2023 年 118 巻 1 号 論文ID: 230327
2023 年 118 巻 1 号 論文ID: 230327
In this study, we investigated the origin and formation process of zeolitized gastropod fossils in Neogene sediments (Shiote Formation) in Minamisoma, Fukushima, Japan using powder X-ray diffraction, SEM-EDS and micro-Raman spectroscopic analysis. The formation of zeolites was particularly pronounced in the upper chamber, which was not filled with detrital particles, of the gastropod fossils, where tabular crystals of heulandite were observed growing directly from the shell wall. The heulandite crystals are often covered by large euhedral crystals of calcite and occasionally by acicular crystals of mordenite. The formation of zeolite (heulandite) was also observed in the matrix of the host sandstone together with clay minerals (mostly montmorillonite), suggesting that the Shiote Formation experienced moderate metamorphism equivalent to zeolite facies during burial diagenesis. The Si/Al ratio of heulandite was found to decrease gradually from the bottom (∼ 4.5) to the top (∼ 3.1) within single crystals across the threshold (4.0) for clinoptilolite/heulandite classification boundary. This may reflect the increase in temperature of the surrounding environment with increase in the burial depth. The extensive growth of zeolites and calcite inside the gastropod fossils indicates that the shell provided semi-closed spaces in which pore fluid could be retained and condensed during diagenesis, thus promoting the crystal growth from the supersaturated solution.
Permineralization of fossils, the phenomenon in which a part of or whole organic tissue is replaced or filled with mineral crystals during or after fossilization, is often observed in nature (e.g., Yushkin et al., 2011; Mustoe, 2018). A typical example is silicified wood in which the internal cellular tissue has been replaced by silica (opal and chalcedony) while retaining the overall appearance and texture of wood (e.g., Mustoe, 2017). Also, the replacements by calcite (carbonatization), pyrite (pyritization), and silica (silicification) of fossilized shells such as mollusks and bivalves are often observed in sedimentary rocks. In all cases, water plays an important role, because it readily dissolves chemical components supplied from the surrounding sediments and the fossils themselves and becomes supersaturated, precipitating minerals in the fossils (Mustoe, 2017). Since silica, calcium carbonate, and iron sulfide are typical components that are abundantly supplied from sediments and biological remains, the permineralization by these minerals is the most common.
On the other hand, although it is far rare compared to those cases, zeolite formation in fossilized gastropod and pelecypod shells (Staple, 1965) and plant tissues (Modreski et al., 1982; Mysliveček et al., 2021) has also been reported. The zeolite-infilled shells reported by Staple (1965) were found in Oligocene sandstone in Oregon, USA and composed mainly of analcime, heulandite, and stilbite. The zeolites are thought to have been formed by the hydrothermal activity in relation to the basalt intrusion in the host rock, because zeolite veins were also found near the basalt dykes. Modreski et al. (1982) reported zeolitized plant fossils in Late Cretaceous to Paleocene sedimentary rocks in Lakewood, USA. The zeolites (mainly, heulandite and stilbite) were found in voids in the cell tissue of the fossilized wood including twigs, seeds, and roots. Since the host sandstone of Denver Formation contains a large amount of volcaniclastic debris, zeolites are likely to have precipitated from the groundwater that are oversaturated with sodium, calcium, and silicate components through the hydrolysis (alteration) of the volcanic glass and penetrated into the open space in the plant tissues. Another example of the zeolitization of fossilized shells was reported by Hashimoto and Taira (2011) from a Miocene sediment in Japan. They described zeolites (heulandite, clinoptilolite-like phase, mordenite, and stilbite) occurring inside the gastropod fossils (Tateiwata, Vicaryella, etc.) based on a macroscopic observation and X-ray diffraction analysis. It is interesting to note that zeolite formation was likely limited to the interior of gastropod fossils and was not found inside bivalve fossils or in the matrix of the host sandstone (Hashimoto and Taira, 2011). There is no clear evidence of volcanic rock intrusion or hydrothermal activity in the surrounding area, which is different from the two previous studies. In order to understand the origin and formation process of the zeolites in gastropod fossils, we studied their mineralogical features by means of X-ray diffraction, Raman spectroscopy, and electron microscopy.
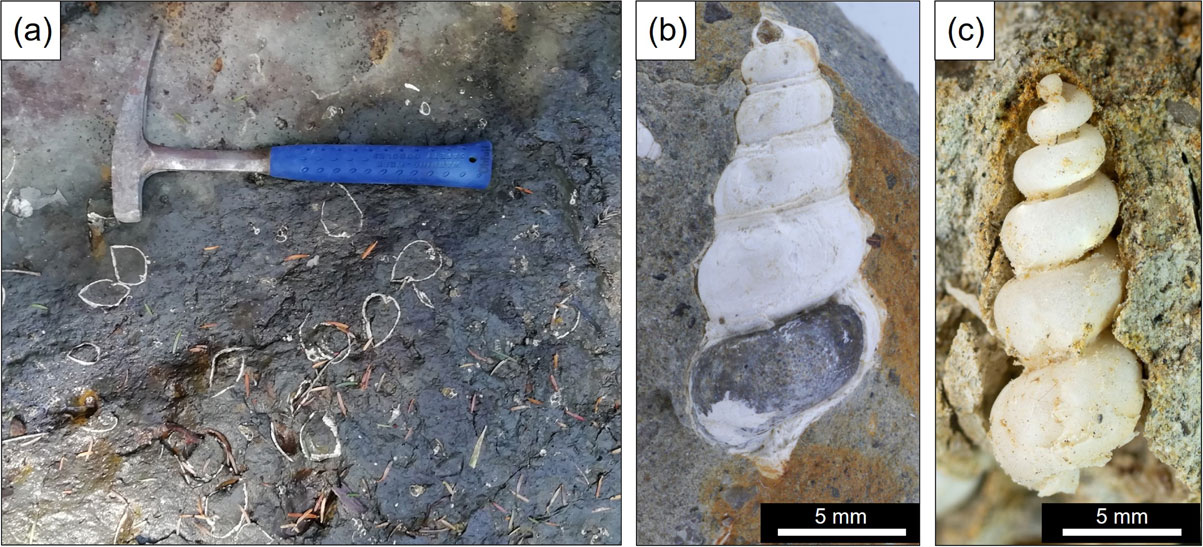
The samples used in this study were collected from outcrops of the Shiote Formation exposed along a stream in Jisahara area, Minamisoma, Fukushima Prefecture, Japan. In the Soma-Nakamura area, which includes the sampling field, Tertiary (Miocene) fluvial, lacustrine, and shallow-marine sediments are distributed over the west side of the Futaba Fault. The bottom part of the sequence is the Shiote Formation, which is unconformably covered by the Tenmyozan Volcanic Rocks and the Ryozen Formation composed mainly of pyroclastic rocks. The Ryozen Formation is unconformably covered by the Ouchi Formation, and then Akashiba Formation, which unconformably contacts the Kameoka Formation (Pliocene). The last three formations are basically nonpyroclastic sediments (Yanagisawa et al., 1996). Shiote Formation yields wood fossils (e.g., Carpinus, Quercus) and shell fossils such as Vicariya, Vicaryella, Cyclina (Yanagisawa et al., 1996), some of which contain zeolites inside (Hashimoto and Taira, 2011). According to their report, zeolite crystals were found exclusively in gastropod fossils such as Tateiwaia, Vicaryella, but were not observed in bivalve fossils (e.g., Cyclina, Hiatula). Along the stream in Jisahara area, fine-grained sandstone to silty mudstone are continuously exposed, and there are a few fossiliferous beds in which a number of gastropod and bivalve fossils were concentrated (Fig. 1a). We collected zeolitized gastropod fossils on the ground (isolated from the host rock), fossils contained in the host rock (sampled together with the rock, Fig. 1b), and fossil-free fine sandstone from the outcrops.
