO32−/CO2mol ratio. The Duan (2014) model only predicts the total CO2 content for silicate melt and does not deal with the speciation reaction.
The Duan (2014) solubility model is constructed on a thermodynamic basis. Consider a situation where a H2O-CO2 binary mixture fluid with a H2O molar fraction of y1 and a CO2 molar fraction of y2 (thus, y1 + y2 = 1) coexists with a silicate melt at constant pressure and temperature. At the fluid-melt equilibrium state, the chemical potential of the volatile component i (H2O or CO2) in the fluid, $\mu_{i}^{\text{fluid}}$, is identical to that of the melt, $\mu_{i}^{\text{melt}}$:
| \begin{equation} \mu_{i}^{\text{fluid}} = \mu_{i}^{\text{melt}} \end{equation} | (3). |
$\mu_{i}^{\text{fluid}}$ is expressed as:
| \begin{equation} \mu_{i}^{\text{fluid}} = \mu_{i}^{\text{fluid,ref}} + RT\ln \frac{f_{i}}{f_{i}^{\text{ref}}} \end{equation} | (4), |
where $\mu_{i}^{\text{fluid},\text{ref}}$ is the chemical potential of volatile component i at a reference state, R is the gas constant (0.083 kbar cm3 mol−1 K−1), T is the temperature (K), fi is the fugacity of volatile component i in the fluid (kbar), and $f_{i}^{\text{ref}}$ is the fugacity of volatile component i at the reference state. fi is written as:
| \begin{equation} f_{i} = \phi_{i}P_{i} = \phi_{i}y_{i}P \end{equation} | (5), |
where ϕi is the partial fugacity coefficient of volatile component i, Pi is the partial pressure of volatile component (kbar), and P denotes the total pressure (kbar). If the pressure of the reference state is set to unity, Eq. (4) is simplified to:
| \begin{equation} \mu_{i}^{\text{fluid}} = \mu_{i}^{\text{fluid,ref}} + RT\ln(\phi_{i}y_{i}P) \end{equation} | (6). |
For the melt, the chemical potential of volatile component i dissolved in the melt is expressed as:
| \begin{equation} \mu_{i}^{\text{melt}} = \mu_{i}^{\text{melt,ref}} + RT\ln a_{i}^{\text{melt}} \end{equation} | (7), |
where $\mu_{i}^{\text{melt},\text{ref}}$ is the chemical potential of volatile component i in the melt at the reference state. $a_{i}^{\text{melt}}$ represents the activity of volatile component i in the melt and is expressed as:
| \begin{equation} a_{i}^{\text{melt}} = \gamma_{i}^{\text{melt}}\,x_{i} \end{equation} | (8), |
where $\gamma_{i}^{\text{melt}}$ is the activity coefficient and xi is the molar fraction of volatile component i. In the Duan (2014) model, xi is defined as the molar fraction on a single oxygen basis, that is, the molar fraction of volatile i calculated on the assumption that the molar mass of volatile-free melt is the mass of melt per mole of oxygen [see Eqs. (17a), (17b), and (18)].
Based on the presented equations, Eq. (3) becomes:
| \begin{equation} \mu_{i}^{\text{fluid,ref}} + RT\ln(\phi_{i}y_{i}P) = \mu_{i}^{\text{melt,ref}} + RT\ln (\gamma_{i}^{\text{melt}}x_{i}) \end{equation} | (9). |
Rearranging Eq. (9), the following equation is obtained:
| \begin{equation} RT\ln \left(\frac{\phi_{i}y_{i}P}{\gamma_{i}^{\text{melt}}\,x_{i}}\right) = \mu_{i}^{\text{melt,ref}} - \mu_{i}^{\text{fluid,ref}} \end{equation} | (10). |
Eq. (10) is the basic solubility equation for the Duan (2014) model. On the left-hand side, ϕi can be calculated from the equation of state. Here, the equation of state by Duan and Zhang (2006) is exclusively used in the Duan (2014) model (see the next section). $\gamma_{i}^{\text{melt}}$ is calculated from the modified version of the Margules-type regular solution model as shown later [see Eqs. (15) and (16)]. For the right-hand side, Duan (2014) assumed $\mu_{i}^{\text{fluid},\text{ref}}$ to be 0 and described $\mu_{i}^{\text{melt},\text{ref}}$ as an empirical function:
| \begin{align} \mu_{i}^{\text{melt,ref}} &= c_{1} + c_{2}T + c_{3}P^{2} \\&\quad+ \frac{c_{4}}{T} + c_{5}\ln T + \frac{c_{6}P^{2}}{T} + c_{7}P \end{align} | (11), |
where c1-c7 are constant values for H2O and CO2 (Table 1). Therefore, the solubility of volatile i (i.e., xi) is obtained by solving Eq. (10) under a given pressure, temperature and composition of coexisting fluid (yi). Note that $\mu_{i}^{\text{melt},\text{ref}}$ was denoted as ‘$\mathit{Par}\,(T, P)$’ in the original paper [Eq. (21) in Duan (2014)].
