2025 Volume 120 Issue 1 Article ID: 240908
2025 Volume 120 Issue 1 Article ID: 240908
Na pyroxene (Napx) of the aegirine-jadeite series occurs as a major metamorphic mineral in metacherts and metabasalts of the lawsonite blueschist (LBS) subfacies in the Kurosegawa belt, Kyushu, Japan. Its jadeite content [XJd = VIAl/(VIAl + Fe3+ + Ca)] is highly variable at the thin section scale and is crucially influenced by associated minerals, such as albite and sodium amphibole (Namp). Whereas the coexistence with albite and quartz imposes an upper limit on XJd in Napx through the equilibrium reaction Ab = Jd + Qz, this paper reveals that uniformly low XJd was observed when albite was absent in the metacherts and the coexistence with Namp also effectively reduced XJd. In the former case available Na and Al content is limited in metacherts and in the latter case the Al/Fe3+ distribution between Napx and Namp controlled the XJd in metacherts and metabaslts. Notably, the distribution coefficient [KD = (Al/Fe3+)Namp/(Al/Fe3+)Napx] systematically varied depending on the mode of growth: KD ∼ 4 in metabasalts, where Napx developed replacing relict igneous pyroxenes (i.e., topotaxial growth), whereas KD ∼ 1 in metacherts and metamorphic veins developed in metabasalts, where no relict phase was involved (i.e., nucleation and growth). Previous studies confirmed this trend, suggesting a convergence of KD to 1 in higher grade rocks, such as epidote-blueschist and eclogite facies. This implies a kinetic control on the distribution process, particularly at lower temperatures (<300 °C). In summary, low-grade Napx associated with igneous pyroxene may represent a metastable state; thus, those in metacherts can serve as a more reliable proxy for the equilibrium conditions of the clinopyroxene system in these grades.
Na pyroxene (Napx) is a major source of pressure-temperature (PT) information in metamorphic rocks. XJd has been used as a crucial indicator of metamorphic pressure, assuming the equilibrium of Ab = Jd + Qz, in early works such as Miyashiro and Banno (1958) and Essene and Fyfe (1967). Several authors have proposed thermodynamic models of the aegirine-jadeite-diopside system based on natural occurrences and synthetic experiments to represent equilibrium states over a wide PT range (Carpenter, 1979a, 1979b, 1980; Holland, 1980; Carpenter, 1982; Holland, 1983; Holland and Powell, 1996a, 1996b; Green et al., 2007).
However, the slow reaction rate at lower temperatures prevented such experiments from yielding reliable results. Additionally, natural low-grade metamorphic rocks occasionally contain Na pyroxenes with compositions falling within the miscibility gap expected by these models. Consequently, a close petrological study on natural samples of metamorphic pyroxenes accurately representing the equilibrium of lower grades is desired. In the interpretation, it is crucial to consider additional compositional controls beyond PT that may influence natural Na pyroxene compositions.
One factor is bulk rock composition. Okay (1978) compared the composition of Na pyroxene in metabasite and metachert from a blueschist facies terrain and found relatively higher Ca contents in the former with comparable jadeite contents. Manzotti et al. (2020) confirmed that the bulk Fe3+ content determines the composition and stability of Na pyroxene and sodium amphibole by constructing pseudosections on various lithotypes. Another factor, the rock fabric, has been discussed by several authors. Maruyama and Liou (1987) illustrated the role of igneous pyroxene as the nuclei of metamorphic Na pyroxene growth. Furthermore, Morata et al. (1994) associated primary rock composition with textual patterns. In tholeiitic metabasites, Na pyroxene is confined to the contact between igneous augite and veinlets, whereas it overgrows Ti-rich pyroxene (epitaxial growth) in transitional-alkaline metabasites. They argued that the Al contents in the latter cases are the product of Ca-Na replacement; thus, they do not directly represent the metamorphic pressure.
In this study, the Na pyroxene compositional trend from low grade [lawsonite blueschist (LBS) subfacies, <300 °C] metacherts and metabasalts was reported. This demonstrates the crucial effect of the coexisting sodium amphibole (Namp), which effectively reduces XJd in association with Na pyroxene. Therefore, Al was concentrated in sodium amphibole through the Al/Fe3+ distribution between the two minerals. Furthermore, the extent of the effect systematically differed according to the mode of growth, i.e., topotaxial replacement in metabasalts as KD = (Al/Fe3+)Namp/(Al/Fe3+)Napx ∼ 4 and nucleation and growth in metacherts as KD ∼ 1, rather than the bulk rock chemistry or oxidation state. Finally, the documented coexistence of Na pyroxene and sodium amphibole was examined to confirm the kinetic aspect of the effect, which diminished in higher grades, where more ideal behavior of cations is expected.
