2023 年 118 巻 ANTARCTICA 号 論文ID: 221130
2023 年 118 巻 ANTARCTICA 号 論文ID: 221130
Spinel + plagioclase symplectites in pelitic metamorphic rocks have been reported from many localities. We report in detail an occurrence of this texture from Tenmondai Rock, Lützow-Holm Complex, East Antarctica. This texture is produced by the following metamorphic reaction; garnet + sillimanite = spinel + plagioclase. This can be described as the following two net-transfer reactions; 5Grs + Alm + 12Als = 3Hc + 15An, and 5Grs + Prp + 12Als = 3Spl + 15An. We propose new geobarometers (GASpP) based on these equations. For example, one of these is P = (−155790 + 587.4T + RT lnKFe) / 29.378, for garnet + sillimanite + spinel + plagioclase assemblage in the CFASZn-system. Where P is pressure in bar, T is temperature in K, and R represents gas constant. The equilibrium constant is, KFe = (aGrs5 aAlm)/(aHc3 aAn15). Our proposed new geobarometers are free from quartz, corundum, orthopyroxene, and cordierite within these equations. These barometers are useful to estimate pressure conditions for spinel-bearing pelitic metamorphic rocks within a wide pressure-temperature range (andalusite, kyanite, and sillimanite fields). We estimate the metamorphic pressure-temperature (P-T) conditions at Tenmondai Rock by applying the GASP, GRIPS, GRAIL, GASpP geobarometers, and the Zr-in-Rt geothermometer. The metamorphic evolution of the rocks at Tenmondai Rock is characterized by a clockwise P-T-t path. The peak P-T condition is about 820 MPa and 850 °C. Spinel + plagioclase symplectite was produced during the decompressional stage of this metamorphic evolution, at about 450 MPa and 700 °C.
Spinel is one of the common rock-forming minerals in pelitic metamorphic rocks. It mainly occurs in the sillimanite field, but also occurs in the kyanite field (e.g., Tuccillo et al., 1992; Zhang et al., 2005; Cruciani et al., 2019) and andalusite field (e.g., Perchuk et al., 1989; Xu et al., 1994; Carson et al., 1997; Kalt et al., 1998; Pitra and de Waal, 2001; Sawaki et al., 2001; Marien et al., 2019).
Spinel coexisting with quartz is commonly regarded as an ultrahigh-temperature (UHT) metamorphic mineral assemblage (e.g., Hensen and Green, 1971; Vielzeuf, 1983; Waters, 1991; Harley, 1998; Shimura et al., 2002; Ganguly et al., 2017) although additional minor chemical components such as Zn can extend the spinel + quartz stability to below 900 °C (Shimura et al., 2002; Harley, 2008; Clark et al., 2011). In spinel + quartz stable cases, spinel occurs in direct contact with quartz. On the other hand, in the case of lower grades, spinel does not occur in direct contact with quartz, although quartz is present in the rocks. In such rocks, spinel coexisting with cordierite is well known (e.g., Clarke and Powell, 1991; Komatsu et al., 1994; Pitra and de Waal, 2001; Johnson et al., 2004).
Sometimes spinel occurs in contact with plagioclase in pelitic metamorphic rocks (e.g., Hiroi et al., 1997; Rötzler and Romer, 2001; Álvarez-Valero et al., 2007; Shimizu et al., 2009; Saki, 2011; Baldwin et al., 2015). For example, Hiroi et al. (1997) reported spinel + plagioclase symplectite around a garnet porphyroblast. This spinel is not in direct contact with quartz. Rötzler and Romer (2001) and Baldwin et al. (2015) describe spinel + plagioclase symplectite occurrences around garnet or kyanite. Álvarez-Valero et al. (2007) observed spinel with a thin rim of plagioclase. Santosh et al. (2007) reported that spinel is surrounded by plagioclase, and is separated from quartz. Saki (2011) reported that spinel + cordierite symplectite occurs around andalusite, and this spinel has a thin rim of plagioclase. These textures suggest that the spinel + plagioclase assemblage may be produced by garnet + aluminosilicate breakdown reactions.
In this paper, we describe an example of microstructural relations between spinel and plagioclase from Tenmondai Rock, Lützow-Holm Complex, East Antarctica, and propose new geobarometers for spinel-bearing pelitic metamorphic rocks.
Abbreviations of minerals in this paper are after Whitney and Evans (2010).
The Lützow-Holm Complex (LHC) is located in eastern Dronning Maud Land, East Antarctica (Fig. 1) and is a medium P/T type metamorphic complex. SHRIMP U-Pb zircon dating of the LHC indicates that peak metamorphism occurred at about 600-500 Ma (Shiraishi et al., 2003, 2008; Dunkley et al., 2020). A two-stage metamorphic history with events at ∼ 600 Ma and 550-530 Ma metamorphism has been reported (e.g., Hokada and Motoyoshi, 2006; Kawakami et al., 2016).

Across the LHC the metamorphic grade increases progressively south-westward from amphibolite-facies to granulite-facies (Hiroi et al., 1991). A clockwise metamorphic P-T-t path is reported by many authors (e.g., Hiroi et al., 1991; Kawakami and Motoyoshi, 2004; Yoshimura et al., 2004, 2008; Kawasaki et al., 2011). The highest-grade rocks of the LHC occur at Rundvågshetta (Fig. 1), where peak P-T conditions reached UHT, about 1.0-1.2 GPa and 1000-1100 °C (Motoyoshi and Ishikawa, 1997; Fraser et al., 2000; Yoshimura et al., 2008; Kawasaki et al., 2011; Takahashi et al., 2018). Spinel + quartz assemblages have been recognized at Rundvågshetta and Skallevikshalsen (Motoyoshi et al., 1985; Kawakami and Motoyoshi, 2004; Kawasaki et al., 2011).
Metamorphic zone mapping of the LHC (Fig. 1) has been studied based on matrix mineral assemblages (Hiroi et al., 1991). Recently, Suzuki and Kawakami (2019) proposed that metamorphism of Akarui Point (Fig. 1) near Tenmondai Rock occurred at higher P-T conditions than suggested by previous studies, and provided P-T estimates of about 1.05 GPa and 834 °C. They suggested that the P-T-t evolution of the LHC needs to be re-evaluated utilizing detailed petrochronological approaches.
