2023 年 118 巻 1 号 論文ID: 230112
2023 年 118 巻 1 号 論文ID: 230112
The crystal structures of α-Na2B4O7 and γ-Na2B4O7 formed during the heating of borax were investigated by single-crystal X-ray diffraction. In addition, the structural stability of the fundamental building blocks (FBBs) was examined using ab initio quantum chemical calculations. α-Na2B4O7 crystallized into the triclinic space group P1 with unit cell dimensions of a = 6.5489(7) Å, b = 8.6261(9) Å, c = 10.4909(11) Å, α = 93.2540(10)°, β = 94.8660(10)°, γ = 90.8380(10)°, V = 589.45(11) Å3. γ-Na2B4O7 crystallized into the triclinic space group P1 with unit cell dimensions of a = 6.7123(11) Å, b = 9.6052(17) Å, c = 13.270(2) Å, α = 104.183(4)°, β = 91.560(4)°, γ = 106.501(4)°, and V = 791.0(2) Å3. In both α-Na2B4O7 and γ-Na2B4O7, Na coordination polyhedra with the same coordination numbers have similar coordination volumes; however, the Na polyhedra in γ-Na2B4O7 possess more distortable environments than those in α-Na2B4O7. The flexibility of the Na coordination environment allows these materials to adopt favorable oxygen positions, leading to an α-γ phase transition. The structural stability of the FBBs in α-Na2B4O7 and γ-Na2B4O7 was lowered by dehydration and recrystallization. Consequently, α-Na2B4O7 and γ-Na2B4O7 possess FBBs with readily changeable connection geometries, which causes a phase transformation between α-Na2B4O7 and γ-Na2B4O7 without requiring a significant amount of energy.
Naturally occurring borates are a major economic source of boron. Currently, the most common commercial sources of borate are colemanite, CaB3O4(OH)3·H2O, ulexite, NaCaB5O6(OH)6·5H2O, kernite, Na2B4O6(OH)2·3H2O, and borax, Na2B4O5(OH)4·8H2O (Helvaci and Palmer, 2017). These are most abundant in Turkey, the United States, and Russia, which supply over 80% of the world’s borates. Borax is one of the most important minerals used in the borate industry. Anhydrous borax, Na2B4O7, has long been studied because of its importance in various applications, such as in borosilicate glasses, glass wool, ceramics, cement enamels, and detergents (Garrett, 1998). From structural and thermodynamic perspectives, studying the dehydration of borax is essential because nearly 50% of the total weight is released from the structure as water (Waclawska, 1995; Ekmekyapar et al., 1997; Dumanli et al., 2022). There are four stages in the thermal transformation of borax to anhydrous borax, which occur over a temperature range of 50-750 °C (Waclawska, 1995). (1) Dehydration of borax starts at 74 °C; upon further heating to 133 °C, (2) it changes to tincalconite Na2B4O5(OH)4·3H2O with the release of 5 mol H2O. Upon heating to 200 °C, (3) tincalconite decomposes into an amorphous phase. Subsequently, (4) the remaining H2O is continuously released. At approximately 420 °C, a part of the amorphous phase crystallizes and finally transforms to anhydrous borax Na2B4O7 at 575 °C. Before Na2B4O7 melts at 723 °C, it undergoes a complicated crystallization process in which many polymorphic modifications occur (Morey and Mervin, 1936; Krogh-Moe, 1974; Li and Liang, 1995; Sennova et al., 2007; Fofanova et al., 2013). Li and Liang (1995) reported that β-Na2B4O7 is formed when the temperature is maintained at 550 °C for 2 days or more, and γ-Na2B4O7 is formed at a temperature range of 560-640 °C. Although the authors proposed that the γ-Na2B4O7 crystallized into the monoclinic space group P2, Pm, or P2/m, it was later corrected by Kanishcheva et al. (2004) to the triclinic space group P1. Above this temperature, γ-Na2B4O7 transforms into stable α-Na2B4O7 with a triclinic space group P1 (Krogh-Moe, 1974). In addition, metastable δ- and ε-Na2B4O7 phases appear depending on the annealing temperature and heating time (Li and Liang, 1995).