Abbreviations for mineral names are from the IMA-CNMNC approved symbols (Warr, 2021), except for Napx, Namp for Na pyroxene and sodium amphibole, respectively.
On Kyushu Island, Japan, the Kurosegawa Belt lies south of the Usuki-Yatsushiro Tectonic Line and is exposed as a narrow area (<20 km width, Fig. 1a). The Yatsushiro area occupies the western region of the Kurosegawa Belt, which is characterized by a serpentinite mélange, including a Jurassic accretionary complex (Shimotake subunit), Permian-Cretaceous sedimentary basin deposits and pre-Jurassic rocks of various origins and ages (Saito et al., 2010). They include mafic metamorphic rocks of prehnite-pumpellyite facies accompanied by Late Palaeozoic limestone blocks (Tobiishi subunit) and high-P/low-T mafic and siliceous metamorphic rocks of lawsonite blueschist subfacies (Hakoishi subunit) (Fig. 1b). White mica K-Ar ages extracted from metamorphic rocks have been reported as 180 Ma for the Tobiishi subunit and 240-290 Ma for the Hakoishi subunit (Sato et al., 2014), and they can be correlated with the Suo and Renge belts, respectively, in the Inner Zone of SW Japan (Tsujimori and Itaya, 1999; Fig. 1a).
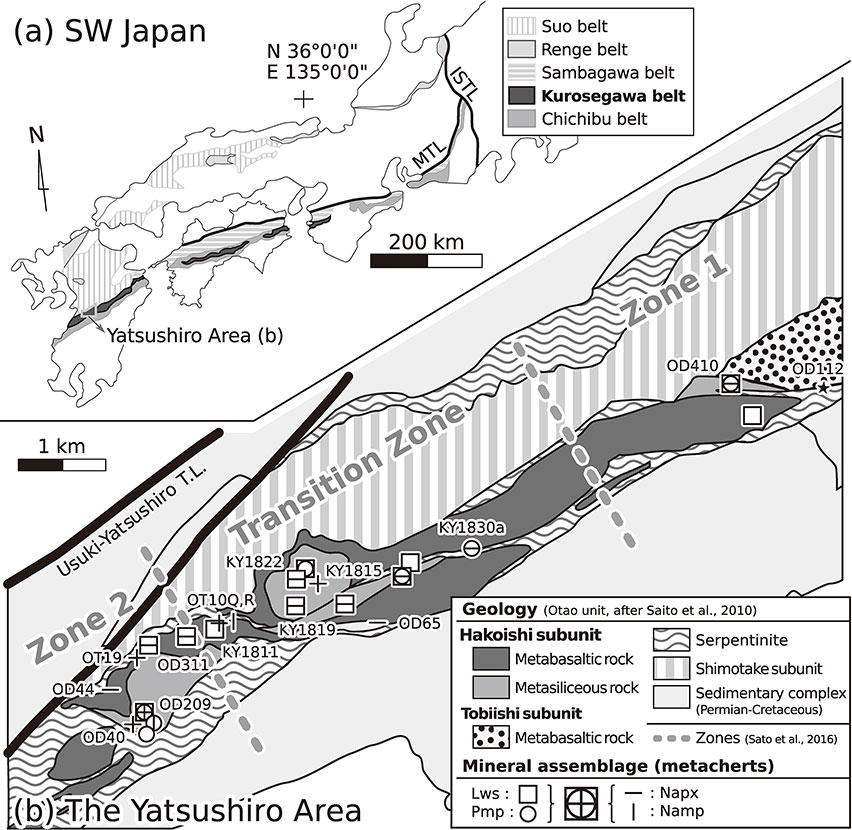
This study focused on the metamorphic rocks within the Hakoishi subunit. Its peak metamorphic condition is estimated as 200-300 °C and 0.45-0.80 GPa (Sato et al., 2016). The meta-mafic rocks (mentioned as ‘metabasalts’ hereafter) are mainly of basaltic lava or hyaloclastite origin and are characterized as massive or weakly foliated fabric, where relict augite or precursor igneous structures are preserved.
Sato et al. (2016) advocated for westward upgrading based on the spatial distribution of low-variance mineral assemblages in metabasalts. This represents the transition from pumpellyite blueschist (Zone 1) to lawsonite blueschist (Zone 2) subfacies, which is controlled by the reaction (1):
| \begin{equation} \text{Napx} + \text{Pmp} + \text{Chl} + \text{H$_{2}$O} = \text{Lws} + \text{Namp} \end{equation} | (1). |
Although the mineral assemblages systematically indicate westward upgrading, the apparent maximum of XJd was observed in the middle of the subunit, which will be discussed later.