| Parameters | H2O | CO2 |
| c1 | 162.95 | 111414.92 |
| c2 | 0.34 | 6.06 |
| c3 | 0.61 | −0.53 |
| c4 | 0 | −10873021.59 |
| c5 | 0 | −15390.5 |
| c6 | 0 | 986.72 |
| c7 | 0 | 25.03 |
Reproduced from Table 4 of Duan (2014).
It should be noted that throughout the calculation of the Duan (2014) model, several different values are used for the gas constant R because the units of pressure and fluid volume differ according to the calculation section. In Eqs. (4)-(10), the value of R is 0.083 kbar cm3 K−1 mol−1, whereas it is 83.14467 bar cm3 K−1 mol−1 when calculating the partial fugacity coefficient through the equation of state by Duan and Zhang (2006). When calculating the activity coefficient of melt in Eqs. (15) and (16) (see later section), R is 8.3 J K−1 mol−1. The values of constants used in the present study are summarized in Supplementary Document (available online from https://doi.org/10.2465/jmps.221224b).
The Duan (2014) solubility model exclusively uses the equation of state developed by Duan and Zhang (2006) to calculate the partial fugacity coefficient (ϕi) of the coexisting fluid. Because the details of the equation of state were reviewed in Part 1 (Yoshimura, 2023), only brief descriptions are provided here.
The Duan and Zhang (2006) equation of state is given as:
| \begin{align} Z = \frac{PV_{\text{m}}}{RT} &= 1 + \frac{BV_{c}}{V_{\text{m}}} + \frac{CV_{\text{c}}^{2}}{V_{\text{m}}^{2}} + \frac{DV_{\text{c}}^{4}}{V_{\text{m}}^{4}} + \frac{EV_{\text{c}}^{5}}{V_{\text{m}}^{5}} \\&\quad+ \frac{FV_{\text{c}}^{2}}{V_{\text{m}}^{2}}\left(\beta + \frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}}\right)\exp\left(-\frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}}\right) \end{align} | (12), |
where Z is the compressibility factor (a dimensionless number), P is the pressure (bar), Vm is the molar volume of the fluid (cm3 mol−1), R is the gas constant (83.14467 bar cm3 K−1 mol−1), and T is the temperature (K). The parameters B, C, D, E, F, β, and γ are temperature-dependent values for H2O and CO2, and two sets of values are prepared: when the pressure is ≤0.2 GPa, the low-pressure values are used, whereas when the pressure is 0.2-10 GPa, the high-pressure values are used (see Supplementary Document). Vc is the molar volume of the fluid at the critical temperature and pressure for pure H2O or CO2 fluid. When the fluid is a mixture of H2O and CO2, the terms BVc, $CV_{\text{c}}^{2}$, … are calculated based on mixing rules (see Supplementary Document).
The partial fugacity coefficient of component i in the fluid, ϕi, is given as:
| \begin{align} &\ln \phi_{i} \\ & \quad = -\ln Z + \frac{(BV_{\text{c}})_{i}{'}}{V_{\text{m}}} + \frac{(CV_{\text{c}}^{2})_{i}{'}}{2V_{\text{m}}^{2}} + \frac{(DV_{\text{c}}^{4})_{i}{'}}{4V_{\text{m}}^{4}} + \frac{(EV_{\text{c}}^{5})_{i}{'}}{5V_{\text{m}}^{5}} \\ & \qquad + \frac{(FV_{\text{c}}^{2})_{i}{'}\beta + \beta_{i}{'}FV_{\text{c}}^{2}}{2\gamma V_{\text{c}}^{2}}\left[1 - \exp\left(-\frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}}\right)\right] \\ & \qquad + {\frac{(FV_{\text{c}}^{2})_{i}{'}\gamma V_{\text{c}}^{2} + (\gamma V_{\text{c}}^{2})_{i}{'}FV_{\text{c}}^{2} - FV_{\text{c}}^{2}\beta[(\gamma V_{\text{c}}^{2})_{i}{'} - \gamma V_{\text{c}}^{2}]}{2(\gamma V_{\text{c}}^{2})^{2}}}\\ & \qquad \times\left[1 - \left(\frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}} + 1\right)\exp\left(-\frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}}\right)\right] \\ & \qquad -\frac{[(\gamma V_{\text{c}}^{2})_{i}{'} - \gamma V_{\text{c}}^{2} ]FV_{\text{c}}^{2}}{2(\gamma V_{\text{c}}^{2})^{2}}\\ & \qquad \times\left[2 - \left(\frac{(\gamma V_{\text{c}}^{2})^{2}}{V_{\text{m}}^{4}} + \frac{2\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}} + 2\right)\exp\left(-\frac{\gamma V_{\text{c}}^{2}}{V_{\text{m}}^{2}}\right)\right] \end{align} | (13) |
where $(BV_{\text{c}})_{i}{'}$, $(CV_{\text{c}}^{2})_{i}{'}$, … are calculated according to the equations given in Supplementary Document. Note that Eq. (13) has long been erroneously presented in previous studies including Duan and Zhang (2006) (see Part 1 for the detail; Yoshimura, 2023).