Despite their industrial and technological importance, there have been no reports on the crystal structures of anhydrous borax phases formed during the heating process. The crystal structure of α-Na2B4O7 was determined using a specimen crystallized from a melt heated at 800 °C (Krogh-Moe, 1974), whereas that of γ-Na2B4O7 was determined using a crystal grown from a mixture of sodium oxide and borate oxide (Kanishcheva et al., 2004; Fofanova et al., 2013). Borates are known to exhibit extreme structural complexity based on fundamental building blocks (FBBs) consisting of Bϕ3 triangles and Bϕ4 tetrahedra (ϕ: O2−, OH−). FBBs form many types of rings consisting of corner-sharing Bϕ3 triangles and Bϕ4 tetrahedra (Hawthorne et al., 1996; Grice et al., 1999). In this study, we investigated the crystal structures of α-Na2B4O7 and γ-Na2B4O7 formed during the heating of borax using single-crystal X-ray diffraction (XRD). In addition, we examined various connection modes between the Bϕ3 triangles and Bϕ4 tetrahedra and their structural stabilities using ab initio quantum chemical calculations.
Commercially available borax, sodium tetraborate decahydrate (guaranteed reagent grade, Fujifilm Wako Pure Chemical Co., Inc., Osaka, Japan) was used as the starting material. It was placed in a Pt crucible and heated in an electronic furnace in the presence of air at temperatures ranging from 40 to 750 °C. After heating for 1 h, the samples were cooled naturally to room temperature. The samples were then stored in a vacuum desiccator until use.
Synchrotron powder XRD measurements were performed at BL8B at the Photon Factory (PF), High-Energy Accelerator Research Organization (KEK), Japan. The samples were removed from the vacuum desiccator, powdered, and inserted into Lindemann glass capillaries (φ = 0.7 mm). The incident beam was monochromatized using an Si(111) double-crystal monochromator. The X-ray beam was collimated to 0.5 mm in diameter. The exposure time was 5 min. The samples were rotated at a 10° φ angle during each exposure. The X-ray wavelength used in this study was determined to be 0.6840(1) Å using CeO2 (NIST Standard Reference Material 674a).
Thermal analysis was performed using thermogravimetric (TG) and differential thermal analysis (DTA) (TG/DTA-7300, Seiko Instruments Inc. Chiba, Japan). Approximately 15 mg of borax was placed in a flat-bottomed platinum pan. The sample was heated from 50 to 800 °C at a rate of 10 °C/min under an argon gas flow at a rate of 200 mL/min.
Single crystals were obtained from samples heated at 600 and 650 °C for 12 h. Single crystals suitable for single-crystal XRD were selected under an optical microscope (SMZ1270, Nikon Corporation, Tokyo, Japan) and mounted on glass fibers. The lattice parameters and intensity data were measured using a single-crystal diffractometer (SMART APEX II ULTRA; Bruker AXS Inc., Germany) equipped with a CCD detector, multilayer optics, and graphite monochromatized MoKα radiation (λ = 0.71073 Å) generated by a rotating anode. The initial lattice parameters and orientation matrix were determined from the 36 frames collected with an exposure time of 10 s. A total of 720 frames were collected with an exposure time per frame of 10 s in the ω-scan mode with Δω = 0.5° at three different ϕ positions. The intensity data were integrated and corrected for Lorentz and polarization effects using the Bruker APEX3 software suite (Bruker, 2018). The collected data was reduced using the SAINT software (Bruker, 2017). Empirical absorption correction was also applied using the SADABS software (Sheldrick, 2012). The structure was solved by combining the direct and difference Fourier methods provided by the program SHELXS-2013/1 (Sheldrick, 2015). The atomic coordinates and atomic displacement parameters were refined using full-matrix least-squares methods on F2 using the software SHELXL-2013/1 (Sheldrick, 2015). The atomic labels for α-Na2B4O7 and γ-Na2B4O7 were obtained from previous studies (Krogh-Moe, 1974; Kanishcheva et al., 2004). All atoms were refined based on an anisotropic displacement model. The details of the data collection and crystal structure refinement are listed in Table 1. The polyhedral parameters were calculated using IVTON software (Balić-Žunić and Vickovic, 1996).