Rock samples containing fossils were cut into chips and embedded in resin (Devcon ET) cured at room temperature. The fossils isolated from the host rock were also embedded in the resin using a mold. To allow the resin to sufficiently penetrate into the sample, these operations were performed in a vacuum jar evacuated by a rotary pump. After curing, two types of polished sections were prepared: standard thin sections (30 µm in thickness) for polarized-light microscope observation and thick sections for scanning electron microscopic (SEM) observation. We also used Ar-ion milling system (JEOL, cross-section polisher IB-19510CP) operated at an accelerating voltage of 4 kV to prepare mechanical damage-free and contamination-free polished sections (ion-milled area: ∼ 0.6 × 0.9 mm). SEM observation was performed by using a field-emission SEM (FE-SEM: JEOL, JSM-7001F) equipped with an energy dispersive X-ray spectrometer (EDS: Oxford Instruments, X-Max 20) and also a filament-type SEM (Hitachi, S-3400N) with Oxford, X-Act EDS, both of which were operated at an accelerating voltage of 15 kV. The detail of EDS analysis will be described later. In this study, all samples for SEM-EDS analysis were coated with a thin layer (5 nm) of osmium using Neoc-STB (Meiwafosis Co. Ltd.) to prevent surface charging. The accuracy of EDS quantitative analysis of Os-coated samples has been confirmed by Ohfuji and Yamamoto (2015).
Part of zeolitized gastropod fossils and host rocks were powdered using an agate mortar and analyzed by powder X-ray diffractometer (XRD, Bruker D8 Advance) equipped with CuKα radiation (λ = 1.5418 Å) operated at 40 kV and 40 mA for phase identification. For some samples, micro-Raman spectroscopy (JASCO, NRS-4100) equipped with a diode-laser (λ = 532 nm) was used for identification of mineral species at local scales.
EDS quantification of zeolite samplesIt is known that the precise chemical quantification of zeolites by electron microprobe analysis is not easy due to electron beam damage, which induces the dehydration and breakdown of the crystal structure (Yoshihara, 2000; Campbell et al., 2018). Therefore, in this study we conducted a series of test measurements under different conditions by changing electron beam current, acquisition time and acquisition mode (point analysis, line scan, and area scan) in the FE-SEM. Then, we found that the standard ‘spot analysis’ caused beam damage even though the beam current was reduced to 0.6 nA, but appropriate quantification data could be obtained by ‘raster scan analysis’ (area analysis) at the beam current of 1.0 nA. The standard deviations, sigma (σ) obtained from five repeated measurements at the same area based on this method were 0.072 (Si), 0.12 (Al), 0.018 (Na), 0.11 (K), and 0.061 (Ca) in wt%, which seems to be precise enough to examine the chemical composition of the zeolites studied in this study. For reliable chemical quantification of the zeolites, we also checked several sets of mineral standards for calibrating the major elements that constitute the zeolites and chose the best one: andalusite (Al2SiO5) for Al, enstatite (MgSiO3) for Si, and wollastonite (CaSiO3) for Ca. Chemical quantification data were obtained from the X-ray spectra that were extracted from the raster scan data using ‘spectrum reconstruction’ function of the AZtec software (Oxford Instruments). The raster scan analyses were conducted so that the target was fully covered by the scanned area with the image resolution: 1024 × 768 pixel, pixel dwell time: 500 µsec and number of frames: 150.
In the Shiote Formation in Jisahara area, the gastropod fossils were observed in two types: directly in the sandstone and in hard carbonate (calcite) concretions in the sandstone. The fossils isolated from or exposed on the surface of the outcrop show a milky transparent appearance (Fig. 1c) due to the dissolution (removal) of calcium carbonate shells and the presence of zeolite minerals formed inside the shell. It looks similar to the Vicarya fossils replaced by silica [known as ‘Tsukino-osagari’ from Mizunami City, Gifu Prefecture, Japan (Itoigawa and Watanabe, 1976)]. On the other hand, fossils in the sandstone and carbonate concretions have retained their shells showing white, non-transparent appearance (Fig. 1b).
Polarizing light microscope and SEM observations of the polished sections showed the cross-section of the spiral structure of the gastropod shells and the presence of fillings/precipitates in the inner space (Fig. 2). The rooms in the lower floors (lower chambers) of the shell are filled with detrital particles such as quartz, feldspar, hornblende, pyroxene, etc., which are basically the same as the constituent particles of the host sandstone. The rooms in the upper floors (upper chambers) are in most cases, empty, but the inner wall was often covered with columnar (tabular) crystals (∼ 500 µm) of zeolite (Fig. 2).