It was found that the calculation of the partial fugacity coefficient is problematic in the Duan (2014) model. Duan and Zhang (2006) instructed that the partial fugacity coefficient should be calculated in the following manner: when the pressure is <0.2 GPa, ϕi is calculated directly from Eq. (13) with low-pressure values, whereas when the pressure is >0.2 GPa, ϕi is calculated as:
| \begin{equation} \ln \phi_{i} = \ln \phi_{i}^{\text{high},P} - \ln \phi_{i}^{\text{high}, 0.2\,\text{GPa}} + \ln \phi_{i}^{\text{low}, 0.2\,\text{GPa}} \end{equation} | (14), |
where $\phi_{i}^{\text{high},P}$ is the partial fugacity coefficient calculated from Eq. (13) with the high-pressure variables and the given pressure, $\phi_{i}^{\text{high}, 0.2\,\text{GPa}}$ is the partial fugacity coefficient calculated from Eq. (13) with the high-pressure variables and a pressure of 0.2 GPa, and $\phi_{i}^{\text{low}, 0.2\,\text{GPa}}$ is the partial fugacity coefficient calculated from Eq. (13) with the low-pressure variables and a pressure of 0.2 GPa. Here, 0.2 GPa is the pressure at which parameter values switch. If this protocol is used, however, the calculated solubility is much higher than that calculated using the Windows executable file previously provided on Duan’s website (Fig. 1).

At pressures >0.2 GPa this discrepancy was always observed irrespective of temperature, pressure, and melt composition. In contrast, at pressures <0.2 GPa no significant discrepancy was observed. After trial and error, it was found that, if ϕi is calculated simply from Eq. (13) without using Eq. (14), even if P > 0.2 GPa, then the solubility results agree well with the values obtained by using the executable file of Duan (2014) (within a relative deviation of <2.5%) (Fig. 1). Therefore, it appears that Duan (2014) established the solubility model without following the official protocol of Duan and Zhang (2006). In the present study, Eq. (13) is only used irrespective of the pressure condition, and Eq. (14) is temporarily abandoned.
In the Duan (2014) solubility model, the activity coefficient for H2O ($\gamma_{\text{H${_{2}}$O}}$) and CO2 ($\gamma_{\text{CO}_{2}}$) of silicate melt is calculated based on a modified Margules-type regular solution model:
| \begin{align} &RT\ln \gamma_{\text{H${_{2}}$O}} \\ & \quad = (1 - x_{\text{H${_{2}}$O}}) x_{\text{CO}_{2}}w_{\text{H${_{2}}$OCO${_{2}}$}} \\ & \qquad + (1 - x_{\text{H${_{2}}$O}})(1 - x_{\text{H${_{2}}$O}} - x_{\text{CO}_{2}})\sum_{j(\ne \text{CO}_{2}) = 1}^{n}(x_{j}w_{\text{H${_{2}}$O}j}) \\ & \qquad - x_{\text{CO}_{2}}(1 - x_{\text{CO}_{2}} - x_{\text{H${_{2}}$O}})\sum_{j(\ne \text{H${_{2}}$O}) = 1}^{n}(x_{j}w_{\text{CO}_{2}j}) \\ & \qquad - (1 - x_{\text{CO}_{2}} - x_{\text{H${_{2}}$O}})^{2}\\ & \qquad \times\sum_{j(\ne \text{CO}_{2}, \text{H${_{2}}$O}) = 1}^{n - 1} \left[\sum_{k(\ne \text{CO}_{2}, \text{H${_{2}}$O}) = j + 1}^{n}(x_{j}x_{k}w_{jk})\right] \end{align} | (15), |