Tenmondai Rock (68°26′S-68°28′S and 41°40′E-41°44′E) is one of the outcrops of the LHC (Figs. 1 and 2), and is the locality where the first appearance of pyroxene is noted in the progressive metamorphic zonation (Hiroi et al., 1991) (Fig. 1). Tenmondai Rock is underlain by gneisses, migmatitic rocks, granite and pegmatite (Shiraishi et al., 1985) (Fig. 2). Among the metamorphic rocks, biotite-hornblende gneiss and hornblende-biotite gneiss are predominant, and lesser amounts of garnet-biotite gneiss and amphibolite are intercalated (Shiraishi et al., 1985). Fe-rich amphibolites at Tenmondai Rock contain orthopyroxene, marking the first appearance of orthopyroxene in the progressive metamorphic sequence in the LHC (Shiraishi et al., 1984) [see isograd Opx-in (1) in Fig. 1]. All aluminosilicate polymorphs (kyanite, sillimanite, and andalusite) are reported from garnet-biotite gneiss from Tenmondai Rock (Hiroi et al., 1983; Shiraishi et al., 1985), and their occurrence is interpreted to result from a clockwise metamorphic P-T-t path (Hiroi et al., 1991; Takamura et al., 2020). Takamura et al. (2020) reported that zircon U-Pb ages from mafic metamorphic rock from Tenmondai Rock show a wide age range, between 582-481 Ma, and post-peak decompression possibly started around 560 Ma.
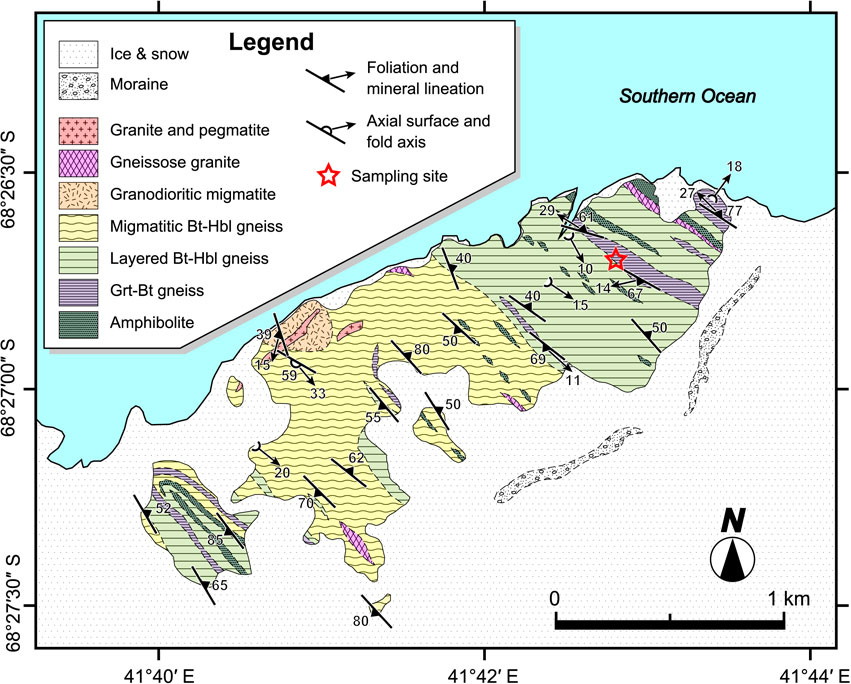
Garnet-biotite gneiss is developed mainly in the eastern area of Tenmondai Rock (Fig. 2). Reddish brown pegmatitic dykes intrude the gneiss (Shiraishi et al., 1985) (Fig. 3a). The garnet-biotite gneiss occurs as a layered migmatite structure composed of biotite-rich melanocratic layers and garnet-aluminosilicate rich leucocratic layers (Fig. 3b). Sample TMD40 (Fig. 3c) was collected from a garnet-sillimanite rich leucocratic layer (star in Fig. 2).

SEM image photographing and qualitative analyses of minerals were carried out by JEOL JSM-6030LA and energy-dispersive X-ray spectroscopy (EDS) (JEOL JED-2300) at the Yamaguchi University. X-ray intensity mapping was also obtained by this EDS, using analytical conditions of 20 kV acceleration voltage, 3.45 nA probe current, 0.0002 s dwell time, 10 sweep time, and 2048 × 1536 pixels.
Quantitative analyses of minerals were carried out by wavelength-dispersive X-ray spectroscopy (WDS) (JEOL JXA-8230) at the Yamaguchi University. Five-channel spectrometers were used simultaneously. Natural and synthesized minerals and oxides were used as standards.
Operating conditions for analyses of garnet, spinel, biotite, feldspars and ilmenite were 15 kV accelerating voltage, 20 nA probe current, beam diameter was 2 µm, counting times were 10 s on the peak and 5 s on the background. Oxide ZAF correction procedures were used. Fe2O3, FeO, Fe3+, and Fe2+ of spinel and ilmenite are calculated by stoichiometry (e.g., Droop, 1987).
The measurement conditions for analyses of rutile referred to Zack et al. (2004). Our operating conditions are as follows; 20 kV accelerating voltage, 120 nA probe current, 5 µm beam diameter. Counting time for zirconium, 300 s on the peak and 150 s on the background. For titanium, 7 s on the peak and 3.5 s on the background. Hafnium was rejected because the measured data was lower than the detection limit in 100 s counting time. For other elements (Si, Sn, Al, Cr, V, Fe, Mg, Zn, Ca, Nb, Sb, Ta, and W), 50-170 s on the peak and 25-85 s on the background. Since the vanadium-LIF-Kα peak is overlapped by the titanium-LIF-Kβ peak, an interference correction was carried out on the intensity of the titanium-LIF-Kα peak. Oxide ZAF correction procedures were used. Quantitative analyses for rutile were confirmed by rutile standards R10 and R19 (Luvizotto et al., 2009).
Petrography and mineral chemistry of a spinel-bearing gneissThe sample TMD40 is about 15 cm sized handspecimen (Figs. 3c and 3d). It is leucocratic medium-grained garnet-sillimanite gneiss. It is mainly composed of garnet, spinel, biotite, sillimanite, plagioclase, K-feldspar, quartz, ilmenite, and rutile (Figs. 4, 5, and 6). It has minor amounts of muscovite, apatite, zircon, and monazite. Very small amounts of hematite, pyrrhotite, pyrite, chalcopyrite, and chlorite occur as retrograde phases. Cordierite is absent.