The siliceous rocks (‘metacherts’) form a series of intercalation with metabasalts and typically display layered or slumping structures with a few centimeters to meter thickness. The matrix is mostly composed of granular quartz (<500 µm in diameter). The major metamorphic minerals in the metacherts [lawsonite, pumpellyite-(Fe3+), chlorite, Na pyroxene and sodium amphibole] were also common in the metabasalts. However, the pumpellyites are significantly richer in Fe3+ and Sr-bearing epidote (up to 9.44 wt% SrO) occurs in the metacherts (Table 1). As accessory minerals, apatite and titanite occur in most samples. The grains are typically arranged parallel to the layered structures. Certain metacherts are rich in Mn, and various Mn-bearing minerals have been reported (howieite, Ibuki et al., 2008; okhotskite, Yabuta and Hirajima, 2020).
| Qz | Ab | Napx | Namp | Lws | Pmp | Ep | Phg | Chl | Hem | Ap | Ttn | Others | |
| OD410 | ● | ● | ● | ● | ○ | ○ | ● | ● | ● | ● | |||
| KY1830a | ● | ● | ● | ● | ● | ● | ● | sursassite | |||||
| OD65 | ● | ● | ● | ● | ● | ● | |||||||
| OD70 | ● | ● | ● | ● | ○ | ● | ● | ● | |||||
| KY1815a | ● | ● | ● | ○ | ● | ○ | howieite, sulphide | ||||||
| KY1822a | ● | ● | ● | ● | ● | ● | ● | ● | |||||
| KY1819 | ● | ● | ● | ● | ● | ● | ○ | tourmaline | |||||
| KY1811 | ● | ● | ○ | ● | ● | ● | ● | piemontite, sursassite, calcite | |||||
| OT10Q | ● | ● | ● | ○ | piemontite, braunite | ||||||||
| OT10R | ● | ● | ● | ● | ● | piemontite, okhotskite | |||||||
| OT19 | ● | ● | ● | ● | ● | ○ | ● | tourmaline, braunite | |||||
| OT19BV | ● | ● | ● | ● | braunite | ||||||||
| OD311 | ● | ● | ● | ○ | ● | piemontite, calcite | |||||||
| OD209 | ● | ● | ● | ● | ● | ● | ● | ● | ● | baryte | |||
| OT27A-b | ● | ● | ● | ● | ● | ● | ● | chalcopyrite, baryte | |||||
| OD40 | ● | ● | ● | ● | howieite, sulphide | ||||||||
| OD44 | ● | ● | ● | ○ | ● | ● | ● | ● | ● | calcite |
Qz, quartz; Ab, albite; Napx, Na pyroxene; Namp, Na amphibole; Lws, lawsonite; Pmp, pumpellyite; Ep, epidote; Phg, phengite; Chl, chlorite; Hem, hematite; Ap, apatite; Ttn, titanite.
Despite common post-peak veins (quartz, albite, and calcite), both the metabasalts and metacherts were scarcely affected by greenschist facies overprinting during the exhumation stage, represented by the development of actinolite or epidote. Fujimoto et al. (2010) and Sato et al. (2016) provide detailed petrography and mineralogy of the metabasalts.
Na pyroxene occurs commonly both in metabasalts and metacherts but displays contrasting modes of occurrence. In metabasalts, igneous augite is typically replaced along fringes or cracks (Sato et al., 2016, Figs. 2 and 3). It rarely occurs as fillings of albite veins, apart from augite grains, in the metabasalt samples (OD112; Fig. 2a). It formed an interfingered structure with acicular sodium amphibole grains coexisting with lawsonite. In considering elemental partitioning, analyses on the outermost rim of neighboring mineral grains (<100 µm apart) are paired.


In metacherts, Na pyroxene rarely formed monomineralic folded veins and light green color (Fig. 2b). It commonly occurs as acicular or columnar grains (<100 µm length, Figs. 2c-2f) in the quartz matrix. Textually, it is closely associated with sodium amphibole, forming bi-mineral layers less than 50 µm thick (Fig. 2g); in addition, parallel twin-like composite grains are occasionally observed (Fig. 2h).