and
| \begin{align} &RT\ln \gamma_{\text{CO}_{2}} \\ & \quad= (1 - x_{\text{CO}_{2}}) x_{\text{H${_{2}}$O}}w_{\text{CO}_{2}\text{H${_{2}}$O}} \\ & \qquad+ (1 - x_{\text{CO}_{2}})(1 - x_{\text{CO}_{2}} - x_{\text{H${_{2}}$O}})\sum_{j(\ne \text{H${_{2}}$O}) = 1}^{n}(x_{j}w_{\text{CO}_{2}j}) \\ & \qquad - x_{\text{H${_{2}}$O}}(1 - x_{\text{H${_{2}}$O}} - x_{\text{CO}_{2}})\sum_{j(\ne \text{CO}_{2}) = 1}^{n}(x_{j}w_{\text{H${_{2}}$O}j}) \\ & \qquad - (1 - x_{\text{CO}_{2}} - x_{\text{H${_{2}}$O}})^{2}\\ & \qquad\times\sum_{j(\ne \text{CO}_{2}, \text{H${_{2}}$O}) = 1}^{n-1} \left[\sum_{k(\ne \text{CO}_{2}, \text{H${_{2}}$O}) = j + 1}^{n}(x_{j}x_{k}w_{jk}) \right] \end{align} | (16). |
Here, the value of the gas constant R is 8.3 (J K−1 mol−1). $x_{\text{H${_{2}}$O}}$ and $x_{\text{CO}_{2}}$ are the molar fractions of H2O and CO2 on a single oxygen basis, respectively (Zhang, 1999):
| \begin{equation} x_{\text{H${_{2}}$O}} = \frac{\cfrac{C_{\text{H${_{2}}$O}}}{M_{\text{H${_{2}}$O}}}}{\cfrac{C_{\text{H${_{2}}$O}}}{M_{\text{H${_{2}}$O}}} + \cfrac{C_{\text{CO}_{2}}}{M_{\text{CO}_{2}}} + \cfrac{1 - C_{\text{H${_{2}}$O}} - C_{\text{CO}_{2}}}{W}} \end{equation} | (17a), |
and
| \begin{equation} x_{\text{CO}_{2}} = \frac{\cfrac{C_{\text{CO}_{2}}}{M_{\text{CO}_{2}}}}{\cfrac{C_{\text{H${_{2}}$O}}}{M_{\text{H${_{2}}$O}}} + \cfrac{C_{\text{CO}_{2}}}{M_{\text{CO}_{2}}} + \cfrac{1 - C_{\text{H${_{2}}$O}} - C_{\text{CO}_{2}}}{W}} \end{equation} | (17b), |
where $C_{\text{H${_{2}}$O}}$ and $C_{\text{CO}_{2}}$ are the weight fractions of H2O and CO2 in the melt, respectively (0 ≤ Ci ≤ 1). $M_{\text{H${_{2}}$O}}$ and $M_{\text{CO}_{2}}$ are the molar masses of H2O (18.02 g mol−1) and CO2 (44.01 g mol−1), respectively. W is the mass of volatile-free melt per mole of oxygen (g per 1 mol oxygen):
| \begin{equation} W = \frac{\displaystyle\sum_{j(\ne \text{CO}_{2},\text{H${_{2}}$O}) = 1}^{n}C_{j}}{\displaystyle\sum_{j(\ne \text{CO}_{2},\text{H${_{2}}$O}) = 1}^{n}\left(\frac{C_{j}}{M_{j}} \times n_{j}^{\text{oxygen}}\right)} \times 100 \end{equation} | (18), |
where $n_{j}^{\text{oxygen}}$ is the number of oxygen atoms in a one-formula oxide component j (e.g., $n_{\text{Al${_{2}}$O${_{3}}$}}^{\text{oxygen}}$ = 3, $n_{\text{MgO}}^{\text{oxygen}}$ = 1). Cj and Mj represent the weight fraction (0 ≤ Cj ≤ 1) and molar mass (g mol−1) of non-volatile oxide j, respectively. Values of Mj used in the present study are summarized in Supplementary Document.
