2024 Volume 119 Issue 1 Article ID: 240216
2024 Volume 119 Issue 1 Article ID: 240216
Low-molecular-weight organic acids are the predominant biological molecules released by microbes in the earth’s surface environments. To elucidate the effect of the molecules on the formation and polymorphism of calcium carbonate (CaCO3) minerals in hot water environments, we studied the formation of CaCO3 minerals in systems containing citric acid, malic acid, or acetic acid at 40, 60, and 80 °C for 24 h. Each system contained 5.0 mmol/L Ca2+, 20.0 mmol/L total carbonate ions, and 0.0, 0.01, 0.05, 0.1, 0.5, and 1.0 mmol/L organic acids. Our results demonstrated that citric acid considerably suppressed aragonite formation and promoted rhombohedral calcite formation with increasing citric acid concentration at temperatures up to 80 °C. Malic acid showed a similar effect on CaCO3 polymorphism to a slightly lesser extent than citric acid, whereas acetic acid exhibited a much lower effect than the other two organic acids. Moreover, the rhombohedral calcite crystals changed to a polyhedral morphology, and then to polyhedral crystals elongated along the c-axis with increasing citric acid concentration at 40 °C. However, very little or no significant effect on the calcite morphology was observed in the systems containing malic acid and acetic acid. The greater effect of citric acid on the CaCO3 polymorphism is likely due to the stronger adsorption affinity of citric acid for the aragonite surface compared to that of malic acid and acetic acid. In the morphology of calcite, citric acid is likely to preferentially adsorb on the {hk0} faces such as {110} and {100} of calcite crystals at 40 °C, resulting in the inhibition of crystal growth in the direction perpendicular to the c-axis and promotion of growth in the direction parallel to the c-axis.
Calcium carbonate (CaCO3) minerals are widely distributed in two polymorphs, calcite and aragonite, in the earth’s surface environments over a wide temperature range from ambient surface temperatures to boiling hot springs and hydrothermal waters (Pentecost, 2005; Fouke, 2011; Luo et al., 2022). Another polymorph, vaterite appears as a metastable phase in the early stages of CaCO3 formation and rapidly transforms into stable phase such as calcite or aragonite depending on the environment. Therefore, its occurrence in natural environment is extremely rare (Rodriguez-Blanco et al., 2011). Calcium carbonate monohydrate and calcium carbonate hexahydrate have been reported to occur in extremely limited regions of low-temperature environments such as sea ice and deep-sea sediments (Dahl and Buchardt, 2006; Dieckmann et al., 2008). Calcite and aragonite are the major polymorphs of CaCO3 minerals, and their formation is influenced by various geochemical factors such as temperature, pH, concentrations of specific cations (e.g., Sr2+, Fe2+, Ba2+, Pb2+, and Mn2+) and anions (e.g., phosphate, selenite, arsenate, and sulfate), and microbial involvement (Folk, 1994; Jones, 2017; Della Porta et al., 2022; Liendo et al., 2022). Among these factors, various biological molecules released by microbes that exhibit monomeric to polymeric forms can significantly influence the formation rate and stable polymorphs depending on their adsorption affinity for the CaCO3 surfaces. It has been revealed that such influence of the biological molecules on the polymorphism is primarily due to the adsorption of the molecules on the aragonite surface, which suppresses the formation of aragonite and consequently promotes calcite formation (Westin and Rasmuson, 2005; Kawano and Tokonami, 2014a; Miyashita et al., 2018).
In hot water environments at >40 °C, aragonite forms favorably over calcite as the predominant polymorph owing to temperature-induced effect when the geochemical factors other than temperature are negligible. In hot water environments, it has been experimentally confirmed that microbial polysaccharides (e.g., alginic acid and gellan gum) and acidic uronic acids (e.g., galacturonic acid and glucuronic acid) strongly suppress the formation of aragonite and promote calcite formation as the predominant polymorph depending on their molecular structures. In particular, alginic acid and galacturonic acid strongly promote calcite formation even in the higher temperature range of 60 to 80 °C (Kawano and Hwang, 2023; Kawano and Koyama, 2023). In the case of low-molecular-weight organic acids, they are major biological molecules released by microbes in the earth’s surface environments. These organic acids are also present in hot spring waters as fermentation products of various microbes such as cyanobacterial mats (Anderson et al., 1987). However, the effects of low-molecular-weight organic acids on CaCO3 polymorphs in hot water environments are not fully understood, and no systematic study has been performed based on their molecular structure. Low-molecular-weight organic acids are abundantly present in various environments on the earth’s surface including hot springs, thus, elucidating their effects on CaCO3 polymorphs is important for a deeper understanding of microbial involvement in CaCO3 formation.