| α-Na2B4O7 | γ-Na2B4O7 | |
| Crystal data | ||
| Crystal size (mm) | 0.20 × 0.10 × 0.10 | 0.10 × 0.05 × 0.05 |
| Crystal system | Triclinic | Triclinic |
| Space group | P1 | P1 |
| a (Å) | 6.5489(7) | 6.7123(11) |
| b (Å) | 8.6261(9) | 9.6052(17) |
| c (Å) | 10.4909(11) | 13.270(2) |
| α (o) | 93.2540(10) | 104.183(4) |
| β (o) | 94.8660(10) | 91.560(4) |
| γ (o) | 90.8380(10) | 106.501(4) |
| V (Å3) | 589.45(11) | 791.0(2) |
| Z | 4 | 2 |
| Data collection and refinement | ||
| Radiation, wavelength (Å) | MoKα, λ = 0.71073 | MoKα, λ = 0.71073 |
| Temperature (°C) | 20 | 20 |
| Maximum observed 2θ (o) | 57.84 | 55.72 |
| Measured reflections | 3539 | 30984 |
| Unique reflections | 2690 | 3746 |
| Reflections Fo > 4σ(Fo) | 2393 | 2615 |
| Range of h, k, l | −8 ≤ h ≤ 8 | −8 ≤ h ≤ 8 |
| −10 ≤ k ≤ 11 | −12 ≤ k ≤ 12 | |
| −13 ≤ l ≤ 12 | −17 ≤ l ≤ 17 | |
| R1 [Fo > 4σ(Fo)] | 0.0320 | 0.0451 |
| wR2 | 0.0853 | 0.1182 |
| Goodness of fit | 1.066 | 1.018 |
| Number of l.s. parameters | 236 | 353 |
| Residual highest and deepest peaks (e/Å3) | 0.74, −0.67 | 0.41, −0.46 |
Ab initio quantum chemical calculations were performed using the quantum chemical calculation software package Gaussian-16 (Frisch et al., 2016). The total energies of the FBBs comprising α-Na2B4O7, γ-Na2B4O7, and representative borate minerals were calculated using the second-order Møller-Plesset perturbation theory (MP2) with the 6-311+G(d,p) basis set. The FBB geometries of the borates used in this study were not composed of infinite extended arrays, such as one-dimensional chains, two-dimensional sheets, and three-dimensional frameworks, but unconnected, isolated borate polyanions. Generally, the edges of FBBs are terminated by adding hydrogen atoms to the terminal oxygen atoms instead of repeating structural unit. However, because the isolated borate polyanions were composed of a single structural unit, no hydrogen atoms were placed at the terminal oxygen atoms of the FBBs. In this study, the total energies of the FBBs were divided by the number of electrons in the FBBs to compare their structural stability (Bochvar et al., 1989; Jiang and Zhang, 1989; Koskinen et al., 1995).
Figure 1 shows the continuous variation in the XRD patterns with temperature. Borax is closely related to tincalconite Na2B4O5(OH)4·3H2O, the dehydration product of borax. Borax and tincalconite are rapidly and reversibly interconverted at a relative humidity of 60% and temperature of 20-25 °C (Christ and Garrels, 1959). The XRD pattern at 40 °C exhibited diffraction peaks for both borax and tincalconite. With increasing temperature, the borax diffraction peaks gradually weakened and finally disappeared at 80 °C. The diffraction peaks of tincalconite decreased at 120 °C and became almost zero at 180 °C. Upon further heating, slight diffraction peaks were observed in the amorphous halo pattern. The slight diffraction peaks remained largely unchanged up to 450 °C but appeared to temporarily increase at 500 °C. As the diffraction peak positions observed between 200 and 550 °C were fully consistent with those of tincalconite, the crystalline phase would possess FBBs similar in structure to those of tincalconite. Above 600 °C, strong diffraction peaks appeared, indicating the crystallization of anhydrous borax Na2B4O7. However, these diffraction patterns corresponded to neither α-Na2B4O7 nor γ-Na2B4O7. This suggests that the XRD patterns show a mixture of several anhydrous borax phases, including α-, β-, γ-, δ-, and ε-Na2B4O7 phases (Li and Liang, 1995). Thereafter, the diffraction peaks weakened at 700 °C and disappeared entirely at 750 °C.