In Eqs. (15) and (16), $x_{j(\ne \text{CO}_{2}, \text{H${_{2}}$O})}$ is the molar fraction of non-volatile oxide component j in the volatile-free melt:
| \begin{equation} x_{j (\ne \text{CO}_{2}, \text{H${_{2}}$O})} = \frac{\cfrac{C_{j}}{M_{j}}}{\displaystyle\sum_{j (\ne \text{H${_{2}}$O}, \text{CO}_{2}) = 1}^{n}\frac{C_{j}}{M_{j}}} \end{equation} | (19). |
$w_{\text{H${_{2}}$O}j}$ and $w_{\text{CO}_{2}j}$ in Eqs. (15) and (16) are the interaction parameters between H2O and non-volatile oxide component j and those between CO2 and non-volatile oxide component j. Values of $w_{\text{H${_{2}}$O}j}$ and $w_{\text{CO}_{2}j}$ are provided in Table 2. wjk is the interaction parameter between two non-volatile oxide components j and k, the values of which are taken from Ghiorso et al. (1983) (Table 3). Note that the unit of $w_{\text{H${_{2}}$O}j}$ and $w_{\text{CO}_{2}j}$ is J, whereas the unit of wjk in Ghiorso et al. (1983) is cal. Therefore, the values of wjk need to be multiplied by 4.2 J cal−1 when input in Eqs. (15) and (16) (e.g., $w_{\text{SiO${_{2}}$-Al${_{2}}$O${_{3}}$}}$ = −78563.2 cal in Ghiorso et al. (1983) → −329965.44 J).
| j | wH2Oj (J) | wCO2j (J) |
| CO2 or H2O | 0 | 0 |
| SiO2 | −41274 | 33754 |
| TiO2 | 0 | 0 |
| Al2O3 | −347360 | −190544 |
| Fe2O3 | −11701 | −36074 |
| FeO | −65722 | −17398 |
| MnO | 0 | 0 |
| MgO | −131718 | −86063 |
| CaO | −202184 | −203394 |
| Na2O | −266067 | −298072 |
| K2O | −308212 | −663279 |
Reproduced from Table 4 of Duan (2014).
| SiO2 | TiO2 | Al2O3 | Fe2O3 | FeO | MnO | MgO | CaO | Na2O | |
| SiO2 | |||||||||
| TiO2 | −29364.5 | ||||||||
| Al2O3 | −78563.2 | −67349.7 | |||||||
| Fe2O3 | 2637.93 | −6821.82 | 1240.32 | ||||||
| FeO | −9630.14 | −4594.59 | −59528.6 | 4524.46 | |||||
| MnO | 5525.36 | −2043.20 | −1917.75 | 212.196 | −703.34 | ||||
| MgO | −30353.6 | 12673.6 | −48674.8 | −1277.03 | −57925.8 | −2810.1 | |||
| CaO | −64068.1 | −102442 | −98428.3 | 1519.81 | −59355.5 | 699.123 | −78924.5 | ||
| Na2O | −73758.3 | −101074 | −135615 | −3717.38 | −36966.2 | 780.15 | −92611.4 | −62779.9 | |
| K2O | −87596.4 | −40700.7 | −175326 | 283.726 | −84579.5 | −60.7241 | −45162.9 | −27908 | −18129.7 |
Values reproduced from Table A4-3 of Ghiorso et al. (1983).
The solubility equation [Eq. (10)] can be solved in the following algorithm. For the coexisting fluid phase, the partial fugacity coefficient is calculated using Eq. (13) at a given pressure, temperature, and fluid composition. For the melt, the activity coefficients for H2O and CO2 ($\gamma_{\text{H${_{2}}$O}}$ and $\gamma_{\text{CO}_{2}}$) are calculated from Eqs. (15) and (16). Because $\gamma_{\text{H${_{2}}$O}}$ and $\gamma_{\text{CO}_{2}}$ are a function of H2O and CO2 content (i.e., $x_{\text{H${_{2}}$O}}$ and $x_{\text{CO}_{2}}$), these four variables ($\gamma_{\text{H${_{2}}$O}}$, $\gamma_{\text{CO}_{2}}$, $x_{\text{H${_{2}}$O}}$, and $x_{\text{CO}_{2}}$) are obtained simultaneously by solving Eqs. (15) and (16) through numerical techniques such as the multivariable Newton-Raphson method (e.g., Albarède, 1995). Then, the solubilities of H2O and CO2, in wt% or ppm, are obtained by converting $x_{\text{H${_{2}}$O}}$ and $x_{\text{CO}_{2}}$ through Eqs. (17a) and (17b). For convenience, an example calculation code written in C-language and a Windows executable program, which was generated by compiling the same code with MS Visual C++ .NET 2003, are provided online (https://doi.org/10.2465/jmps.221224b), and readers may freely use and modify it for their purposes.
Although the solubility calculated without using Eq. (14) agrees with the values obtained by using the executable file previously provided on Duan’s website, a small deviation (∼ 2.5% for rhyolite and ∼ 2.0% for basalt; Fig. 1) still exists. It was found, after some trial and error, that the calculation results are highly sensitive to a small change of constant values, in particular gas constant and cal-J conversion value (∼ 4.2 J cal−1). For example, when the value of 4.184 J cal−1 is used instead of 4.2, the consistency worsened (with deviation of ∼ 4.2% for rhyolite and ∼ 4.1% for basalt). When the gas constants of 0.08314 and 8.314 were used, respectively, in Eqs. (10) and (15)-(16), instead of 0.083 and 8.3, the consistency again worsened (with deviation of ∼ 3.9% for rhyolite and ∼ 3.7% for basalt). Therefore, the choice of constant values is considered the main cause for the small deviation. Different values of the molar mass of oxides may improve or worsen the results. However, because the model contains so many constant values and because there is no guide for adjusting these values, no further attempt was made to search for the best values. Values tentatively used in the present study are provided in Supplementary Document.