TMD40 is subdivided into two domains. One is a matrix domain consisting of plagioclase + quartz + K-feldspar (lower part of Fig. 5a). This domain shows granoblastic texture. Spinel does not occur in this domain. The other is a garnet- and sillimanite- rich domain. This domain contains spinel (upper part of Fig. 5a). Spinel occurs as vermicular intergrowth with plagioclase (Figs. 3d and 5a-5i). In this paper, we call such texture and related minerals as ‘spinel + plagioclase symplectite’, ‘symplectite spinel’, and ‘spinel-bearing plagioclase’, hereafter.
Representative chemical compositions of minerals are shown in Supplementary Table S1 (Supplementary Tables S1, S2, and S3 are available online from https://doi.org/10.2465/jmps.221130).
Generally, garnet occurs as porphyroblast (Figs. 3c, 3d, and 4a). Sometimes it is embayed by spinel + plagioclase symplectite (Fig. 5f) or by sillimanite + ilmenite + quartz (Fig. 6). Porphyroblastic garnet {XMg [= Mg/(Fe2+ + Mg)] = 0.244-0.306, Prp [= Mg/(Fe2+ + Mn + Mg + Ca) mol%] = 23.5-29.4, Grs [= Ca/(Fe2+ + Mn + Mg + Ca) mol%] = 2.01-3.63} is euhedral to subhedral (Figs. 4, 5, and 6). It encloses quartz, sillimanite, rutile, ilmenite, apatite and zircon (Fig. 4). Very small amounts of biotite and muscovite are occasionally enclosed (Fig. 4a). Chemical zoning in porphyroblast garnet is shown in Figure 7. The grossular content of porphyroblast garnet is rich in the core and decreases towards the rim. The XMg is low in the core and high in the mantle, and then decreases towards the rim.

Spinel + plagioclase symplectite occurs associated with garnet- and sillimanite- rich domains (Fig. 5). Garnet compositions near the symplectite are lower in Mg and Ca (XMg = 0.204-0.256, Grs = 2.11-2.75) than in the porphyroblasts (Fig. 8 and Table S1-1). Garnet compositions in contact with biotite or muscovite are lower in XMg (XMg = 0.156-0.286, Prp = 19.6-24.6, Grs = 1.97-2.69) (Fig. 8 and Table S1-1).

Sometimes sillimanite + ilmenite + quartz domains are embayed into garnet (Fig. 6). In this case sillimanite and garnet are bounded by quartz (Fig. 6d). Spinel does not occur around such texture.
Sillimanite occurs as prismatic crystals (Figs. 5 and 6). Kyanite and andalusite are not found in sample TMD40, but these were reported from almost the same locality (Hiroi et al., 1983; Shiraishi et al., 1985).
Dark green spinel occurs as spinel + plagioclase symplectite (Fig. 5). Spinel and quartz are always separated by plagioclase and spinel is not in direct contact with quartz (Fig. 5). Garnet near this texture is enveloped by plagioclase (Figs. 5a-5f).
Oxide spinels (AB2O4) can be classified as ‘2-3 spinels’ (A2+B3+2O4) and ‘4-2 spinels’ (A4+B2+2O4) (e.g., Biagioni and Pasero, 2014; Colas et al., 2016). The spinel series is B = Al3+ solid solution and the general formula is (Fe, Mn, Mg, Zn, Ni)2+Al3+2O4 (e.g., Deer et al., 2013). Among naturally occurring spinels, A may include Fe2+, Mn, Mg, Ca, Zn, Ni, Co, and Cu, whereas B may include Al, Cr, V, Fe3+, Ti, and Si. R4+ is occupied in ‘4-2 spinel’ (A4+B2+2O4) structure, such as a result of the substitution R4+ + R2+ = 2R3+ (Deer et al., 2013), where R is a generalized symbol for a group of metal ions. Spinel in TMD40 has low XMg (= 0.201-0.313), Fe3+/Fetotal (= 0.084-0.147), and Fe3+/R3+ (= 0.033-0.062) in composition (Fig. 9 and Table S1-2). It is hercynite (FeAl2O4). It contains small amounts of ZnO (1.72-2.34 wt%), Cr2O3 (0.42-1.79 wt%), V2O3 (0.23-0.39 wt%), NiO (0.06-0.20 wt%), and CoO (0.07-0.18 wt%) (Fig. 9 and Table S1-2). CuO is lower than the detection limit.

Two main occurrences of plagioclase can be observed. One is ‘matrix-plagioclase’ (lower part of Fig. 5a) which shows granoblastic texture. The other is ‘spinel-bearing plagioclase’ (Fig. 5). Spinel-bearing plagioclase is richer in anorthite content (An = 25.4-31.2 mol%), compared with matrix-plagioclase which is poorer in anorthite content (An = 22.5-25.0 mol%) (Fig. 10 and Table S1-3). K-feldspar (Or = 81.5-90.9 mol%) (Fig. 10 and Table S1-3) represents granoblastic texture with plagioclase and quartz in the matrix (Figs. 4a and 5a).

Large euhedral or subhedral biotite (XMg = 0.488-0.555) occurs in the matrix or cuts the garnet porphyroblasts, and it has X and Y = brown, Z = reddish brown pleochroism (right of Fig. 4a and Table S1-4). Sometimes small anhedral biotite (XMg = 0.625-0.631) occurs within spinel + plagioclase symplectite (Figs. 5b, 5f, 5g, and 5h). It has X and Y = light brown, Z = brown pleochroism. Composition of this biotite is rich in XMg and Al, and poor in Ti (Table S1-4). A small amount of fine-grained muscovite occurs in the pressure-shadows or along cracks in garnet (Fig. 4) or near areas with chlorite (Fig. 5j).
Ilmenite occurs in the matrix (Fig. 4a), in inclusions in garnet (Fig. 4c), in Sil + Ilm + Qz domains (Fig. 6), and with Spl + Qz symplectite (Fig. 5i). Representative chemical compositions of ilmenite are shown in Table S1-5.