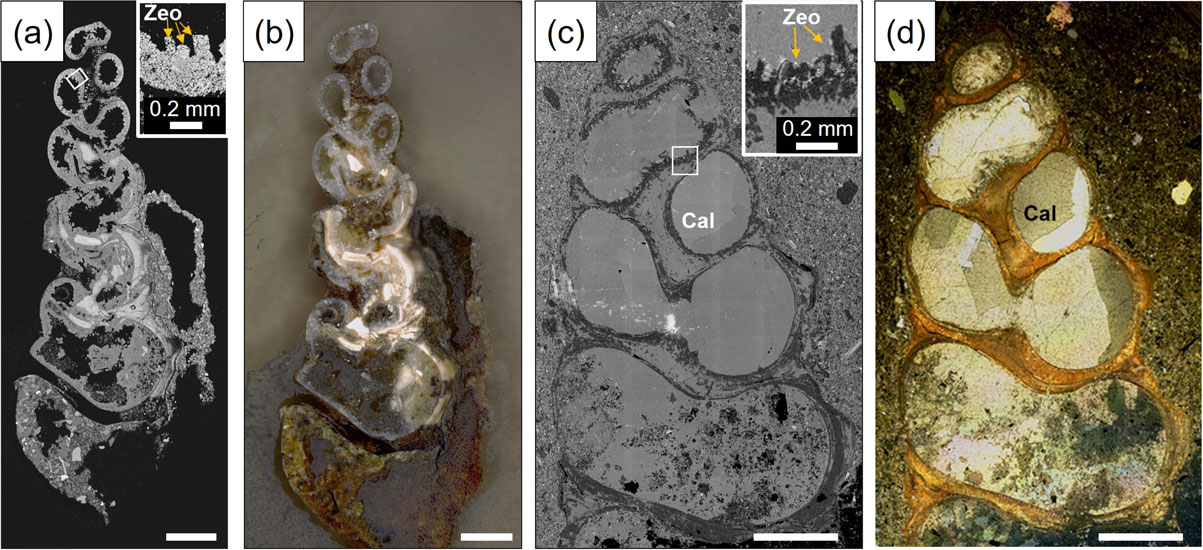
In the fossils embedded in carbonate concretion, the detrital particles infilling the lower chamber of the shell are, in most cases, cemented by anhedral calcite, and large euhedral calcite crystals (up to 1 mm) partly or completely fill inside the upper chambers of the shell. In some cases, tabular crystals of zeolite were observed between the shell wall and large calcite crystals, but they were not found in all gastropod fossils in the carbonate concretion and in many cases, the size is smaller (100-200 µm) than those observed in fossils directly embedded in the sandstone (without concretion).
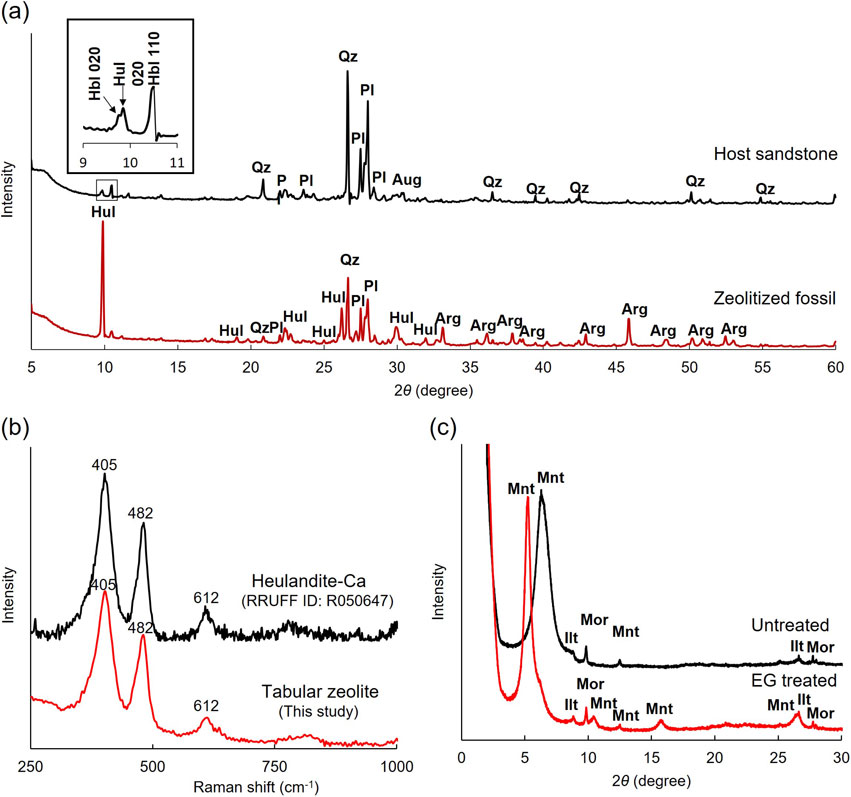
XRD pattern of an isolated zeolitized fossil showed the diffraction peaks of heulandite, aragonite, quartz, and plagioclase (Fig. 3a), among which aragonite is derived from the shell and the latter two are from the detrital particles filling inside the lower chambers of the shell (see Fig. 2). This confirms that the zeolite crystals formed inside the gastropod shells are mainly heulandite as previously reported by Hashimoto and Taira (2011), which was also checked by Micro-Raman analysis (Fig. 3b). We also measured the host sandstone sampled near one of the fossiliferous beds by XRD and identified the peaks of quartz, plagioclase, hornblende, and augite (Fig. 3a), which were indeed observed to be the major detrital particles comprising the sandstone by SEM-EDS as described later. In addition to the peaks of these detrital minerals, a small peak of heulandite was also detected; the strongest heulandite 020 peak (2θ = 9.85) can be seen right neighbor of the hornblende 020 peak (2θ = 9.77) (see the inset of Fig. 3a). Since a broad small peak originating from clay mineral was also observed at the lowest angle (2θ = ∼ 6°), we extracted particles smaller than 2 µm from the sandstone by elutriation and prepared an oriented sample (although the amount obtained was very small). Figure 3c shows XRD patterns of the oriented sample before and after the ethylene glycol (EG) treatment. The intense broad peak (centered at 6.3°, d = 14 Å) originating from the basal plane was shifted to the lower angle side (at 5.2°, d = 17 Å) after the EG treatment, suggesting that the clay mineral contained in the sandstone is mainly of the smectite group.