To examine the predictability of the Duan (2014) model, isobars (solubilities at constant pressures) were calculated and compared with the experimental data (Fig. 2). The same colours for isobar and data point in Figure 2 represent the same melt composition and temperature. The melt compositions examined here are summarized in Supplementary Document.
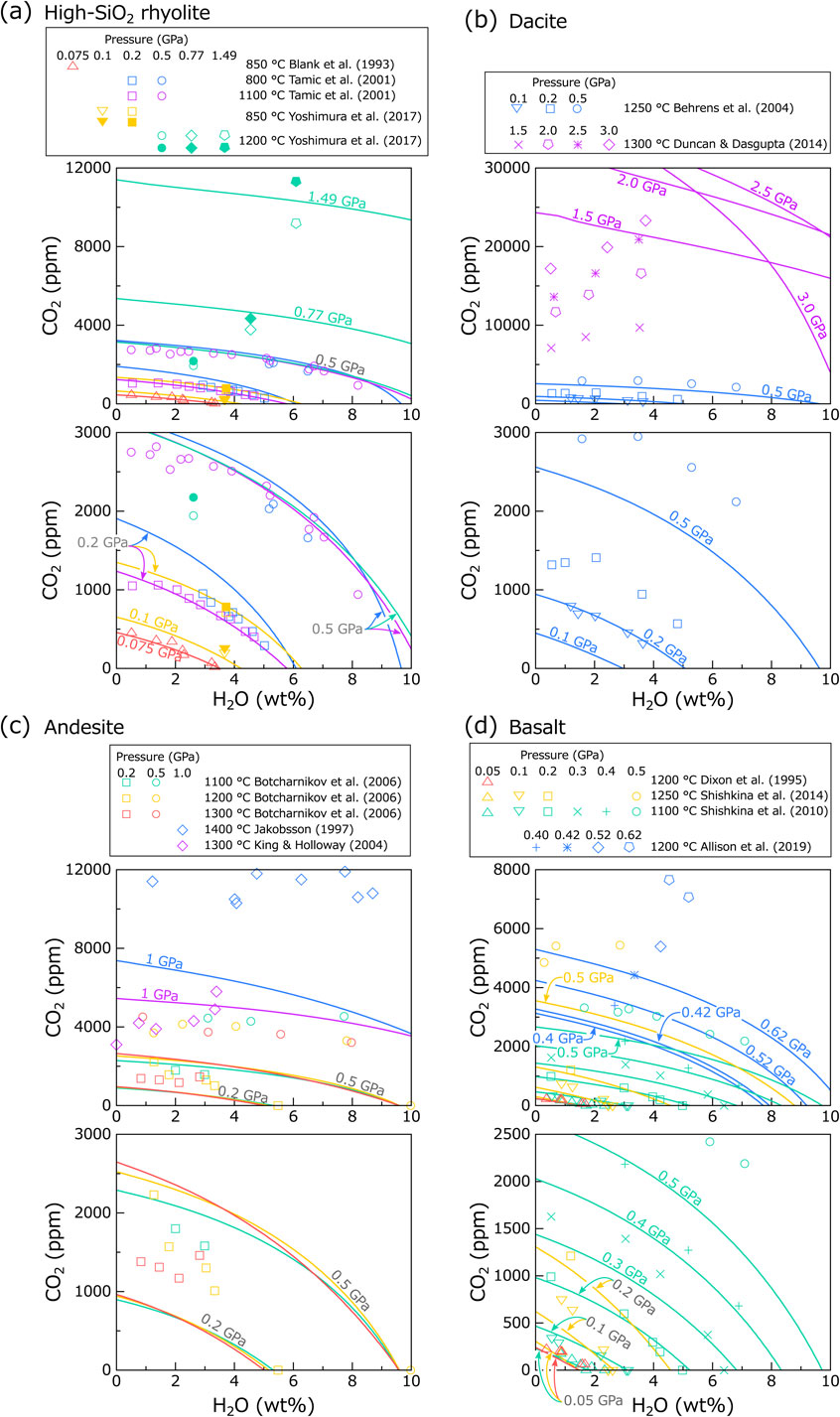
Figure 2a shows the experimental data and calculated isobars for high-SiO2 rhyolitic melts. While Blank et al. (1993) and Tamic et al. (2001) reported that CO2 dissolved solely as CO2mol in their experimental samples, Yoshimura et al. (2017) observed presence of both CO2mol and CO32−. Therefore, the data for CO2mol + CO32− are also shown for Yoshimura et al. (2017). The comparison shows that the data of Blank et al. (1993) and the 1100 °C data of Tamic et al. (2001) are reproduced well by the model with a deviation of ∼ 10%. The 800 °C data of Tamic et al. (2001), in particular those at 0.2 GPa, are less accurately reproduced (deviation is ∼ 25%), despite that all these data were used for calibrating the Duan (2014) model. The results of the 0.77 GPa experiment and 1.49 GPa experiment of Yoshimura et al. (2017), which were not used for the model calibration, are acceptably reproduced by the model with a deviation of ∼ 20%, whereas the 0.5 GPa experiment of Yoshimura et al. (2017) was less well reproduced by the model (with a deviation of ∼ 25%).