Rutile occurs as subhedral large crystals in the matrix (Figs. 4a and 5c), or it occurs as small crystals in garnet porphyroblasts (Fig. 4). The c-axis of large rutile defines a weak lineation (Figs. 5c and 5f) and the zirconium content shows a relatively wide range (466-2821 ppm) (Figs. 5c, 11, and Table S1-6). Rutile inclusions in garnet porphyroblasts have relatively high zirconium content, Zr = 1251-2517 ppm (Figs. 4, 11, and Table S1-6). Rutile within spinel + plagioclase symplectite has relatively low zirconium content, Zr = 637-703 ppm (Figs. 5g, 11, and Table S1-6). Rutile occurs as very small euhedral crystals around ilmenite associated with chlorite (Figs. 5j and 5k). Sometimes this chlorite forms a fine-grained intergrowth with rutile (Fig. 5k). Such rutile has low zirconium content; lower than 622 ppm (Figs. 5j, 11, and Table S1-6).

We propose multiple metamorphic stages (Fig. 12) based on the textural evidence and mineral compositions documented in this study as well as previous petrological analyses of garnet-biotite gneiss at Tenmondai Rock. The main stages are the following; stage-I (prograde), stage-II (peak-temperature), and stage-III (retrograde).

Stage-I is subdivided to the two sub-stages Ia and Ib. Stage-Ia is inferred from the presence of relict kyanite (Hiroi et al., 1983; Shiraishi et al., 1985). Stage-Ib is represented by the core of garnet porphyroblasts which enclosed quartz, sillimanite, rutile, ilmenite, apatite, and zircon (Fig. 4a). However, sample TMD40 preserves relatively poor evidence for the prograde stage, because the sample is re-equilibrated by later stage metamorphism.
Stage-II is a peak-temperature metamorphic stage. It is defined by a garnet + K-feldspar tie-line (Fig. 12). We interpret the composition of some matrix rutile to have been partially affected by subsequent stages of metamorphism and re-equilibration, and regard the matrix rutile that contains the highest zirconium content to provide the best estimate of Stage-II temperature conditions (Fig. 11).
Stage-III is subdivided into three sub-stages; IIIa, IIIb, and IIIc. Stage-IIIa is defined by disappearance of garnet + K-feldspar association (Fig. 12) and the occurrence of embayed garnet (Fig. 6). The embayed garnet is replaced by Ilm + Sil + Qz (Fig. 6). We interpret that rutile originally occurred as inclusions in garnet. This texture is interpreted as the beginning of a garnet breakdown reaction, Grt + Rt = Ilm + Sil + Qz. This is the GRAIL geobarometer reaction (Bohlen et al., 1983) progressing to the lower-pressure conditions. These garnet crystals which experienced the GRAIL reaction are separated from the matrix by quartz (Fig. 6). Since the garnet and sillimanite are separated by quartz, the formation reaction of spinel (Grt + Sil = Spl + Pl) does not occur. The reaction Grt + Sil = Spl + Pl is observed at grain boundaries between garnet and sillimanite (Figs. 5g and 5h). This can be understood using the tetrahedral diagram of Figure 13f. Quartz and spinel plot on opposite sides of the Prp/Alm-Grs-Als plane. As a result, such garnet constitutes a spinel-free domain.

Stages-IIIb and IIIc are defined by appearance of spinel and disappearance of garnet, respectively (Fig. 12). Coexistence of garnet + sillimanite + spinel + plagioclase is restricted to stage-IIIb (Fig. 12), and the observed spinel + plagioclase symplectite (Fig. 5) is interpreted to have formed in this stage. Small amounts of fine-grained muscovite in the pressure-shadows around garnet (Fig. 4a) is produced at stage-IIIc (Fig. 12).
Many authors studied spinel-forming reactions in pelitic metamorphic rocks. Some of these were solved as net-transfer reactions (geobarometers). Many geobarometers have been proposed for spinel-bearing pelitic metamorphic rocks (Table 1). For example, net-transfer reaction in Table 1b and Figure 13b is, Grt + 2Als = Spl + Crd. This reaction means that the product minerals are spinel and cordierite. As a result, the spinel must be in contact with cordierite. Such textures are well-known from the amphibolite-facies or granulite-facies metamorphic rocks (e.g., Clarke and Powell, 1991; Komatsu et al., 1994). However, our sample TMD40 is cordierite absent.
| Reaction number |
Net-transfer reaction | System | Als | Reference of geobarometer |
| (a) | Crd = 2(Hc, Spl) + 5Qz | FAS | - | Harris (1981) |
| FAS | - | Seifert and Schumacher (1986) | ||
| MAS | ||||
| MAS | - | Perchuk et al. (1989) | ||
| FMAS | - | Vielzeuf (1983) | ||
| FASZn MASZn |
- | Nichols et al. (1992) | ||
| (b) | (Alm, Prp) + 2Als = (Hc, Spl) + Crd |
FAS | Sil | Harris (1981) Droop and Charnley (1985) |
| MAS | Sil | Perchuk et al. (1989) | ||
| (c) | 5En + 10Als = 2Spl + 4Crd | MAS | Sil | Harris and Holland (1984) |
| (d) | (Alm, Prp) + 2Als = 3(Hc, Spl) + 5Qz |
FAS | Sil | Bohlen et al. (1986) |
| MAS | Sil | Perchuk et al. (1989) | ||
| FASZn MASZn |
Sil, Ky | Nichols et al. (1992) | ||
| (e) | Alm + 5Crn = 3Hc + 3Als | FAS | Sil | Bohlen et al. (1986) Shulters and Bohlen (1989) |
| (f) | 5Grs + (Alm, Prp) + 12Als = 3(Hc, Spl) + 15An |
CFASZn CMASZn |
And, Sil, Ky | This Study |
On the other hand, net-transfer reactions which related spinel + quartz assemblage are, Crd = Spl + Qz (Fig. 13a and Table 1a) and Grt + 2Als = 3Spl + 5Qz (Fig. 13d and Table 1d). These equations mean that the product minerals are spinel and quartz. As a result, the spinel must be in contact with quartz directly (e.g., Vielzeuf, 1983; Shimura et al., 2002; Kawakami and Motoyoshi, 2004; Kawasaki et al., 2011). Generally, almost all pelitic metamorphic rocks contain quartz. However, spinel does not occur in contact with quartz in the amphibolite facies and/or in the granulite facies (lower than the UHT) conditions. One reason for this is that cordierite lies on the spinel-quartz tie line, in the composition-paragenesis diagram (Fig. 13a). Since the paragenesis is subdivided into two assemblages, cordierite + quartz and spinel + cordierite, spinel + quartz assemblage is not stable (Fig. 13a). Therefore, reactions (a) and (d) geobarometers cannot be used for such metamorphic conditions.