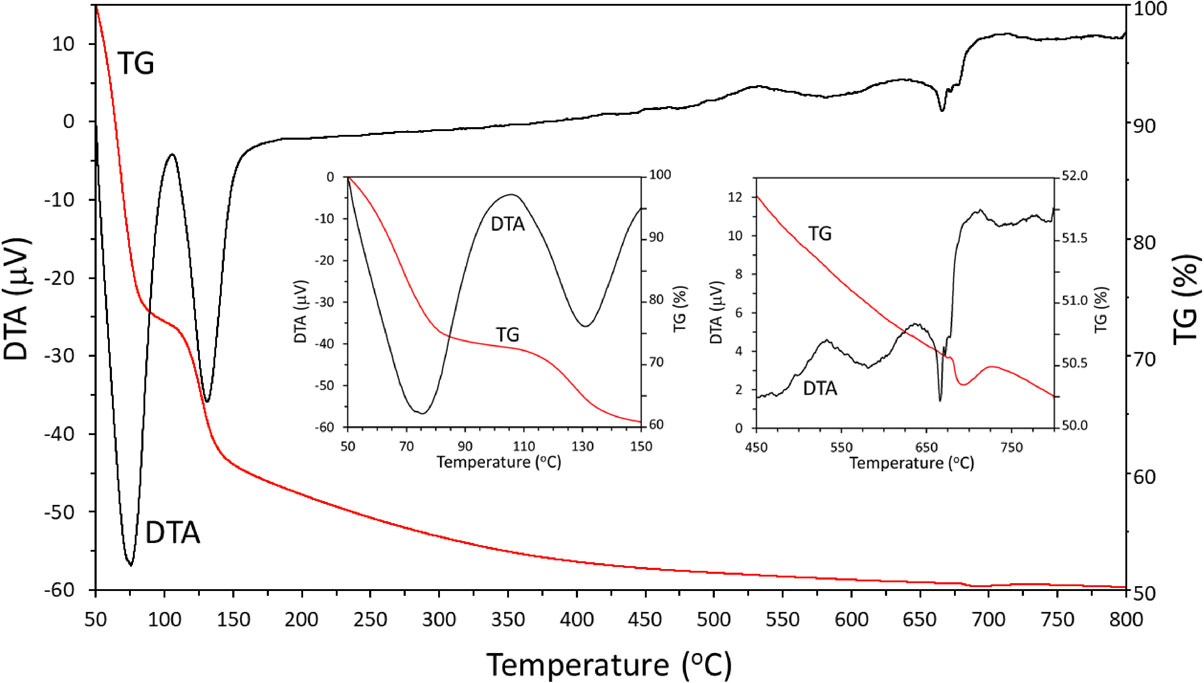
Figure 2 shows the TG and DTA curves of borax. The two dehydration temperatures of borax and tincalconite are consistent with those reported by Waclawska (1995). The first and second mass losses of approximately 25% and 10% agree with the theoretical mass losses of 23.6% corresponding to 5H2O in borax and 9.4% corresponding to 2H2O in tincalconite. Thereafter, the TG curve showed a continuous mass loss of the remaining water molecules, finally showing a mass loss of 49.5%, which is in approximate agreement with the mass of ten water molecules in borax corresponding to 47.2%. The exothermic peak not accompanied by a clear mass loss was observed at approximately 530 °C, which indicates that dehydrated borax crystallized at this temperature. The XRD result supported this result that the diffraction peaks temporarily increased at 500 °C (Fig. 1). Sharp endothermal peaks were observed at approximately 670 °C, indicating that the Na2B4O7 phases began to decompose at these temperatures. After a small mass loss at 700 °C, the anhydrous borax melted at approximately 725 °C. The melting temperature was consistent with that reported by Waclawska (1995); however, the cause of the mass loss just before melting requires further detailed study.
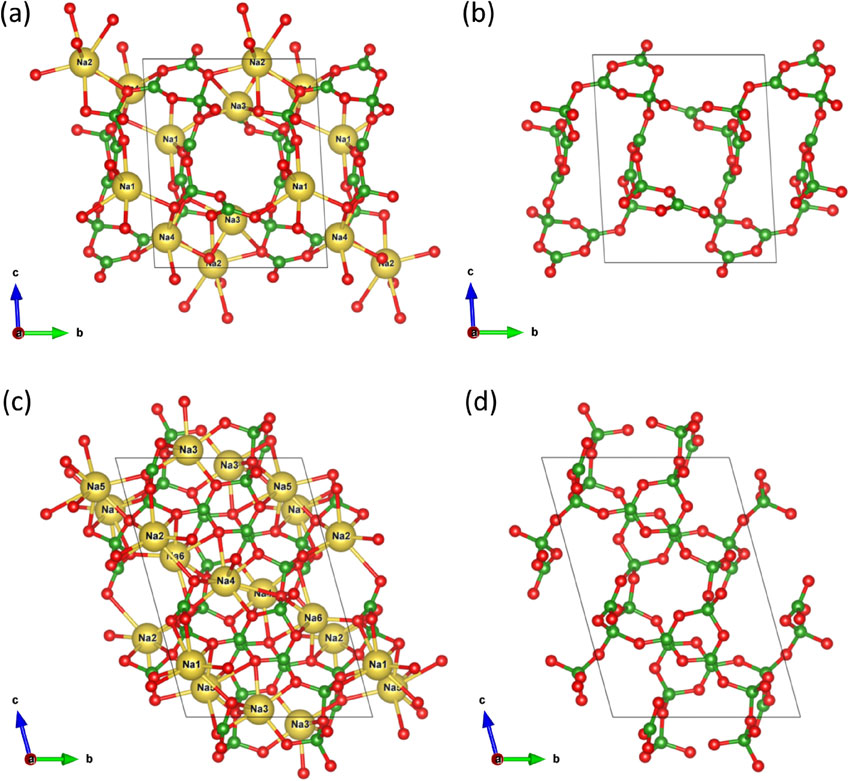
Single crystals extracted at 600 and 650 °C were investigated using single-crystal XRD measurements. The crystal structures of the crystals extracted at 600 and 650 °C matched those of γ-Na2B4O7 and α-Na2B4O7, respectively. γ-Na2B4O7 crystallized in triclinic space group P1 with unit cell dimensions of a = 6.7123(11) Å, b = 9.6052(17) Å, c = 13.270(2) Å, α = 104.183(4)°, β = 91.560(4)°, γ = 106.501(4)°, and V = 791.0(2) Å3 (Table 1). α-Na2B4O7 crystallized into the triclinic space group P1 with unit cell dimensions of a = 6.5489(7) Å, b = 8.6261(9) Å, c = 10.4909(11) Å, α = 93.2540(10)°, β = 94.8660(10)°, γ = 90.8380(10)°, V = 589.45(11) Å3 (Table 1). The fractional atomic coordinates, displacement parameters, and selected bond distances are listed in Supplementary Tables S1 and S2 (Tables S1 and S2 are available online from https://doi.org/10.2465/jmps.230112), respectively. Figure 