In this study, therefore, precipitation experiments on CaCO3 minerals in the presence of citric acid, malic acid, and acetic acid at temperatures in the range of 40-80 °C were performed to confirm the effect of these organic acids on the formation and polymorphism of CaCO3 minerals. These organic acids are widely present in natural environment, and exhibit different molecular structures classified as tri-, di-, and mono-carboxylic acids, respectively. The findings of this study further advance our understanding of microbial involvement in CaCO3 formation in the earth’s surface environment, including hot springs.
The low-molecular-weight organic acids used in this study were citric acid (C6H8O7), malic acid (C4H6O5), and acetic acid (C2H4O2), all of which were guaranteed-grade reagents obtained from Nacalai Tesque, Inc. (Japan). These organic acids are classified as tri-carboxylic, di-carboxylic, and mono-carboxylic acids, respectively, and are known as typical organic molecules present in the earth’s surface such as soil environments (Adeleke et al., 2017). The dissociation constants (pK) of the carboxyl functional groups of these organic acids are citric acid: pK1 = 3.13, pK2 = 4.76, pK3 = 6.40, malic acid: pK1 = 3.46, pK2 = 5.10, and acetic acid: pK = 4.76 (Dawson et al., 1986). The molecular structures of these organic acids are shown in Figure 1. In addition, the guaranteed-grade reagents (Nacalai Tesque, Inc., Japan) of CaCl2·2H2O and NaHCO3 were used as raw materials for Ca2+ and carbonate ions for the experiments, respectively.

The CaCO3 precipitation experiments were conducted according to a previously reported experimental method, and citric acid, malic acid, and acetic acid were used as organic molecules in this study (Kawano and Hwang, 2023). Specifically, using a 100 mL flask, initial solutions containing 5.0 mmol/L Ca2+, 20.0 mmol/L total carbonate ion, and 0-1.0 mmol/L of each organic acid were prepared, and their pH values were adjusted to 7.0 using 1.0 M HCl. The glass flasks were sealed with aerated caps to allow the release of degassed CO2, and incubated statically in water baths at 40, 60, and 80 °C for 24 h. The systems containing citric acid, malic acid, and acetic acid were named C, M, and A systems, respectively (Table 1). After incubation, the flasks were cooled to 25 °C under running water, and the solution pH and concentrations of Ca2+ and total carbonate ions were measured. The saturation indices [SI = log(IAP/K)] of the solutions with respect to the CaCO3 minerals were calculated to evaluate the saturation states. The CaCO3 precipitates in the flasks were washed twice with distilled water and centrifuged. A portion of the precipitates was placed on a glass slide for morphological observation. The remainder was washed with ethanol, pulverized in an agate mortar, and dried on a glass slide for X-ray powder diffraction (XRD) measurements. The relative abundances of CaCO3 polymorphic minerals in the precipitates were calculated by the Rietveld quantification method.