Reactions (b) and (c) in Table 1 and Figure 13 have cordierite as a product. Reactions (c) and (e) in Table 1 and Figure 13 have orthopyroxene and corundum as reactants, respectively. However, our sample TMD40 is absent of cordierite, orthopyroxene, and corundum. Therefore, for our sample TMD40, all spinel-related previously proposed geobarometers in Table 1a-e cannot be used. The pressure condition of our spinel-bearing texture cannot be solved by the those previously proposed geobarometers.
Garnet-aluminosilicate-spinel-plagioclase (GASpP) geobarometerTextural evidence from our sample suggests that the reactants are garnet and sillimanite, and the products are spinel and plagioclase (Fig. 5). This texture implies the following reaction;
| \begin{equation} \text{Grt} + \text{Sil} = \text{Spl} + \text{Pl} \end{equation} | (1). |
This reaction can be explained within the CaO-FeO-Al2O3-SiO2 system and the CaO-MgO-Al2O3-SiO2 system. The four-component CFAS or CMAS system is illustrated by a tetrahedral-plot diagram (e.g., Shimura and Kemp, 2015) (Fig. 13f). In this diagram, garnet solid-solution is plotted on a (Prp/Alm)-Grs line. Reactants garnet solid solution + sillimanite are shown as a triangular plane of (Prp/Alm)-Grs-Sil. On the other hand, products spinel + plagioclase are shown as a tie-line of Spl-An. As shown in Figure 13f, the reactants plane is cut by the products line. It must be solved as a net-transfer reaction. We solved this reaction as the following two net-transfer reactions;
| \begin{align} &\text{CFAS system:} \\&\quad \text{5Grs} + \text{Alm} + \text{12Als} = \text{3Hc} + \text{15An} \\ &\quad \text{5Ca$_{3}$Al$_{2}$Si$_{3}$O$_{12}$} + \text{Fe$_{3}$Al$_{2}$Si$_{3}$O$_{12}$} + \text{12Al$_{2}$SiO$_{5}$} \\&\qquad= \text{3FeAl$_{2}$O$_{4}$} + \text{15CaAl$_{2}$Si$_{2}$O$_{8}$} \\ &\quad \text{and}\\ &\text{CMAS system:} \qquad \\&\quad \text{5Grs} + \text{Prp} + \text{12 Als} \\ &\qquad= \text{3Spl (Mg-endmember)} + \text{15An} \\ &\quad \text{5Ca$_{3}$Al$_{2}$Si$_{3}$O$_{12}$} + \text{Mg$_{3}$Al$_{2}$Si$_{3}$O$_{12}$} + \text{12Al$_{2}$SiO$_{5}$} \\&\qquad= \text{3MgAl$_{2}$O$_{4}$} + \text{15CaAl$_{2}$Si$_{2}$O$_{8}$} \end{align} | (2). |
| \begin{equation} P = (\varDelta H - T\varDelta S + RT \ln K_{\text{eq}})/ (-\varDelta V) \end{equation} | (3). |
| \begin{align} &P_{\text{[CFAS, Ky-bearing]}} \\&\quad = (-241950 + 730.2T + RT{\ln}K_{\text{Fe}})/36.242, \\ &P_{\text{[CMAS, Ky-bearing]}} \\&\quad = (-218730 + 707.0T + RT{\ln}K_{\text{Mg}})/36.163, \\ &P_{\text{[CFAS, Sil-bearing]}} \\&\quad = (-155790 + 587.4T + RT{\ln}K_{\text{Fe}})/29.378, \\ &P_{\text{[CMAS, Sil-bearing]}} \\&\quad= (-132570 + 564.2T + RT{\ln}K_{\text{Mg}})/29.299, \\ &P_{\text{[CFAS, And-bearing]}} \\&\quad= (-190350 + 619.8T + RT{\ln}K_{\text{Fe}})/27.374,\\ &\quad \text{and} \\ &P_{\text{[CMAS, And-bearing]}} \\&\quad= (-167130 + 596.6T + RT{\ln}K_{\text{Mg}})/27.295 \end{align} | (4). |
| \begin{align} &K_{\text{Fe}} = (a_{\text{Grs}}^{5}\ a_{\text{Alm}})/(a_{\text{Hc}}^{3}\ a_{\text{An}}^{15})\quad \\ &\quad \text{and} \\ &K_{\text{Mg}} = (a_{\text{Grs}}^{5}\ a_{\text{Prp}})/(a_{\text{Spl}}^{3}\ a_{\text{An}}^{15}) \end{align} | (5). |
| End-member | H (kJ/mol) | S (J/mol K) | V (J/bar) | |
| Grs | −6643.01 | 255.0 | 12.535 | |
| Alm | −5260.70 | 342.0 | 11.525 | |
| Prp | −6281.96 | 269.5 | 11.313 | |
| Sps | −5693.49 | 335.3 | 11.792 | |
| Ky | −2592.97 | 83.5 | 4.414 | |
| Sil | −2585.79 | 95.4 | 4.986 | |
| And | −2588.67 | 92.7 | 5.153 | |
| Hc | −1953.03 | 113.9 | 4.075 | |
| Spl | −2301.19 | 82.0 | 3.978 | |
| An | −4232.69 | 200.5 | 10.079 | |
| CFAS-system | H (kJ/mol) | S (J/mol K) | V (J/bar) | ΔS/ΔV |
| 5Grs + Alm + 12Ky | −69591.39 | 2619.0 | 127.168 | |
| 5Grs + Alm + 12Sil | −69505.23 | 2761.8 | 134.032 | |
| 5Grs + Alm + 12And | −69539.79 | 2729.4 | 136.036 | |
| 3Hc + 15An | −69349.44 | 3349.2 | 163.410 | |
| Δ [Ky] | −241.95 | −730.2 | −36.242 | 20.15 |
| Δ [Sil] | −155.79 | −587.4 | −29.378 | 19.99 |
| Δ [And] | −190.35 | −619.8 | −27.374 | 22.64 |
| CMAS-system | H (kJ/mol) | S (J/mol K) | V (J/bar) | ΔS/ΔV |
| 5Grs + Prp + 12Ky | −70612.65 | 2546.5 | 126.956 | |
| 5Grs + Prp + 12Sil | −70526.49 | 2689.3 | 133.820 | |
| 5Grs + Prp + 12And | −70561.05 | 2656.9 | 135.824 | |
| 3Spl + 15An | −70393.92 | 3253.5 | 163.119 | |
| Δ [Ky] | −218.73 | −707.0 | −36.163 | 19.55 |
| Δ [Sil] | −132.57 | −564.2 | −29.299 | 19.26 |
| Δ [And] | −167.13 | −596.6 | −27.295 | 21.86 |
Original thermodynamic dataset (H, S, V) of each endmembers of minerals are employed from Table 2a of Holland and Powell (2011) and their ‘tc-ds62’ file (which date description is 6 Feb, 2012) in the THERMOCALC.