3 shows the crystal structures of α-Na2B4O7 and γ-Na2B4O7. The crystal structure of α-Na2B4O7 is characterized by a framework structure with open channels, whereas that of γ-Na2B4O7 is characterized by a complicated three-dimensional framework. The Na-O and B-O bond distances in α-Na2B4O7 and γ-Na2B4O7 were identical to those reported by Krogh-Moe (1974) and Kanishcheva et al. (2004). The broad distribution of Na-O distances is a characteristic of borate structures (Krogh-Moe, 1974; Fofanova et al., 2013). Moreover, the Na-O interatomic distances around the Na4 site in γ-Na2B4O7 are continuously distributed up to 3.187 Å, forming 10-fold coordination. However, as this is unrealistic, it is difficult to determine the coordination number only from the coordination environments around the Na atoms. In this study, the bond valence sum (Brese and O’Keeffe, 1991) was used as an indicator to estimate the coordination number of the Na atom. The bond valence analysis results are listed in Supplementary Tables S3 and S4 (Tables S3 and S4 are available online from https://doi.org/10.2465/jmps.230112). Consequently, we concluded that the Na atom at the Na4 site in γ-Na2B4O7 is coordinated by six oxygen atoms in the 2.320-2.643 Å. The seven-fold coordination at the Na2 site in α-Na2B4O7 exhibits a low bond valence sum (0.930 v.u.), but the next neighbor Na-O interatomic distance of 3.638 Å is too large to form a bond between Na and O atoms. The polyhedral parameters of the coordination environments of the Na atoms are presented in Table 2. In α-Na2B4O7 and γ-Na2B4O7, Na coordination polyhedra with the same coordination number have similar coordination volumes and radii as the fitted spheres. Na polyhedra with the largest and smallest cation eccentricities (distance between the centroid position and the Na atom in the Na polyhedron) are formed in γ-Na2B4O7. However, the average eccentricity in γ-Na2B4O7 is almost equal to that in α-Na2B4O7, which implies that the Na polyhedra in γ-Na2B4O7 possess more distortable environments than those in α-Na2B4O7. The flexibility of the Na coordination environment allows these materials to adopt favorable oxygen positions, which leads to the α-γ phase transition.