Figures 4a and 4b show light-microscope and back-scattered electron images of the interface between the shell wall and heulandite crystals, respectively. It is obvious that the heulandite crystals (<100-500 µm in size) grew directly from the surface of the inner shell wall, elongating inward. Individual heulandite crystals show typical tabular morphology, which is clearly seen from the top-view of the heulandite layer (Fig. 4c), similar to those observed commonly in hydrothermal veins and druses in volcanic rocks. In some cases, small calcite crystals were observed in contact with the inner shell wall and covered by heulandite (Figs. 4a and 4b), suggesting that such calcite crystals formed slightly earlier than or at almost the same timing as heulandite. Occasionally, needle crystals were found on the top of heulandite crystals, rarely with tiny (∼ 5 µm) crystals composed of Fe, Mn, C, and O (most likely, Mn-bearing siderite) (Fig. 4f) and large euhedral crystals of calcite (Fig. 4e). The needle crystals were identified as mordenite based on the morphological and chemical features and were found to be relatively Na-rich compared to the underlying heulandite by EDS (Fig. 4d).
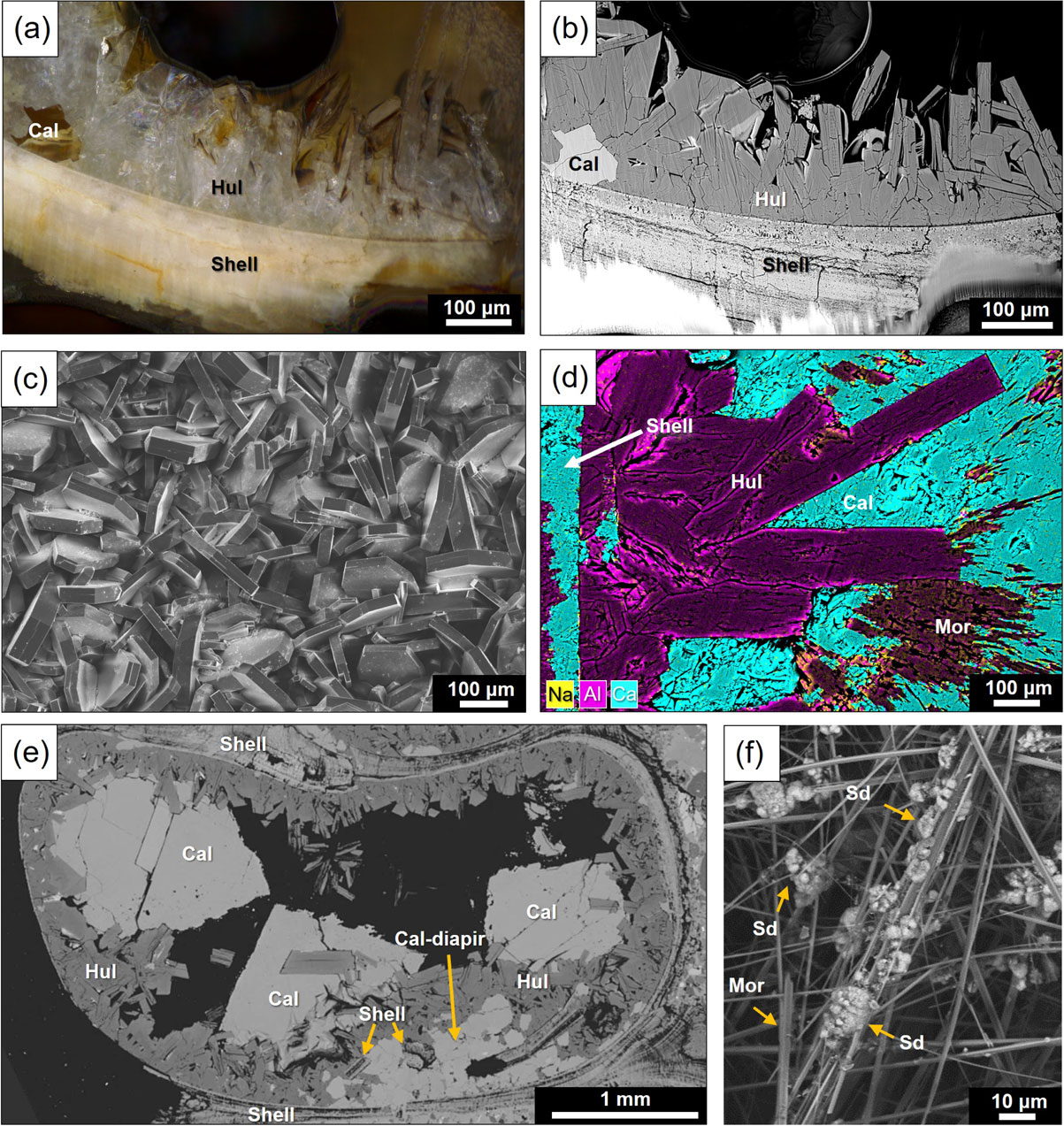
In the gastropod fossils embedded in carbonate concretions, the formation of heulandite was also observed inside the upper chambers, and the secondary growth of large calcite crystals over the zeolite layer was found to be more extensive (up to a few mm in size) (Fig. 4e), in some cases, entirely filling inside the internal chambers (Figs. 2c and 2d). In addition, there are a number of veins and diapir-like structures composed by polycrystalline calcite cutting across the shell (Fig. 4f). Figure 4f shows an example of a diapir-like intrusion of calcite from the outside of the shell, which appears to have pushed up small fragments of the shell and overlying heulandite layer. In the lower chambers filled with detrital particles such as quartz and feldspars of the shells, those particles are cemented by microcrystalline calcite and the formation of heulandite crystals was also observed, although the amount of heulandite is far smaller than the upper chambers.