Activity-composition relationships (a-x relation) of garnet are explained by the regular solution model by many authors (e.g., Ganguly and Saxena, 1984; Berman, 1990; Mukhopadhyay et al., 1997; White et al., 2014). Garnet solid solution can be written as A2+3B3+2Si3O12. The divalent cations such as Fe2+, Mn2+, Mg2+, and Ca2+ occupy the A-site, and the trivalent cations such as Fe3+, Cr3+, and Al3+ occupy the B-site. Wu (2015) described that ‘Fe3+ and Cr3+ cations in metapelitic garnet are negligible due to extreme dilution, therefore, only the mixing properties of the divalent cations on the dodecahedral sites are considered’. Wood and Fraser (1976) described that ‘it is rarely necessary to extend the symmetric solution treatment beyond three or four components despite the presence of many minor components in phases of geological interest’. Consequently, we consider garnet as pyralspite garnet, (Fe, Mn, Mg, Ca)3Al2Si3O12. Then;
| \begin{align} &X_{\text{Alm}} = \text{Fe}/(\text{Fe} + \text{Mn} + \text{Mg} + \text{Ca}), \\ &X_{\text{Prp}} = \text{Mg}/(\text{Fe} + \text{Mn} + \text{Mg} + \text{Ca}),\\ &\quad \text{and} \\ &X_{\text{Grs}} = \text{Ca}/(\text{Fe} + \text{Mn} + \text{Mg} + \text{Ca}) \end{align} | (6). |
Mukhopadhyay et al. (1993) and Berman (1990) described ternary (three components i, j, and k) asymmetric regular solution model;
| \begin{align} RT \ln \gamma_{\text{i}} &= 2(X_{\text{i}}X_{\text{j}}W_{\text{ji}} + X_{\text{i}}X_{\text{k}}W_{\text{ki}}) + X_{\text{j}}^{2}W_{\text{ij}} + X_{\text{k}}^{2}W_{\text{ik}} \\ &\quad - 2[X_{\text{i}}X_{\text{j}}(X_{\text{j}}W_{\text{ij}} + X_{\text{i}}W_{\text{ji}}) \\ &\quad + X_{\text{i}}X_{\text{k}}(X_{\text{k}}W_{\text{ik}} + X_{\text{i}}W_{\text{ki}}) \\ &\quad + X_{\text{j}}X_{\text{k}}(X_{\text{k}}W_{\text{jk}} + X_{2}W_{\text{kj}})] \end{align} | (7). |
| \begin{align} RT{\ln}\gamma_{\text{Fe}} &= 2(X_{\text{Fe}}X_{\text{Ca}}W_{\text{CaFe}} + X_{\text{Fe}}X_{\text{Mg}}W_{\text{MgFe}}) \\ &\quad + X_{\text{Ca}}^{2}W_{\text{FeCa}} + X_{\text{Mg}}^{2}W_{\text{FeMg}} \\ &\quad - 2[X_{\text{Fe}}X_{\text{Ca}}(X_{\text{Ca}}W_{\text{FeCa}} + X_{\text{Fe}}W_{\text{CaFe}}) \\ &\quad + X_{\text{Fe}}X_{\text{Mg}}(X_{\text{Mg}}W_{\text{FeMg}} + X_{\text{Fe}}W_{\text{MgFe}}) \\ &\quad + X_{\text{Ca}}X_{\text{Mg}}(X_{\text{Mg}}W_{\text{CaMg}} + X_{\text{Ca}}W_{\text{MgCa}})], \\ RT{\ln}\gamma_{\text{Mg}} &= 2(X_{\text{Mg}}X_{\text{Fe}}W_{\text{FeMg}} + X_{\text{Mg}}X_{\text{Ca}}W_{\text{CaMg}}) \\ &\quad + X_{\text{Fe}}^{2}W_{\text{MgFe}} + X_{\text{Ca}}^{2}W_{\text{MgCa}} \\ &\quad - 2[X_{\text{Mg}}X_{\text{Fe}}(X_{\text{Fe}}W_{\text{MgFe}} + X_{\text{Mg}}W_{\text{FeMg}}) \\ &\quad + X_{\text{Mg}}X_{\text{Ca}}(X_{\text{Ca}}W_{\text{MgCa}} + X_{\text{Mg}}W_{\text{CaMg}}) \\ &\quad + X_{\text{Fe}}X_{\text{Ca}}(X_{\text{Ca}}W_{\text{FeCa}} + X_{\text{Fe}}W_{\text{CaFe}})],\quad \\ &\text{and}\\ RT{\ln}\gamma_{\text{Ca}} &= 2(X_{\text{Ca}}X_{\text{Mg}}W_{\text{MgCa}} + X_{\text{Ca}}X_{\text{Fe}}W_{\text{FeCa}}) \\ &\quad + X_{\text{Mg}}^{2}W_{\text{CaMg}} + X_{\text{Fe}}^{2}W_{\text{CaFe}} \\ &\quad - 2[X_{\text{Ca}}X_{\text{Mg}}(X_{\text{Mg}}W_{\text{CaMg}} + X_{\text{Ca}}W_{\text{MgCa}}) \\ &\quad + X_{\text{Ca}}X_{\text{Fe}}(X_{\text{Fe}}W_{\text{CaFe}} + X_{\text{Ca}}W_{\text{FeCa}}) \\ &\quad + X_{\text{Mg}}X_{\text{Fe}}(X_{\text{Fe}}W_{\text{MgFe}} + X_{\text{Mg}}W_{\text{FeMg}})] \end{align} | (8). |