| α-Na2B4O7 | γ-Na2B4O7 | |||||||||
| Na1O6 | Na2O7 | Na3O6 | Na4O6 | Na1O7 | Na2O7 | Na3O6 | Na4O6 | Na5O7 | Na6O6 | |
| V | 14.91 Å3 | 22.81 Å3 | 13.82 Å3 | 12.84 Å3 | 20.27 Å3 | 21.18 Å3 | 14.72 Å3 | 13.72 Å3 | 21.37 Å3 | 14.11 Å3 |
| r | 2.495 Å | 2.575 Å | 2.470 Å | 2.414 Å | 2.488 Å | 2.534 Å | 2.401 Å | 2.420 Å | 2.579 Å | 2.476 Å |
| s | 0.9455 | 0.9495 | 0.9385 | 0.9499 | 0.9277 | 0.9104 | 0.9796 | 0.9731 | 0.9488 | 0.9305 |
| Δ | 0.153 Å | 0.225 Å | 0.277 Å | 0.148 Å | 0.065 Å | 0.357 Å | 0.198 Å | 0.164 Å | 0.290 Å | 0.181 Å |
V, polyhedron volume; r, radius of the least-squares sphere fitted to the polyhedron; s, sphericity equals to (1 − σr/r), where σr is standard deviation of r; Δ, distances between the centroid and central atom of the polyhedron (cation eccentricity).
Figure 4 shows the connection modes between BO3 triangles and BO4 tetrahedra. The crystal structure of α-Na2B4O7 comprises two types of FBBs. One consists of a three-membered ring composed of two BO3 triangles and one BO4 tetrahedron (Fig. 4a). According to the description proposed by Burns et al. (1995), this FBB is expressed by ⟨2△□⟩, where △ and □ indicate Bϕ3 triangles and Bϕ4 tetrahedra (ϕ: O2−, OH−), respectively. The other consists of a double three-membered ring connected by two types of three-membered rings. One comprises two BO3 triangles and one BO4 tetrahedron, and the other comprises one BO3 triangle and two BO4 tetrahedra connected by a common BO4 tetrahedron (Fig. 4b). The FBB is expressed as ⟨2△□⟩-⟨△2□⟩. The crystal structure of γ-Na2B4O7 comprises only one type of FBB: a double three-membered ring observed in α-Na2B4O7 expressed by ⟨2△□⟩-⟨△2□⟩ (Fig. 4b). It is important to emphasize that the double three-membered rings are a common connection mode in α-Na2B4O7 and γ-Na2B4O7. Considering the phase transition between α-Na2B4O7 and γ-Na2B4O, it is reasonable to assume that they consist of common FBBs. In addition, this suggests the possibility that the β-, δ-, and ε-Na2B4O7 phases include the same double three-membered rings expressed by ⟨2△□⟩-⟨△2□⟩ in the structures.

The total energies of the FBBs in anhydrous borates and representative borate minerals are listed in Table 3. Burns (1995) previously performed molecular orbital calculations for FBBs in borax using the Hartree-Fock method with the 3-21G* basis set. The total energy obtained by Burns (1995) was −771.24 Hartree, almost identical to our result (Table 3). The relative structural stability of the FBBs is shown in Figure 5. Borax comprises two three-membered rings, each containing a BO3 triangle and two BO4 tetrahedra. Because these rings have two BO4 tetrahedra in common, this FBB is expressed by ⟨△2□⟩=⟨△2□⟩, where = indicates two polyhedra common to both rings. Tincalconite Na2B4O5(OH)4·3H2O, which is formed by the dehydration of borax, is composed of the same FBB as borax (Giacovazzo et al., 1973). The connection mode of borax shows relatively low energy in the diagram (Fig. 5). Colemanite CaB3O4(OH)3·H2O, one of the most common borate minerals, includes three-membered rings composed of one Bϕ3 triangle and two Bϕ4 tetrahedra, expressed