Figure 2b shows the comparison for dacitic melts (Behrens et al., 2004) and alkali-rich low-SiO2 rhyolitic melts (Duncan and Dasgupta, 2014). In these melts, both CO2mol and CO32− exist as dissolved species. For dacitic melt (Behrens et al., 2004), the model significantly underestimates the CO2 content (deviation is ∼ 40%), despite that these experimental data were used for the model calibration. For low-SiO2 rhyolite (Duncan and Dasgupta, 2014), the model resulted in extremely high CO2 content, which is clearly incompatible with the data. Moreover, the calculated isobars intersect each other despite the identical melt composition and temperature, which seems to be unrealistic for solubility behaviour.
Figure 2c shows the comparison for andesitic melts. These melts contain both CO2mol and CO32− as dissolved species. The CO2 content of Botcharnikov et al. (2006) and Jakobsson (1997) is significantly higher than the calculated isobars, despite the data being used for the model calibration. The data of King and Holloway (2002) are to some extent consistent with the model.
Figure 2d shows the data for basaltic melts. All these melts contain solely CO32− as dissolved species. It is known that CO2 solubility in basaltic melt increases with increasing alkali and alkali earth content (e.g., Dixon, 1997; Moore, 2008), and the observation that the data of Shishkina et al. (2014) and Allison et al. (2019), both for alkali basalt, show much higher CO2 content than the data of Shishkina et al. (2010), for tholeiitic basalt, is consistent with this relationship. The calculated isobars also show the same compositional dependency. However, the comparison shows that the model only reproduces well the experimental data for tholeiitic basalt (Shishkina et al., 2010) at <0.2 GPa with a deviation of ∼ 10%, and at higher pressures, the model failed to reproduce the data. For alkali basalt, the deviation is more severe at all pressure ranges.
In conclusion, the Duan (2014) model provides acceptable solubility prediction for rhyolitic melts (and tholeiitic melt at low pressures), whereas the model mostly underestimates the CO2 content for other melts. Therefore, caution is required when applying the Duan (2014) model to dacite to alkali-rich basaltic melts. Nevertheless, the model can be used appropriately for natural andesitic to dacitic magmas because the interstitial melt of these magmas (or groundmass of the rocks) is typically high-SiO2 rhyolitic in composition.
For reference, isobars calculated using Eq. (14) are compared with the experimental data (see Supplementary Document): it was found that the data fit did not improve significantly.
Finally, the Duan (2014) model was compared with the MagmaSat model (Ghiorso and Gualda, 2015), which is similarly applicable to wide pressure and temperature conditions and various melt compositions (Fig. 3). The MagmaSat calculation was carried out using the VESIcal web application version 1.0.1 released by Iacovino et al. (2021).