| \begin{align} &a_{\text{Alm}} = (X_{\text{Fe}}\ \gamma_{\text{Fe}})^{3},\ a_{\text{Prp}} = (X_{\text{Mg}}\ \gamma_{\text{Mg}})^{3},\ \\ &\quad \text{and}\quad a_{\text{Grs}} = (X_{\text{Ca}} \ \gamma_{\text{Ca}})^{3} \end{align} | (9). |
Feldspar is CaAl2Si2O8-NaAlSi3O8-KAlSi3O8 solid solution. The a-x relation of plagioclase is not simple, because it has a miscibility gap by phase transition (e.g., Carpenter and McConnell, 1984). Holland and Powell (1992) describes the a-x relation of plagioclase based upon Darken’s quadratic formalism (e.g., Powell, 1987) and Landau’s theory of phase transitions (e.g., Carpenter and McConnell, 1984). The following equations (Eqs. 10-14) are after Holland and Powell (1992);
| \begin{align} &X_{\text{Ca}} = \text{Ca}/(\text{Ca} + \text{Na} + \text{K}),\quad \\ &\quad \text{and}\quad X_{\text{Na}} = \text{Na}/(\text{Ca} + \text{Na} + \text{K}) \end{align} | (10). |
| \begin{equation} X_{\text{An}} = (1/16)\ X_{\text{Ca}}\ (1 + X_{\text{Ca}})^{2}\ (3 - X_{\text{Ca}})^{2} \end{equation} | (11a) |
| \begin{equation} X_{\text{b}} = 0.12 + 0.00038T \end{equation} | (11b). |
| \begin{align} I_{\text{An}} &= -(W_{\text{c}} - W_{\text{i}})\ (1 - X_{\text{b}})^{2} \\&\quad- RT{\ln}\ [(1 + X_{\text{b}})^{2}\ (3 - X_{\text{b}})^{2}\ {/}16] \end{align} | (12). |
Finally, for the anorthite a-x relations, they proposed two kinds of equations,
If XCa ≥ Xb conditions, a-x relation is;
| \begin{equation} RT{\ln}\gamma_{\text{An}} = W_{\text{c}}\ X_{\text{Na}}^{2} + I_{\text{An}}\quad \text{and}\quad a_{\text{An}} = X_{\text{An}}\ \gamma_{\text{An}} \end{equation} | (13). |
If XCa ≥ Xb conditions, a-x relation is;
| \begin{equation} RT{\ln}\gamma_{\text{An}} = W_{\text{i}}\ X_{\text{Na}}^{2}\quad \text{and} \quad a_{\text{An}} = X_{\text{Ca}}\ \gamma_{\text{An}} \end{equation} | (14). |
a-x relation of spinel was described by many authors (e.g., White et al., 2002; Holland and Powell, 2011). For our geobarometers, not only A-site composition, but also aluminum occupancy in the B-site is important, within the A2+B3+2O4 stoichiometry. Therefore, we use the approach of Harris (1981) and Goscombe et al. (1998);
| \begin{align} &X_{\text{Fe}} = \text{Fe}^{2+}/A^{2+},\ X_{\text{Mg}} = \text{Mg}/A^{2+},\ X_{\text{Zn}} = \text{Zn}/A^{2+},\ \\&\quad\text{and}\quad X_{\text{Al}} = \text{Al}/B^{3+} \end{align} | (15). |
| \begin{align} &RT \ln \gamma_{\text{Hc}} = W_{\text{MgFe}}\ (X_{\text{Mg}}^{2} + X_{\text{Mg}}X_{\text{Zn}}) \\&\qquad\qquad\quad + W_{\text{FeZn}}\ (X_{\text{Zn}}^{2} + X_{\text{Mg}}X_{\text{Zn}}) \\&\qquad\qquad\quad + W_{\text{MgZn}}\ (-X_{\text{Mg}}X_{\text{Zn}}), \\ &RT \ln \gamma_{\text{Spl}} = W_{\text{MgFe}} (X_{\text{Fe}}^{2} + X_{\text{Fe}}X_{\text{Zn}}) \\&\qquad\qquad\quad + W_{\text{MgZn}} (X_{\text{Zn}}^{2} + X_{\text{Fe}}X_{\text{Zn}}) \\&\qquad\qquad\quad + W_{\text{FeZn}} (-X_{\text{Fe}}X_{\text{Zn}}), \\ &\quad\text{and}\\ &a_{\text{Hc}} = X_{\text{Al}}^{2}X_{\text{Fe}}\gamma_{\text{Hc}},\quad \text{and}\quad a_{\text{Spl}} = X_{\text{Al}}^{2}X_{\text{Mg}}\gamma_{\text{Spl}} \end{align} | (16). |
Because the ΔS/ΔV is very small (19.26-22.64) (Table 2), dP/dT slopes of the above-mentioned equations are very gentle, making these reactions good geobarometers (Fig. 14).