| Run | Ca2+ (mmol/L) |
Total carbonate (mmol/L) |
Citric acid (mmol/L) |
Malic acid (mmol/L) |
Acetic acid (mmol/L) |
Initial pH | Reaction temp. (°C) |
| C system | |||||||
| C0 | 5.0 | 20.0 | 0.00 | - | - | 7.0 | 40, 60, 80 |
| C001 | 5.0 | 20.0 | 0.01 | - | - | 7.0 | 40, 60, 80 |
| C005 | 5.0 | 20.0 | 0.05 | - | - | 7.0 | 40, 60, 80 |
| C01 | 5.0 | 20.0 | 0.10 | - | - | 7.0 | 40, 60, 80 |
| C05 | 5.0 | 20.0 | 0.50 | - | - | 7.0 | 40, 60, 80 |
| C1 | 5.0 | 20.0 | 1.00 | - | - | 7.0 | 40, 60, 80 |
| M system | |||||||
| M0 | 5.0 | 20.0 | - | 0.00 | - | 7.0 | 40, 60, 80 |
| M001 | 5.0 | 20.0 | - | 0.01 | - | 7.0 | 40, 60, 80 |
| M005 | 5.0 | 20.0 | - | 0.05 | - | 7.0 | 40, 60, 80 |
| M01 | 5.0 | 20.0 | - | 0.10 | - | 7.0 | 40, 60, 80 |
| M05 | 5.0 | 20.0 | - | 0.50 | - | 7.0 | 40, 60, 80 |
| M1 | 5.0 | 20.0 | - | 1.00 | - | 7.0 | 40, 60, 80 |
| A system | |||||||
| A0 | 5.0 | 20.0 | - | - | 0.00 | 7.0 | 40, 60, 80 |
| A001 | 5.0 | 20.0 | - | - | 0.01 | 7.0 | 40, 60, 80 |
| A005 | 5.0 | 20.0 | - | - | 0.05 | 7.0 | 40, 60, 80 |
| A01 | 5.0 | 20.0 | - | - | 0.10 | 7.0 | 40, 60, 80 |
| A05 | 5.0 | 20.0 | - | - | 0.50 | 7.0 | 40, 60, 80 |
| A1 | 5.0 | 20.0 | - | - | 1.00 | 7.0 | 40, 60, 80 |
The pH values of the solutions before and after incubation were measured using a pH meter S220 (Mettler Toledo, Switzerland) equipped with a pH electrode. The concentrations of Ca2+ and total carbonate ions were measured by high-performance liquid chromatography with a Hitachi LaChrom Elite (Hitachi High-Tech Co., Japan) equipped with a TSKgel IC-Cation I/II HR column for Ca2+ and a TSKgel OApak-P column for total carbonate ions. PHREEQC calculations of the saturation indices of calcite and aragonite were conducted using the Minteq.dat database (Parkhurst and Appelo, 1999). XRD analysis was performed with a Rigaku RINT2000 diffractometer (Rigaku Co., Japan) using CuKα radiation generated at 40 kV and 30 mA with a scanning speed of 2° 2θ/min. Rietveld quantification of aragonite, calcite, and vaterite in the precipitates was performed using the powder X-ray diffraction profile analysis software PDXL equipped on a RINT2000 diffractometer. The structural data of CaCO3 minerals used in this quantitative calculation were based on COD ID: 2100187 (aragonite: Caspi et al., 2005), 1010928 (calcite: Elliott, 1937), and 9013565 (vaterite: Wang and Becker, 2009) in the Crystallography Open Database (Gražulis et al., 2018). The morphologies of the reaction products were observed using an optical microscope (DN-107T, WanTong Precision Instruments Co., Ltd.). Calcite crystals exhibiting some different characteristic morphologies were observed using a scanning electron microscope (SEM) at an accelerating voltage of 15 kV (VE-9800, Keyence Co., Japan).