We also examined the matrix of the host sandstone and found that zeolite (heulandite) and clay mineral filling the interstices of the detrital particles (i.e., they are authigenic) and occur in veins (Fig. 5), although the grain size of heulandite crystals is much smaller (a few to several tens µm) than those inside the fossils. The chemical composition of the clay mineral was estimated by EDS analysis to be Ca0.15(Al1.52,Mg0.33,Fe0.23)Si4.00O10(OH)2·nH2O. Although the amount of alkaline elements (mostly Ca in this case) may have been partly lost during analysis due to the electron beam damage, cations in the tetrahedral and octahedral sheets (Si, Al, Mg, and Fe) seems to be estimated correctly. The obtained chemical composition shows approximately 90% montmorillonite and 10% nontronite and therefore, the clay mineral filling the interstices of the detrital particles in the sandstone can be identified as montmorillonite following the 50% rule for mineral naming.

In the next step, we investigated the chemical composition of heulandite inside the shell by SEM-EDS. Since zeolite minerals are susceptible to electron beam damage, we obtained the quantitative data from the X-ray spectra reconstructed from raster scan data by post analysis (see EXPERIMENTAL section for details).
We found that the chemical composition of heulandite in gastropod fossils varied slightly from place to place, even within a single crystal. Table 1 shows a list of the chemical quantification data obtained from the bottom to the top of a heulandite crystal (Fig. 6b) at a regular interval. Although the total values (in wt%) of some data points are apparently lower than that expected from the stoichiometry (without H2O) due to the influence of the surface conditions such as cleavage and cracks of the sample, the ratio of cations (assuming the number of oxygens is 72) seems to be not influenced by such errors. Figure 6a shows the variation of Si/Al ratio of three heulandite crystals grown from the inner wall of a shell along the elongation direction of the individual crystals (see Fig. 6b).
| Distance from the bottom (µm) |
Chemical composition (wt%) | Cation numbers (O=72) | |||||||||||
| O | Na | Al | Si | K | Ca | Total | Na | Al | Si | K | Ca | Si/Al ratio | |
| 0 | 42.72 | 0.19 | 6.94 | 30.38 | 0.65 | 4.35 | 85.23 | 0.22 | 6.94 | 29.16 | 0.45 | 2.93 | 4.20 |
| 4.8 | 43.55 | 0.25 | 6.99 | 30.91 | 0.61 | 4.75 | 87.06 | 0.29 | 6.85 | 29.11 | 0.41 | 3.13 | 4.25 |
| 9.6 | 42.54 | 0.23 | 6.85 | 30.08 | 0.71 | 4.91 | 85.32 | 0.27 | 6.87 | 28.99 | 0.49 | 3.31 | 4.22 |
| 14.4 | 41.94 | 0.30 | 6.52 | 29.71 | 0.57 | 5.16 | 84.21 | 0.36 | 6.64 | 29.06 | 0.40 | 3.54 | 4.38 |
| 19.2 | 42.80 | 0.19 | 6.73 | 30.34 | 0.58 | 5.16 | 85.80 | 0.22 | 6.71 | 29.08 | 0.40 | 3.47 | 4.33 |
| 24.0 | 42.69 | 0.23 | 6.89 | 30.11 | 0.65 | 5.11 | 85.68 | 0.27 | 6.89 | 28.93 | 0.45 | 3.44 | 4.20 |
| 28.8 | 40.64 | 0.46 | 6.56 | 28.59 | 0.68 | 4.86 | 81.79 | 0.56 | 6.89 | 28.84 | 0.49 | 3.43 | 4.19 |
| 33.6 | 41.94 | 0.27 | 6.70 | 29.67 | 0.74 | 4.84 | 84.16 | 0.32 | 6.82 | 29.02 | 0.52 | 3.31 | 4.25 |
| 38.4 | 41.97 | 0.24 | 6.72 | 29.63 | 0.74 | 5.02 | 84.32 | 0.29 | 6.83 | 28.95 | 0.52 | 3.44 | 4.24 |
| 43.2 | 41.87 | 0.18 | 6.64 | 29.61 | 0.59 | 5.14 | 84.03 | 0.21 | 6.76 | 29.01 | 0.41 | 3.53 | 4.29 |
| 48.0 | 41.79 | 0.18 | 6.60 | 29.57 | 0.75 | 5.07 | 83.95 | 0.21 | 6.74 | 29.01 | 0.53 | 3.48 | 4.30 |
| 52.8 | 42.56 | 0.20 | 6.96 | 29.95 | 0.75 | 5.04 | 85.47 | 0.24 | 6.98 | 28.87 | 0.53 | 3.41 | 4.14 |
| 57.6 | 42.93 | 0.19 | 6.91 | 30.29 | 0.76 | 5.17 | 86.25 | 0.22 | 6.87 | 28.93 | 0.53 | 3.46 | 4.21 |
| 62.4 | 43.43 | 0.20 | 7.19 | 30.60 | 0.65 | 4.93 | 86.99 | 0.22 | 7.07 | 28.89 | 0.44 | 3.27 | 4.09 |
| 67.2 | 43.76 | 0.20 | 7.05 | 30.96 | 0.62 | 5.07 | 87.66 | 0.22 | 6.87 | 29.02 | 0.41 | 3.33 | 4.22 |
| 72.0 | 44.36 | 0.15 | 7.20 | 31.32 | 0.68 | 5.21 | 88.93 | 0.17 | 6.93 | 28.96 | 0.46 | 3.38 | 4.18 |
| 76.8 | 43.07 | 0.22 | 6.89 | 30.41 | 0.80 | 5.15 | 86.55 | 0.26 | 6.83 | 28.96 | 0.55 | 3.44 | 4.24 |