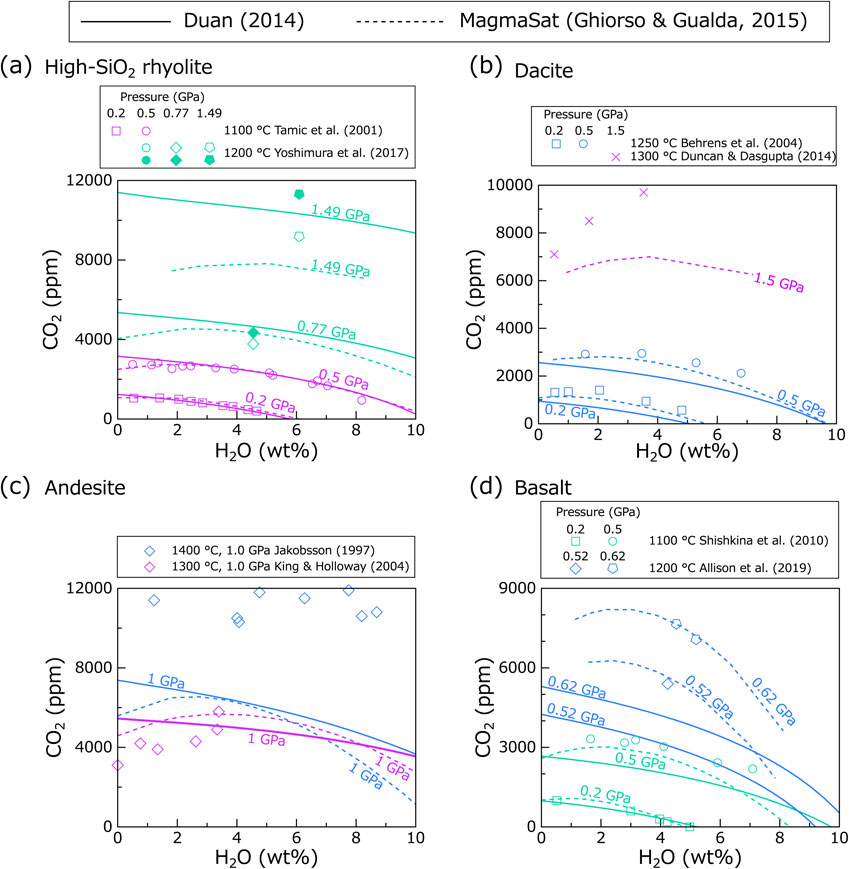
For rhyolite (Fig. 3a), both MagmaSat and Duan (2014) models generally accurately reproduce the experimental data. For the 1.49 GPa experiment (Yoshimura et al., 2017), MagmaSat underestimates the total CO2 content (deviation is ∼ 25%), in contrast to the Duan (2014) model which acceptably reproduced the 1.49 GPa data (deviation is ∼ 10%).
For dacitic melt of Behrens et al. (2004) (Fig. 3b), MagmaSat results are more consistent with the data at 0.2 and 0.5 GPa than the Duan (2014) model. For low-SiO2 rhyolitic melt of Duncan and Dasgupta (2014), MagmaSat well predicts the H2O-poorest data (deviation is <10%). However, as the water content increases, the MagmaSat model significantly underestimates the CO2 content.
For andesitic melts (Fig. 3c), data from King and Holloway (2002) are to some extent consistent with both models. Data from Jakobsson (1997) are reproduced by neither MagmaSat nor the Duan (2014) model.
For basaltic melts (Fig. 3d), data from the 0.2 GPa experiments are reproduced well by both models, whereas data from higher pressures (>0.5 GPa) are only reproduced well by MagmaSat. In particular, the data from Allison et al. (2019) for alkali basalt at high pressures (0.52 and 0.62 GPa) are excellently reproduced by MagmaSat, in contrast to the Duan (2014) model.
It can be concluded that MagmaSat appears to predict H2O-CO2 solubility better for most magmas. Therefore, MagmaSat can be seen as a safe choice to study, for example, pre-eruptive magmatic conditions based on melt inclusion data. Since the Duan (2014) model is simpler and provides reasonable solubility predictions at pressures of the shallow to middle crust for rhyolite and at pressures of the shallow crust for tholeiitic basalt, the model can be adequately used in simulation studies where the fundamental characteristics of degassing processes, rather than quantitative pressure estimation, are the main research target.
This paper reviewed the solubility model developed by Duan (2014). The calculation procedure and some modifications of the calculation method for the partial fugacity coefficient at pressures >0.2 GPa were presented. The calculation results reproduced well those computed using the Windows executable file, which was previously provided on Duan’s website, within 2.5%. Comparing the model with experimental data showed that the Duan (2014) solubility model accurately predicted the solubility for high-SiO2 rhyolitic melts, but it underestimated CO2 content for other melts including dacitic, andesitic, and basaltic melts, especially when the pressure exceeded 0.5 GPa. Comparison with MagmaSat (Ghiorso and Gualda, 2015) revealed that MagmaSat provides values that are more consistent with experimental data than the Duan (2014) model. Therefore, MagmaSat may be preferred for quantitatively estimating magma chamber conditions based on melt inclusion data.
I thank Junji Yamamoto and an anonymous reviewer for constructive comments. Editor Tetsu Kogiso carefully reviewed the manuscript and provided helpful suggestions. This study was supported by JSPS KAKENHI Grant Number JP20H01989.
Supplementary Document, code, and executable file are available online from https://doi.org/10.2465/jmps.221224b.