After incubation for 24 h, the concentrations of Ca2+ and total carbonate ions and the pH of the solutions varied depending on the temperature and organic acids in the solutions. For the C system, Ca2+ concentrations decreased to 2-3 mmol/L at 40 °C, and to 0.1-0.6 mmol/L at both 60 and 80 °C (Fig. 2a). Such decreases in Ca2+ concentrations were due to the formation of CaCO3 minerals during incubation at each temperature. The smaller decreases in the Ca2+ concentration at 40 °C indicated that the rate of CaCO3 formation was slower than that at 60 and 80 °C. The total carbonate concentrations decreased to 9.1-12.6, 7.5-8.7, and 5.3-6.2 mmol/L by the incubation at 40, 60, and 80 °C, respectively (Fig. 2b). The decrease in total carbonate were more than 2.5 times that of Ca2+, suggesting the significant contribution of CO2 degassing in the reduction in total carbonate concentrations. Therefore, the pH of the solution increased from the initial pH of 7.0 to 7.8-8.3, 8.7-9.1, and approximately 10.0 at 40, 60, and 80 °C, respectively, mainly due to the CO2 degassing (Fig. 2c). The saturation indices with respect to the calcite (SIcalcite) and aragonite (SIaragonite) obtained by PHREEQC calculation reveled that the initial solutions of the C system showed SIcalcite = 0.94, 1.18, and 1.40, and SIaragonite = 0.79, 1.00, and 1.16 at temperatures of 40, 60, and 80 °C, respectively. These initial SI values increased with times and citric acid concentrations, and finally reached to SIcalcite = 1.08-1.56, 1.40-1.29, and 1.39-1.32, and SIaragonite = 0.93-1.41, 1.21-1.10, and 1.16-1.08 at 40, 60, and 80 °C, respectively, after incubation for 24 h. Overall, the SI values tended to increase after incubation for 24 h compared to the corresponding initial values. This was thought to be due more to the significant increase in pH caused by CO2 degassing than to the consumption of Ca2+ and carbonate ions by the carbonate mineral formation. Furthermore, the SI value also showed a tendency to increase slightly with increasing citric acid concentration, which is likely due to that citric acid inhibited the formation of carbonate minerals and suppressed the consumption of Ca2+ and carbonate ions to some extent. In the M and A systems, changes in Ca2+ and total carbonate concentrations and solution pH were observed similar to those in the C system, while the effect of differences in acetic acid concentration on Ca2+ consumption was extremely small (Figs. 2d-2i).

In all systems containing no organic acid at 40 °C, XRD showed that aragonite was mainly formed as the predominant product with small amounts of calcite and vaterite (C0, M0, and A0 in Fig. 3). However, the formation of aragonite was suppressed and a successive increase in calcite formation was confirmed with increasing citric acid concentration to 0.01-1.0 mmol/L (Fig. 3a). Vaterite was observed at relatively low citric acid concentrations (0.01 mmol/L). In the M and A systems, aragonite formation was also suppressed and calcite formation progressed slowly as the concentrations of malic acid and acetic acid increased, similar to that in the C system. The results indicated that these organic acids had a significant effect on the CaCO3 polymorphism, with the degree of their effect is as follows: citric acid > malic acid > acetic acid (Figs. 3b and 3c). At 60 and 80 °C, only aragonite was formed as the main product with small amounts of calcite and vaterite in the reaction with no organic acids. As the organic acid concentration increased, XRD demonstrated that aragonite formation was inhibited and calcite formation was promoted depending on the organic acid species. Specifically, the significant effect of citric acid on the polymorphism was almost maintained as temperature increased, whereas the effect of malic acid on the polymorphism decreased significantly, and almost no effect of acetic acid was observed at 60 and 80 °C.

The degree of influence of organic acids on polymorphism can be clearly recognized by the quantitative calculation of each polymorphic mineral (aragonite, vaterite, and calcite) in the precipitates, as shown in Figure 4. In the C system, aragonite was the predominant product in the absence of citric acid at 40-80 °C. However, aragonite amount decreased rapidly and calcite amount increased as the citric acid concentration increased. Quantitative calculations showed that the relative abundance of calcite exceeded 90% at citric acid >0.05 mmol/L (Figs. 4a-4c). This result indicates that citric acid significantly affects CaCO3 polymorphism even at low concentrations in this temperature range. In the M system, the amount of aragonite decreased rapidly and that of calcite increased up to 97% as malic acid concentration increased to 0.5 mmol/L at 40 °C (Fig. 4d). However, the amount of polymorphic changes tended to decrease gradually as the temperature increased (Figs. 4e and 4f). In the A system, the amount of polymorphic changes with organic acid concentration was smaller than that in the C and M systems, and 42% of aragonite amount was observed with 1.0 mmol/L acetic acid at 40 °C (Fig. 4g). Furthermore, almost no aragonite was formed up to 1.0 mmol/L acetic acid at 60 and 80 °C (Figs. 4h and 4i).