As described earlier, previously proposed spinel-bearing geobarometers (Table 1a-e) cannot be used for the spinel-bearing texture of our sample TMD40. On the other hand, garnet-aluminosilicate-silica-plagioclase geobarometer (GASP) is represented by the reaction;
| \begin{align} &\text{Grs} + \text{2Als} + \text{Qz} = \text{3An}\quad \\ &\quad \text{and} \\ &\text{Ca$_{3}$Al$_{2}$Si$_{3}$O$_{12}$} + \text{2Al$_{2}$SiO$_{5}$} + \text{SiO$_{2}$} = \text{3CaAl$_{2}$Si$_{2}$O$_{8}$} \end{align} | (17). |
GASP was first proposed by Ghent (1976). After that, many authors proposed new models (e.g., see review in Holdaway, 2001). The GASP equation includes quartz, but does not include spinel (Eqs. 17). On the other hand, our GASpP equation includes spinel, but does not include quartz (Eqs. 1 and 2). Both geobarometers include garnet, plagioclase, and aluminosilicate. We arranged GASP by thermodynamic data (Holland and Powell, 2011) and their ‘tc-ds62’ file in the THEMOCALC. These equations are;
| \begin{align} &P_{\text{[Ky-bearing]}} = (40770 - 136.5T - RT{\ln}K)/(-6.605), \\ &P_{\text{[Sil-bearing]}} = (26290 - 112.5T - RT{\ln}K)/(-5.461),\ \\ &\quad \text{and} \\ &P_{\text{[And-bearing]}} = (32050 - 118.1T - RT{\ln}K)/(-5.127) \end{align} | (18). |
Figure 15a shows calculation results of GASpP geobarometer by 54 metamorphic samples in Supplementary Table S2. Figure 15b shows GASP versus GASpP calculation results with the same data. Although equilibrium conditions between spinel and quartz are not established for certain in GASP equations, except for UHT assemblages (see Table S2 and its references), the calculation results are consistent with wide P-T conditions (Fig. 15 and Table S2). GASpP is free from quartz, corundum, orthopyroxene, and cordierite (Table 1 and Fig. 13). The GASpP barometer is useful to estimate pressure conditions for spinel-bearing pelitic metamorphic rocks across a wide range of P-T conditions (andalusite, kyanite, and sillimanite fields) and mineral assemblages (Figs. 14, 15, and Table S2). P(GASpP, CFASZn)-P(GASpP, CMASZn) diagrams are also consistent (Fig. 15c). Calculation results of CFASZn and CMASZn from our TMD40 sample are almost the same (Table S2 and S3-4). We note that careful observations of petrography and quality of analysis data are important for such geothermo-barometric calculations.

Previous studies reported that the peak P-T conditions of Tenmondai Rock were about 750 °C and 720-750 MPa (e.g., Hiroi et al., 1983). Recently, several authors reported higher peak conditions of 750-800 °C and 800-900 MPa from pelitic metamorphic rock (Baba et al., 2019a, 2019b) or 850-860 °C and 780-840 MPa from mafic metamorphic rock (Takamura et al., 2019a, 2019b, 2020). Takamura et al. (2020) reported metamorphic rock from Tenmondai rock, and they described a clockwise P-T-t path.
For our sample TMD40, metamorphic P-T conditions of each stage (Fig. 12) are calculated from related textures and their mineral compositions (see Tables S1 and S3). For example, Stage-II pressure condition from mantle of porphyroblast garnet and matrix plagioclase by GASP (method by this study) and GRIPS (Ghent and Stout, 1984) barometers (Tables S3-1 and S3-2), and temperature by Zr-in-Rt thermometer (Fig. 11 and Table S1-6). Stage-IIIa pressure condition from embayed garnet texture (Fig. 6) by GRAIL barometer (Table S3-3) and temperature by Zr-in-Rt thermometer (Fig. 11 and Table S1-6). Stage-IIIb pressure condition from spinel + plagioclase symplectite texture by GASpP barometer (method by this study, Table S3-4) and temperature by Zr-in-Rt thermometer (Fig. 11 and Table S1-6).
The metamorphic peak (stage-II) P-T conditions are estimated as about 820 MPa and 850 °C (Fig. 16). These conditions are broadly consistent with recent studies of Tenmondai Rock (Takamura et al., 2020). The retrograde stage-IIIa P-T conditions are slightly lower than the peak P-T conditions (Fig. 16).

In contrast to the peak conditions, results from our GASpP geobarometer indicate a narrow range of pressure conditions, around 400-500 MPa. Occurrence of rutile in spinel + plagioclase symplectite texture (Fig. 5i) is rare; we found only two rutile grains in many thin-sections. The mineral paragenesis of rutile is not clear, and analyzed data of the rutile is poor, but the zirconium content is between that of inclusions in garnet and that around ilmenite associated with chlorite (Figs. 5j, 5k, and 11). As a result, we regard the Zr-in-Rt temperature of stage-IIIb to be lower than that of the lower limit of inclusions in garnet, and higher than that of the upper limit of around ilmenite (Fig. 11). We estimate the Stage-IIIb temperature is about 675-730 °C. The assemblage ‘garnet + sillimanite + spinel + plagioclase’ is stable only at stage-IIIb (Fig. 12). We interpret our spinel + plagioclase symplectite is produced by decompression from a garnet + sillimanite assemblage at stage-IIIb, at pressures defining the lower stability limit of garnet + sillimanite during the retrograde stage (Figs. 12 and 16).
Conclusions and implicationsDecompressional metamorphic P-T-t paths produce spinel + plagioclase textures from the reaction of garnet + aluminosilicate (e.g., this study; Hiroi et al., 1997; Rötzler and Romer, 2001; Álvarez-Valero et al., 2007). An example is provided here from Tenmondai Rock, East Antarctica, which experienced a clockwise P-T-t path. In contrast, counter-clockwise metamorphic P-T-t paths produce garnet + aluminosilicate from reaction of spinel + feldspar assemblages (e.g., Boger and White, 2003; Zhang et al., 2012; Sarkar and Schenk, 2014).
We propose a new geobarometer (GASpP) based on the reaction ‘garnet + aluminosilicate = spinel + plagioclase’, with application across a wide range of P-T conditions. Application of this geobarometer provides new constraints on the magnitude of decompression at Tenmondai Rock.
We (Tsuchiya, Shimura, and Fraser) visited Tenmondai Rock, 11-13 February 1994, during the geological field survey of the 35th Japanese Antarctic Research Expedition (JARE-35) (1993-1994). The sample TMD40 of this study was collected at that time. We wish to thank all members of JARE-35 and the crew of the icebreaker SHIRASE, and the National Institute of Polar Research, for their support in the fieldwork. We would like to thank all members of the JARE geology group for their fruitful discussions. We wish to thank Prof. George L. Luvizotto, who kindly distributed rutile standard samples to us. Mr. Youji Morihuku helped us with EPMA analysis. Constructive reviews by two anonymous reviewers and editorial comments by Prof. T. Kawakami improved the manuscript drastically and are gratefully acknowledged. Part of this study was supported by JSPS KAKENHI Grant Numbers JP24540509 and JP15K05343 to T. Shimura.
Table S1 (Representative mineral compositions) Table S2 and its references (original data of Figure 15), and calculation examples of geobarometers Table S3 (calculation examples of geobarometers) are available online from https://doi.org/10.2465/jmps.221130.