| 81.6 | 43.28 | 0.14 | 6.86 | 30.61 | 0.66 | 5.32 | 86.87 | 0.17 | 6.77 | 29.01 | 0.45 | 3.54 | 4.28 |
| 86.4 | 43.71 | 0.22 | 7.23 | 30.69 | 0.78 | 5.22 | 87.85 | 0.25 | 7.06 | 28.80 | 0.53 | 3.43 | 4.08 |
| 91.2 | 44.15 | 0.21 | 7.25 | 31.08 | 0.73 | 5.21 | 88.62 | 0.24 | 7.01 | 28.87 | 0.48 | 3.39 | 4.12 |
| 96.0 | 44.24 | 0.19 | 7.19 | 31.11 | 0.76 | 5.45 | 88.96 | 0.22 | 6.94 | 28.84 | 0.50 | 3.54 | 4.16 |
| 100.8 | 44.59 | 0.22 | 7.58 | 31.13 | 0.64 | 5.47 | 89.62 | 0.25 | 7.25 | 28.63 | 0.43 | 3.52 | 3.95 |
| 105.6 | 44.20 | 0.18 | 7.43 | 30.91 | 0.69 | 5.44 | 88.84 | 0.20 | 7.17 | 28.69 | 0.46 | 3.54 | 4.00 |
| 110.4 | 43.83 | 0.19 | 7.23 | 30.75 | 0.80 | 5.34 | 88.13 | 0.21 | 7.05 | 28.78 | 0.54 | 3.50 | 4.08 |
| 115.2 | 44.29 | 0.23 | 7.46 | 30.91 | 0.79 | 5.51 | 89.19 | 0.26 | 7.19 | 28.63 | 0.53 | 3.58 | 3.98 |
| 120.0 | 41.45 | 0.78 | 6.95 | 28.65 | 0.88 | 5.44 | 84.15 | 0.95 | 7.17 | 28.35 | 0.62 | 3.77 | 3.96 |
| 129.6 | 44.09 | 0.21 | 7.50 | 30.76 | 0.77 | 5.38 | 88.70 | 0.24 | 7.25 | 28.62 | 0.52 | 3.51 | 3.94 |
| 134.4 | 43.98 | 0.20 | 7.66 | 30.58 | 0.65 | 5.32 | 88.40 | 0.22 | 7.43 | 28.52 | 0.44 | 3.48 | 3.84 |
| 139.2 | 44.04 | 0.15 | 7.66 | 30.53 | 0.84 | 5.56 | 88.78 | 0.18 | 7.43 | 28.43 | 0.56 | 3.62 | 3.83 |
| 144.0 | 43.92 | 0.24 | 7.73 | 30.44 | 0.83 | 5.27 | 88.44 | 0.27 | 7.52 | 28.43 | 0.56 | 3.45 | 3.78 |
| 148.8 | 43.90 | 0.23 | 7.71 | 30.42 | 0.89 | 5.32 | 88.47 | 0.26 | 7.50 | 28.42 | 0.59 | 3.48 | 3.79 |
| 153.6 | 44.11 | 0.31 | 7.83 | 30.47 | 0.87 | 5.38 | 88.97 | 0.35 | 7.58 | 28.33 | 0.58 | 3.51 | 3.74 |
| 158.4 | 44.18 | 0.25 | 7.93 | 30.42 | 1.01 | 5.45 | 89.24 | 0.28 | 7.67 | 28.24 | 0.67 | 3.55 | 3.68 |
| 163.2 | 44.01 | 0.24 | 7.95 | 30.22 | 0.96 | 5.60 | 88.98 | 0.28 | 7.71 | 28.16 | 0.64 | 3.65 | 3.65 |
| 168.0 | 43.97 | 0.24 | 8.21 | 30.01 | 1.17 | 5.39 | 88.99 | 0.27 | 7.97 | 28.00 | 0.78 | 3.52 | 3.51 |
| 172.8 | 43.83 | 0.23 | 8.53 | 29.60 | 1.11 | 5.53 | 88.83 | 0.26 | 8.31 | 27.70 | 0.74 | 3.63 | 3.33 |
| 177.6 | 43.21 | 0.31 | 8.60 | 28.98 | 1.23 | 5.51 | 87.83 | 0.36 | 8.49 | 27.50 | 0.84 | 3.66 | 3.24 |
| 182.4 | 42.69 | 0.38 | 8.68 | 28.48 | 1.22 | 5.37 | 86.82 | 0.45 | 8.67 | 27.37 | 0.85 | 3.61 | 3.15 |
| 187.2 | 42.62 | 0.31 | 8.66 | 28.45 | 1.40 | 5.29 | 86.74 | 0.36 | 8.68 | 27.38 | 0.97 | 3.57 | 3.16 |
| 192.0 | 41.84 | 0.34 | 8.66 | 27.67 | 1.42 | 5.53 | 85.46 | 0.41 | 8.83 | 27.13 | 1.00 | 3.80 | 3.07 |

Polarizing microscope and SEM observations revealed that the formation of zeolites (heulandite and mordenite) in gastropod fossils in the Shiote Formation is obviously prevalent in the upper chambers of the shells, which have not been filled with detrital particles. The heulandite crystals show euhedral shapes and have grown directly from the inner shell wall. Small amounts of heulandite crystals were also found in the lower chambers, but they occur exclusively in the interstices between the detrital particles filling inside the chambers (Figs. 2c and 2d). Furthermore, heulandite crystals were also found together with flaky crystals of montmorillonite in the matrix of the host sandstone (Fig. 5), although the grain size is much smaller than those observed inside the fossils. Also note that montmorillonite was observed to have grown inward from the pore walls built up of detrital particles (Fig. 5), suggesting that it is authigenic but not detrital. The formation of such authigenic montmorillonite and heulandite is most likely as a result of alteration/weak metamorphism of volcanic glass and clasts (Hay and Sheppard, 2001), which were indeed contained in the Shiote Formation as secondarily deposited particles, as reported by Yanagisawa et al. (1996). It has been known that the hydrolytic alteration (palagonitization) of volcanic glass provides many cations such as Si, Al, and alkaline elements (Na, K, Ca, etc.) into the pore water, which promotes the formation of zeolites under moderate temperature during the burial diagenesis (Coombs et al., 1959; Iijima, 1980; Sheppard and Hay, 2001).