The CaCO3 minerals formed in the C system showed a typical morphological future corresponding to their polymorphism. At 40 °C, aragonite crystals exhibiting a needle-like habit elongated along the c-axis with <200 µm in length and <20 µm in width were abundantly observed in the absence of citric acid (Fig. 5a). In this precipitate, a small amount of rhombohedral calcite crystals with <50 in size and leaf-like vaterite particles with <100 µm in size coexisted with the aragonite crystals. As citric acid concentration increased to 0.01 mmol/L, the amount of rhombohedral calcite crystals with a size of <100 increased significantly, and leaf-like vaterite particles with <100 µm in size were also observed (Fig. 5b). At >0.1 mmol/L citric acid, calcite crystals exhibiting a rhombohedral habit gradually changed to a polyhedral morphology with a size of <100 µm, and tended to elongate in a length of <100 µm along the c-axis (Figs. 5c and 5d). These three different characteristic morphologies of calcite crystals exhibiting rhombohedral, polyhedral, and elongated polyhedral habits were clearly observed by SEM as presented in Figures 5b-5d. At higher temperatures, needle-like aragonite crystals were also formed in the absence of citric acid, and they tended to increase in the c-axis elongation up to a length of <300 µm with increasing temperature at 60 and 80 °C (Figs. 5e and 5i). As citric acid concentration increased, rhombohedral calcite with <50 µm size was abundantly formed at 0.01 mmol/L at 60 and 80 °C (Figs. 5f and 5j). At >0.1 mmol/L citric acid, these calcite crystals gradually changed to the polyhedral shape with a smaller size than the rhombohedral crystals, but the c-axis elongation did not progress much compared to 40 °C (Figs. 5g, 5h, 5k, and 5l).

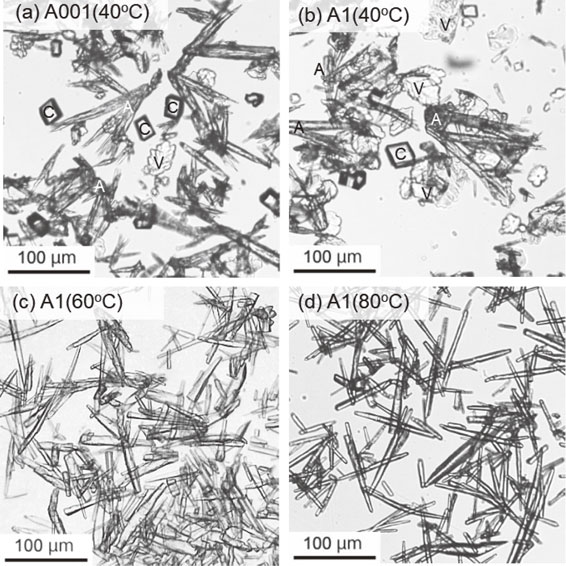
In the M system at 40 °C, aragonite needles with a length of <100 µm were mainly formed together with leaf-like vaterite and rhombohedral calcite crystals with both sizes of <50 µm at 0.01 mmol/L malic acid (Fig. 6a). Rhombohedral calcite increased in abundance with a small amount of non-elongated polyhedral crystals as the malic acid concentration increased (Fig. 6b). A similar trend was also observed at 60 and 80 °C, while aragonite needles remained abundantly at 0.01 mmol/L malic acid concentrations, respectively (Figs. 6c and 6e), and rhombohedral calcite crystals were mainly formed at 1.0 mmol/L malic acid concentration (Figs. 6d and 6f). In the A system, aragonite crystals exhibiting a needle-like habit with <100 µm in length were predominantly formed throughout the acetic acid concentrations of 0.01-1.0 mmol/L at 40-80 °C with significant amounts of rhombohedral calcite and leaf-like vaterite crystals at 40 °C (Figs. 7a-7d).

Precipitation experiments of CaCO3 minerals confirmed that needle-like aragonite was well formed as the main polymorph in the temperature range of 40-80 °C in the absence of organic acids. The predominant formation of aragonite over calcite at >40 °C is a well-known temperature-induced effect on the formation of CaCO3 polymorphs (Kitano, 1962; Burton and Walter, 1987; Ogino et al., 1987; Sawada, 1997). However, experiments in the presence of citric acid, malic acid, and acetic acid demonstrated that these low-molecular-weight organic acids significantly suppressed aragonite formation and promoted calcite formation depending on their structure and concentration (see Figs. 3 and 4). Remarkably, citric acid showed a stronger inhibitory effect on aragonite formation even at a low concentration (0.01 mmol/L), resulting in favorable calcite formation at concentration ≥0.01 mmol/L. Malic acid also exhibited similar effect on CaCO3 polymorphism to citric acid to a lesser extent than citric acid at 40-60 °C. Acetic acid had a much lower effect on the polymorphism at 40 °C, and no significant effect was observed at 50 and 60 °C. Therefore, the degree of influence of these organic acids on CaCO3 polymorphism was in the order of citric acid > malic acid > acetic acid. This effect was more pronounced at 40 °C, as the temperature-induced effect on aragonite formation was more enhanced at 60 and 80 °C.