by ⟨△2□⟩. The colemanite connection mode exhibited the lowest total energy among the borate minerals (Fig. 5). Ulexite, NaCaB5O6(OH)6·5H2O, and kernite, Na2B4O6(OH)2·3H2O, are also common borate minerals. They are composed of double three-membered rings consisting of two three-membered rings of one Bϕ3 triangle and two Bϕ4 tetrahedra, expressed by ⟨△2□⟩-⟨△2□⟩. The relatively low total energies of FBBs in borax, ulexite, and kernite are consistent with their widespread occurrence. Notably, these major borate minerals possess common FBBs of three-membered rings of one Bϕ3 triangle and two Bϕ4 tetrahedra, expressed by ⟨△2□⟩. Burns et al. (1995) examined the frequency of occurrence of various FBB geometries in borate minerals and concluded that ⟨△2□⟩ rings are strongly favored in FBBs. Burns et al. (1995) concluded that three-membered rings occur in the following order of preference: ⟨△2□⟩ >> ⟨2△□⟩ > ⟨3□⟩ > ⟨3△⟩. This order of frequency is partially consistent with the ab initio quantum chemical calculations. As mentioned above, the ⟨△2□⟩ ring in colemanite exhibits the lowest total energy, which is in good agreement with the results of Burns et al. (1995). The ⟨2△□⟩ ring, consisting of two Bϕ3 triangles and one Bϕ4 tetrahedron in ameghinite, also exhibits relatively lower total energy. Unexpectedly, sborgite had an FBB composed of two ⟨2△□⟩ rings, with the second lowest total energy. This was because sborgite is a rare mineral. Thus far, sborgite has been found in only two known locations: California, USA, and Larderello, Italy (Cipriani, 1957; McAllister, 1961), and no relationship has been found between the frequency of occurrence and structural stability. Metaborite possesses an FBB of the ⟨3□⟩ ring consisting of three BO4 tetrahedra with relatively high total energy. The ⟨3△⟩ ring is the least common structure in borate minerals (Burns et al., 1995; Hawthorne et al., 1996). To the best of our knowledge, no borate minerals with an FBB consisting of only 3△ rings have been reported. However, one of the most noteworthy findings of this study was that the total energy of the ⟨3△⟩ ring in NaBO2 was lower than that of the ⟨2△□⟩ ring in ameghinite. The FBB of the ⟨3△⟩ ring is not necessarily an energetically unstable arrangement in the borate structures. Therefore, borate minerals with ⟨3△⟩ rings may be found.
| FBB | |||
| Name | Chemical formula | Chemical formula | Connectivity (Burns et al., 1995) |
| α-Na2B4O7 | Na2B4O7 | [B5O11]7− | ⟨2△□⟩-⟨△2□⟩ |
| α-Na2B4O7 | Na2B4O7 | [B3O7]5− | ⟨2△□⟩ |
| γ-Na2B4O7 | Na2B4O7 | [B5O11]7− | ⟨2△□⟩-⟨△2□⟩ |
| Borcarite | Ca4MgB4O6(CO3)2(OH)6 | [B4O6(OH)6]6− | ⟨4□⟩ |
| Uralborite | CaB2O2(OH)4 | [B4O4(OH)8]4− | ⟨3□⟩□ |
| Metaborite | CaB3O4(OH)3·H2O | [B3O3(OH)6]3− | ⟨3□⟩ |
| Priceite | Ca2B5O7(OH)5·H2O | [B5O8(OH)5]6− | ⟨△2□⟩-⟨3□⟩ |
| Sassolite | H3BO3 | B(OH)3 | 1△ |
| Hydrochlorborite | Ca2B3O3(OH)4·BO(OH)3Cl·7H2O | [B3O3(OH)4·BO(OH)3]3− | ⟨△2□⟩□ |
| Biringuccite | Na2B5O8(OH)·H2O | [B5O10(OH)]6− | ⟨2△□⟩-⟨△2□⟩ |
| Ulexite | NaCaB5O6(OH)6·5H2O | [B5O6(OH)6]3− | ⟨△2□⟩-⟨△2□⟩ |
| Ameghinite | NaB3O3(OH)4 | [B3O3(OH)4]− | ⟨2△□⟩ |
| Borax | Na2B4O5(OH)4·8H2O | [B4O5(OH)4]2− | ⟨△2□⟩=⟨△2□⟩ |