Temperature is also an important factor in zeolite formation. The Miocene paleothermal gradient in the Soma Nakamura area including the studied area, is considered to be 3-5 °C/100 m (Suzuki, 1989). The burial temperature of the Shiote Formation estimated from the total thickness (500-600 m) of the sequence of all the Miocene sediments (from Shiote Formation to Akashiba Formation, see EXPERIMENTAL section) in Soma-Nakamura area is around 35-50 °C (Yanagisawa et al., 1996). However, since the original thickness of the uppermost Akashiba Formation, which is unconformably covered by the Pliocene Kameoka Formation, could have been thicker (Yanagisawa et al., 1996), the burial temperature may be even higher (>50 °C) than this estimate. On the other hand, smectite (pure, not mixed layer with illite) is reported to be stable up to 60 °C (Hoffman and Hower, 1979). The burial temperature of the Shiote Formation inferred from the palaeothermal gradient and the formation condition for authigenic montmorillonite is within the range of the condition suitable for heulandite-clinoptilolite formation (50-100 °C) reported by Iijima (1980).
Furthermore, pH of the growth medium is another factor affecting zeolite formation, because it influences the solubility of Si and Al in solution, and higher pH promotes the precipitation of zeolite (Hay and Sheppard, 2001). Experimental studies on the interaction of basalt and seawater demonstrated that in the system where water/rock ratio was low the dissolution of silicate glass buffered the pH, which resulted in the precipitation of montmorillonite and basic pH due to the consumption of H+ ions (Seyfried and Bischoff, 1979). Thus, assuming the initial pH of the pore water in the Shiote Formation was equivalent to the seawater (currently, pH ∼ 8), the solution is expected to have remained alkaline as a result of the hydrolysis of volcaniclastic particles in the sediment, providing a suitable condition for zeolite formation. In particular, in the gastropod shells (semi-closed spaces) such alkaline solution could have trapped and concentrated during the diagenesis. As a result, the nucleation and subsequent growth of heulandite crystals were promoted in the rooms inside the shells. It is consistent with the lack of obvious zeolite formation in the bivalve fossils which are also contained in the sediment, because such a semi-closed environment was not maintained by the open shell. The source of the cations is thought to be the product of the hydrolysis of the volcaniclastic debris in the sandstone, while the supply from the fossilized shells seems to have been limited, since no signs of dissolution of the shell were observed even at the boundary between the shell wall and overlying zeolite (Figs. 4a and 4b).
On the other hand, in gastropod fossils embedded in carbonate concretions, zeolite formation is moderate; zeolite was not observed in all fossilized shells, and the size of the crystals is apparently smaller than those formed inside the fossils in the sandstone. This is probably due to the difference in permeability and mobility of the fluid in the sandstone and in the carbonate concretion. According to recent studies (Yoshida et al., 2015, 2018), the formation of carbonate concretion around fossilized shells occurs rapidly in the very early stage of diagenesis through the reaction of Ca2+ with HCO3− produced by the decomposition of the soft (organic) tissues under a diffusion-controlled environment. Therefore, in the present case it is very likely that the formation of carbonate concretions occurred prior to the precipitation of zeolite in the shells and caused a decrease in permeability around the shells, which limited the transport of cations into the shell. On the other hand, most of the gastropod fossils contained in the carbonate concretion has large (millimeter-sized) euhedral crystals of calcite, which have grown over the zeolite crystals (Figs. 2d and 4f). These morphological features indicate that their growth occurred in free spaces inside the shell under relative low driving force conditions where the crystal growth rate was more dominant than the nucleation rate after the zeolite formation. The intrusion of calcite ‘diapir’ (Fig. 4f) suggests that the secondary calcite crystals were precipitated from the fluid that penetrated into the interior through cracks in the shells. Thus, it is concluded that in the present case, the calcite formation around (inside and outside) the fossilized shells occurred at least three times, 1) during the formation of carbonate concretion, 2) during the formation of zeolites (Fig. 4b), and 3) after the zeolite formation.
An interesting chemical feature shown by the heulandite in gastropod fossils in this study is that the Si/Al ratio varies remarkably from ∼ 4.4 to 3.2 along the elongation direction of the crystals (Fig. 6a). This compositional change crosses the threshold for the classification of heulandite (Si/Al < 4.0) and clinoptilolite (Si/Al ≥ 4.0) proposed by Coombs et al. (1997). They both have a similar topological crystal framework composed of TO4 tetrahedra (T = Si, Al) linked to each other forming cages and show some differences such as in thermal stability (Mumpton, 1960), distortion of TO4 tetrahedra (Yang et al., 2010), and optical property (Rodríguez-Iznaga et al., 2022), but their discrimination is not easy.
Since heulandite-clinoptilolite series does not have clear compositional end members, the ‘50% rule’ cannot be applied, and the classification based on the Si/Al ratio is widely used (Sandimirova et al., 2022; Nikitczuk et al., 2022). The compositional change within single crystals of ‘heulandite’ observed in this study indicates the transition from clinoptilolite to heulandite occurred during the crystal growth in the shell.
A similar case was reported by Ogihara and Iijima (1989) who studied zeolites formed in drilling core samples (Tertiary-Quaternary marine sediments) from offshore of the northeast Japan. They described a transition from the clinoptilolite zone to analcite-heulandite zone with increasing burial depth (i.e., temperature) in the drilling cores and found a zoning structure in zeolites in which the composition changed from clinoptilolite-Na to heulandite from the core to rim of single crystals. Since, their earlier study (Iijima, 1980) found that clinoptilolite formed shallower, lower-temperature environments than heulandite, they inferred that the zoning structure was produced through the dissolution of pre-formed clinoptilolite crystals from the rim and subsequent overgrowth by heulandite in response to the temperature rise with increasing burial depth.