Structurally, citric acid, malic acid, and acetic acid are typical monomeric organic acids with three, two, and one functional carboxyl groups, respectively. These carboxyl groups tend to deprotonate with increasing solution pH under acidic conditions and are almost entirely deprotonated at pH > 7. Hence, these organic acids exist in trivalent, divalent, and monovalent anionic species in the pH range of this experiment (pH 7-10). Thus, the trivalent anions of citric acid would bind more strongly by electrostatic interactions with the terminated Ca2+ sites on the CaCO3 surface relative to the divalent malic acid and monovalent acetic acid anions. Therefore, it can be inferred that the binding of the organic acids to the CaCO3 surface significantly influenced the formation of polymorphic minerals. The effects of organic anions, including monomeric and polymeric carboxylic acids, on the inhibition of CaCO3 precipitation by their interactions have been previously reported by many researchers (Kitano and Hood, 1965; Berner et al., 1978; Amjad et al., 1998; Wada et al., 1999; Hoch et al., 2000; Klepetsanis et al., 2000; Wada et al., 2001; Lin et al., 2005; etc). It is widely accepted that the binding of organic anions to Ca2+ sites on the CaCO3 surface blocks the growth sites of the crystals and inhibits CaCO3 formation, depending on the degree of binding. Furthermore, comparing the interaction of organic anions with aragonite and calcite, aragonite surface has a greater adsorption affinity for organic anions than the calcite surface. This is because the aragonite surface has more abundant terminal Ca2+ sites than the calcite surface (Mucci and Morse, 1985; Westin and Rasmuson, 2005). Indeed, many previous studies have confirmed that various anionic organic substances and inorganic anions are preferentially adsorbed on the surface of aragonite over that of calcite. For example, organic molecules such as acidic amino acids, low-molecular-weight organic acids, and uronic acids (Kawano and Tokonami, 2014a, 2014b; Kawano and Miyashita, 2018), and inorganic anions such as phosphate, selenite, arsenate, and silicate (Millero et al., 2001; Kawano and Maeda, 2020). Consequently, it can be concluded that the organic acid used in this study preferentially adsorbed on the aragonite surface according to their molecular structure, which significantly suppressed the formation of aragonite, resulting in the favorable formation of calcite. Similar effects of low-molecular-weight organic acids such as citric acid and malic acid on CaCO3 polymorphs have been confirmed by precipitation experiments in the presence of Mg2+ at 25 °C (Kawano and Tokonami, 2014a; Miyashita et al., 2018). In this case, Mg2+ acts as a catalyst to promote aragonite formation even at 25 °C (Berner, 1975), however organic acids tend to favor calcite formation with their concentrations because these molecules are preferentially adsorbed on the aragonite surface and inhibit aragonite crystal growth more strongly.