| NaBO2 | NaBO2 | [B3O6]3− | ⟨3△⟩ |
| Sborgite | NaB5O6(OH)4·3H2O | [B5O6(OH)4]− | ⟨2△□⟩-⟨2△□⟩ |
| Colemanite | CaB3O4(OH)3·H2O | [B3O5(OH)3]2− | ⟨△2□⟩ |
| FBB | |||||
| Name | # of atoms | # of electrons | Total energy (Hartree) |
Total energy/ electrons (Hartree) |
References |
| α-Na2B4O7 | 16 | 120 | −948.30 | −7.903 | This study |
| α-Na2B4O7 | 10 | 76 | −597.22 | −7.858 | This study |
| γ-Na2B4O7 | 16 | 120 | −945.43 | −7.879 | This study |
| Borcarite | 22 | 128 | −1002.59 | −7.833 | Burns and Hawthorne (1995) |
| Uralborite | 24 | 128 | −1004.72 | −7.849 | Simonov et al. (1977) |
| Metaborite | 18 | 96 | −753.81 | −7.852 | Freyhardt et al. (2000) |
| Priceite | 23 | 140 | −1102.10 | −7.872 | Sun et al. (2011) |
| Sassolite | 7 | 32 | −251.97 | −7.874 | Gajhede et al. (1986) |
| Hydrochlorborite | 22 | 118 | −929.51 | −7.877 | Brown and Clark (1978) |
| Biringuccite | 17 | 120 | −949.51 | −7.913 | Corazza et al. (1974) |
| Ulexite | 23 | 130 | −1028.78 | −7.914 | Ghose et al. (1978) |
| Ameghinite | 14 | 76 | −602.81 | −7.932 | Dal Negro et al. (1975) |
| Borax | 17 | 98 | −777.83 | −7.937 | Levy and Lisensky (1978) |
| NaBO2 | 9 | 66 | −524.83 | −7.952 | Borisova et al. (1978) |
| Sborgite | 19 | 110 | −877.91 | −7.981 | Merlino and Sartori (1972) |
| Colemanite | 14 | 84 | −676.94 | −8.059 | Burns and Hawthorne (1993) |
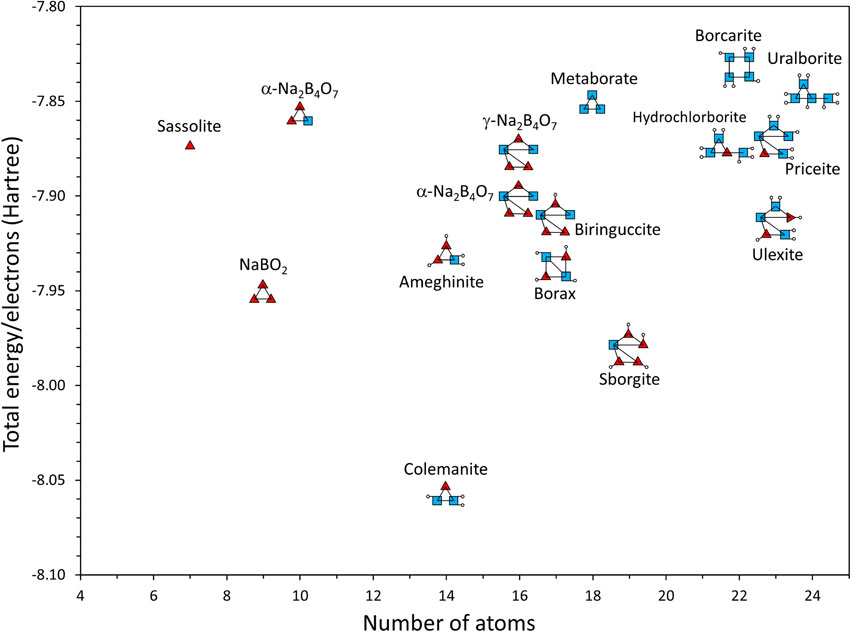
As shown in Figure 5, the total energies of the FBBs in both α-Na2B4O7 and γ-Na2B4O7 are higher than those in borax. Upon thermal treatment, borax transforms to α-Na2B4O7 or γ-Na2B4O7, but the ⟨△2□⟩ ring in borax survives as part of the FBBs in α-Na2B4O7 and γ-Na2B4O7. One of the FBBs in α-Na2B4O7 consists of three-membered rings, expressed as ⟨2△□⟩. This is the same FBB found in ameghinite. The critical difference between α-Na2B4O7 and ameghinite is the bonding of oxygen atoms on the vertices to hydrogen. The FBBs in α-Na2B4O7 and γ-Na2B4O7 contained no hydrogen at the oxygen vertices of the FBBs. Dehydration and recrystallization reduces the structural stability of the FBBs in α-Na2B4O7 and γ-Na2B4O7 from that of borax. Consequently, α-Na2B4O7 and γ-Na2B4O7 possess FBBs with readily changeable connection geometries, which would cause phase transformations among the α-, β-, γ-, δ-, and ε-Na2B4O7 phases without requiring a significant amount of energy. Grew et al. (2017) proposed that boron mineral diversity increases with geological time and is accelerated during supercontinent assembly accompanied by high-temperature metamorphism. Our results suggest that new borate minerals may be found where borates are exposed to high temperatures because borates dehydrated at high temperatures are highly likely to reconstruct the connection mode of BO3 triangles and BO4 tetrahedra.
The synchrotron radiation experiment performed at BL8B at KEK-PF was approved by the Photon Factory Program Advisory Committee (Proposal Nos. 2019G115 and 2021G521). This study was partially supported by the JSPS KAKENHI Grant Number JP20K04124.
Supplementary Tables S1-S4 are available online from https://doi.org/10.2465/jmps.230112.