Figure 7a is a plot showing the relationship between the framework cation ratio (Si/Al) and extra-framework cation ratio [(Ca + Sr + Mg)/(Ca + Sr + Mg + Na + K)] of clinoptilolite-heulandite crystals reported by Ogihara and Iijima (1989) and observed in this study. The data of Ogihara and Iijima shows a clear negative correlation, indicating that the change in Si/Al ratio is mainly accompanied by (Na, K) + Si ⇄ Ca + Al substitution. On the other hand, our data do not show such a trend; the (Ca + Sr + Mg)/(Ca + Sr + Mg + Na + K) ratio is almost constant over the wide range of Si/Al, which suggests a different substitution mechanism for the compositional change. Then, we checked other cations and found a positive correlation between the Si/Al and Ca/K ratios (Fig. 7b), which suggests that the substitution of TO4 tetrahedra by Al occurs partly by Si + Ca ⇄ 3K + Al. The difference may be related to the fact that clinoptilolite observed in Ogihara and Iijima’s study was rich in Na, while that of this study is rich in Ca due to the abundant supply of Ca, presumably from biogenic carbonate (e.g., micro fossils) in the host rocks.
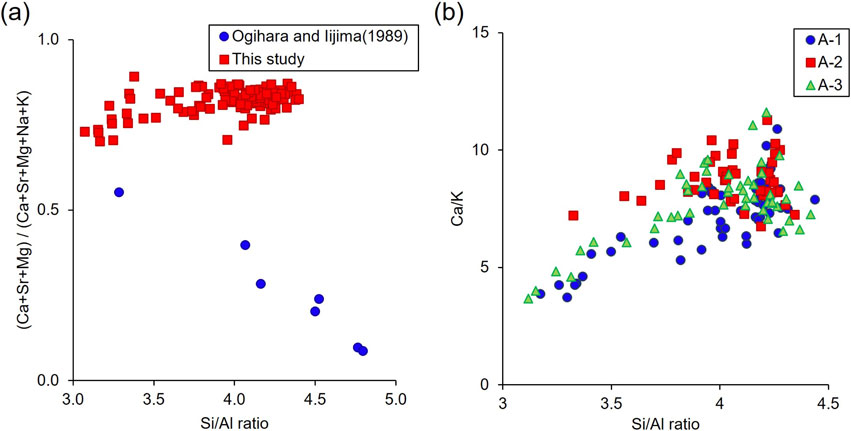
Then, what is responsible for the continuous change in Si/Al ratio in clinoptilolite-heulandite crystals? One possibility is the change in chemical composition of the fluid from which they crystalized. If a closed system is assumed to have been maintained within the shells (particularly in the upper chambers), the Si/Al ratio of the fluid composition may have gradually decreased, resulting in the change in Si/Al ratio of the growing crystals to the heulandite composition. However, according to this rule, the Si/Al ratio of the fluid is expected to become lower, which contradicts the fact that the mordenite with higher Si/Al ratio (>4) formed after heulandite (Fig. 6a). Another possibility is the change (increase) in surrounding temperature associated with an increase in burial depth, as reported by Iijima (1980) and Ogihara and Iijima (1989). However, the temperature effect on the stability of the clinoptilolite-heulandite series has not been studied by experimental or thermodynamic approaches, but is rather empirical based on observations of natural samples. Therefore, further research is needed to understand the essential mechanism and conditions of the clinoptilolite-heulandite formation.
Silicification, replacement by silica such as opal and chalcedony, is known to be the most common permineralization of fossilized shells in sedimentary rocks. It occurs by the concurrent dissolution of calcium carbonate shell and precipitation of silica under conditions of excessive silica supply (Butts and Bring, 2011) such as by dissolution of biogenic silica (e.g., radiolarians) (Ernest, 1960) or siliceous volcanic glass (Tsintsov et al., 2001) in the host rock, and intrusion of silica-rich hydrothermal fluids (Sanchez-Munoz et al., 2009). It is interesting to note that in the present case, no silica, but instead, zeolite was observed extensively in gastropod fossils, which suggests the high activity of Al as well as Si in the solution trapped inside the shells. The high Al activity in the pore water is also evidenced by the formation of montmorillonite in the interstitial spaces of the matrix of the host sandstone and also of the filling of the lower chambers (near the inlet) of many gastropod shells. The source of Al is most likely the hydrolytic alteration of volcaniclastic particles in the sediments, as discussed above. Since montmorillonite formation was not observed in the upper chambers of the shells, where heulandite formation is pronounced, it is possible that there was a difference in the relative activity of Si and Al between the interior and exterior of the shells in the sandstone. Experimental study investigating the dissolution rate of elements from basaltic rocks showed that Si and Na dissolved faster than the other elements including Al (Zhi and Ying, 1993). It is therefore expected that the composition of the pore water was relatively rich in Si in the early stages of burial, and gradually became Al-rich by further alteration of the volcaniclastic particles. In this case, montmorillonite with a lower Si/Al ratio was formed in the sandstone (including near the inlet of the shells) where the mobility of elements was large due to the fluid flow, while zeolites with higher Si/Al ratios was formed preferentially inside the shells in a semi-closed system where the movement of elements was relatively slow due to fluid retention.
Shiote Formation containing zeolitized gastropod fossils in the studied area experienced weak metamorphism equivalent to zeolite facies through burial diagenesis. As a result of the hydrolytic alteration of volcanoclastic particles (most likely, volcanic glass) in the sediments, large numbers of Al and alkaline cations in addition to Si were supplied to the pore water, resulting in the formation of zeolite as well as montmorillonite in the interstitial spaces between detrital particles that constitutes the host sandstone and the interior of the shell fossils.
The formation of zeolites is particularly pronounced in the upper chambers of the gastropod shells, in which pore water was trapped and condensed during diagenesis. It seems that the chemical enrichment in such fluid retained in nearly closed system of the shell interior (Fig. 8) under appropriate temperature and pH conditions is the key for the preferential growth of zeolites. In this process, clinoptilolite, which is likely stable at lower temperature and has higher Si/Al ratio, crystallized in the initial stage, and then gradually changed to heulandite, which is stable at higher temperature and has lower Si/Al ratio, through the gradual temperature increase with burial depth.

We thank M. Taira and H. Inose for guiding us to the outcrop and assisting with the sampling. We also thank M. Tokunaga for drawing the illustration of a gastropod used in one of the figures. We appreciate the constructive comments from Prof. H. Yoshida of Nagoya University and an anonymous reviewer.