Another important effect of organic acids on CaCO3 formation was the progressive morphological change in calcite crystals with their concentration. Calcite crystals exhibiting a typical rhombohedral morphology were formed at low citric acid concentrations. However, they changed to a polyhedral morphology as the citric acid concentration increased, and subsequently to a polyhedral shape elongated along the c-axis as the concentration further increased (see Fig. 5). These morphological changes in the calcite crystals occurred more remarkably at <1.0 mmol/L citric acid at 40 °C than at 60 and 80 °C. However, no significant morphological changes were observed in the systems containing malic acid and acetic acid, regardless of their concentrations. Thus, the order of influence of organic acids on calcite morphology was citric acid > malic acid ≈ acetic acid, which was almost the same as the order of influence on CaCO3 polymorphism. Similar c-axis elongation of calcite crystals has been previously reported in the system containing various anionic organic molecules such as citric acid, malic acid, and tartaric acid (Meldrum and Hyde, 2001; Kurra and Mukkamala, 2013; Lu et al., 2023), EDTA and dextrin (Ukrainczyk et al., 2013), polysaccharides (Kayano et al., 2011), peptides and proteins (Geider et al., 1996; Gerbaud et al., 2000), and synthetic copolymers consisting of polyethylene glycol and polyacrylic acid (Su et al., 2009). It is thought that such morphological changes in the calcite crystals are probably caused by the adsorption of dissolved organic substances on the calcite surface, while the detailed molecular-level adsorption mechanism has not been fully elucidated. As is well known, the morphology of calcite crystals is significantly influenced by the adsorption of ionic molecules such as organic acids on specific crystal faces of the calcite surface. Adsorption of ionic molecules to specific faces suppresses the growth of the faces by blocking the growth sites, which significantly changes the crystal morphology depending on the degree of adsorption (Titiloye et al., 1993; Ukrainczyk et al., 2015). In the present study, the formation of polyhedral crystals and their elongated forms can be attributed to the preferential adsorption of citric acid on the specific surface faces of the calcite crystals. The surface of typical rhombohedral calcite crystals is mainly composed of the crystallographically identical faces of {104} and other minor faces of {hk0} such as {110} and {100} depending on the irregularity of the crystal shape (Titiloye et al., 1993). The {104} crystal face obliquely intersect the c-axis, whereas the {hk0} faces extend parallel to the c-axis. Therefore, citric acid is preferentially adsorbed on the {hk0} faces over the {104} face, forming polyhedral calcite crystals by the suppression of crystal growth in the direction perpendicular to the c-axis. As citric acid adsorption on the {hk0} faces progresses further, growth of these crystal faces is more strongly suppressed and the formation of polyhedral calcite crystals elongated in the c-axis direction is likely to be promoted as illustrated in Figure 8. Although the {hk0} faces of calcite crystals could not be identified in this study, this formation process of the calcite morphology is consistent with molecular dynamics calculations showing that the adsorption of anionic polymers on the calcite surface proceeds preferentially to the {110} face rather than to over the {104} face (Choi and Kim, 2017).

The CaCO3 polymorphic minerals are widely formed in various earth’s surface environments from low to high temperatures including soils and hot springs. Similarly, low-molecular-weight organic acids such as citric acid, malic acid, and acetic acid are ubiquitously present in the earth’s surface environments as biological organic components originating from microbes and plants (Kördel et al., 1997; Kosobucki and Buszewski, 2014; Adeleke et al., 2017). Therefore, this study contributes to considering the influence of biological molecules on the formation of CaCO3 polymorphic minerals in the earth’s surface environments.
The precipitation experiments of CaCO3 minerals demonstrated that needle-like aragonite was mainly formed as the predominant polymorph in the absence of organic acids at 40-80 °C. In the presence of organic acids, citric acid strongly inhibited aragonite formation and favored formation of rhombohedral calcite with increasing citric acid concentration. This effect on the polymorphic change was significantly more pronounced at 40 °C and slightly decreased as the temperature increased to 60 and 80 °C. Malic acid also showed a similar effect on the polymorphic changes to a lesser extent than citric acid in this temperature range. Acetic acid exhibited a much lower effect than citric acid and malic acid at 40 °C, and no significant effect was observed at 60 and 80 °C. Consequently, it was revealed that these organic acids had a significant effect on the CaCO3 polymorphism in the order of citric acid > malic acid > acetic acid at temperatures of 40-80 °C. These polymorphic changes in the CaCO3 minerals appeared to be due to the preferential adsorption of the organic acids on the aragonite surface.
Moreover, the rhombohedral calcite changed to a polyhedral morphology and further transformed into polyhedral crystals elongated along the c-axis with increasing citric acid concentration. Malic acid and acetic acid exhibited very little or no effect on the calcite morphology even at higher concentrations. This morphological change in the calcite crystals is possibly due to the preferential adsorption of citric acid to the {hk0} faces such as {110} and {100} faces extending parallel to the c-axis. This adsorption suppressed crystal growth perpendicular to the c-axis resulting in the formation and elongation of polyhedral crystals depending on the amount of citric acid adsorption.
The authors would like to thank Prof. J. Hwang, Pusan National University, for his help and cooperation. We are grateful to the editor for handling our manuscript and the reviewer for their valuable reviews.