While chemical compositions may vary within a thin section, individual grains are largely homogeneous. No textural evidence, such as zoning or core-rim relationships, suggests a multi-stage growth history, except for later-stage growth associated with cross-cutting quartz veins, which are easily distinguishable both chemically and texturally (Figs. 2b and 2c). Consequently, most Na pyroxene grains are interpreted to have formed during the peak metamorphic event. The paragenetic relationships between Na pyroxene and adjacent minerals are thus considered apparent.
Compositions of minerals were determined by electron microprobe analyses (EPMA), using a Hitachi S3500H scanning electron microscope with an energy-dispersive X-ray analyser (EDAX®) at the Department of Geology and Mineralogy, Kyoto University. The acceleration voltage was 20 kV, the beam current was 500 pA, and the spot size was 5 µm. Natural and synthetic materials were used as standards, and a ZAF correction scheme was applied to process the X-ray intensity data.
The discussion of cation partitioning may be significantly influenced by the accuracy of Fe3+/Fetotal estimation. To evaluate uncertainties arising from different estimation methods, two approaches were applied to Na pyroxene and sodium amphibole. For Na pyroxene, the methods of Droop (1987) and Morimoto et al. (1988) were employed, assuming six oxygen atoms, four total cations, and Na = VIAl + Fe3+. For sodium amphibole, the methods of Droop (1987) and Matsumoto and Hirajima (2005) were used, assuming 23 oxygen atoms, Σ(Si, Ti, Al, Cr, Fe, Mn, Mg) = 13, or BNa (Na in the B site) = VIAl + Fe3+. For chlorite, total Fe is regarded as divalent.
Many of the analyzed Na pyroxenes were poor in Ca and, thus, belonged to the Aeg-Jd series. The Ca content was lower [<0.25 per formula unit (p.f.u.); Fig. 3a; Table 2] in metacherts than in metabasalts (<0.50; Fig. 3c). XJd was variable, particularly in metacherts, ranging from 0.13 to 0.60 within a single hand-specimen (KY1830a). While Ca content varies significantly among samples, it remains relatively constant within individual samples, particularly in metabasalts. Consequently, a negative correlation exists between XJd and Ca content across different samples (lower XJd corresponds to higher Ca content), as suggested by Endo and Wallis (2017) in the Mikabu-Northern Chichibu accretionary wedge, central Shikoku, whereas the correlation is weak within a single sample. Several Na pyroxenes suffered from later-stage fluid infiltration; however, such alterations could be discerned both textually and chemically. For instance, a mono-mineral vein mainly composed of Na pyroxene with XJd ∼ 0.4 was cut by a later-stage quartz vein, and an aegirine-richer part with XJd < 0.1 (Fig. 3a) developed at the contact (Fig. 2c).
| # | KY1819 | OT10Q | KY1815a | OD311 | KY1830a | KHK31*** | |
| Coexistence | Ab | Namp | Namp Ab | Vein | Vein** | Namp Ab | |
| Oxide wt% | |||||||
| SiO2 | 54.81 | 55.84 | 52.73 | 53.65 | 54.64 | 53.37 | 54.06 |
| TiO2 | 0.11 | 0.32 | 0.25 | 0.24 | n.d. | n.d. | 0.29 |
| Al2O3 | 8.26 | 11.69 | 2.71 | 4.68 | 7.78 | 2.25 | 5.83 |
| Cr2O3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.03 |
| Fe2O3* | 20.78 | 14.81 | 27.55 | 19.93 | 19.22 | 24.46 | 21.27 |
| FeO* | 0.00 | 0.00 | 1.62 | 0.94 | 0.45 | 0.32 | 0.00 |
| MnO | 0.10 | 0.40 | 0.60 | 1.54 | 0.49 | 1.51 | 0.16 |
| MgO | 1.19 | 1.80 | 0.37 | 2.98 | 1.61 | 2.62 | 2.35 |
| CaO | 1.43 | 1.51 | 1.73 | 4.89 | 3.30 | 4.87 | 3.96 |
| Na2O | 13.15 | 13.66 | 12.34 | 10.58 | 12.17 | 10.84 | 11.96 |
| K2O | 0.03 | n.d. | n.d. | n.d. | 0.03 | 0.03 | 0.02 |
| Total | 99.86 | 100.03 | 99.90 | 99.43 | 99.70 | 100.27 | 99.93 |
| Cations for O = 6 | |||||||
| Si | 2.005 | 1.999 | 2.003 | 2.006 | 2.008 | 2.007 | 1.997 |
| Ti | 0.003 | 0.009 | 0.007 | 0.007 | - | - | 0.008 |
| Al | 0.356 | 0.493 | 0.121 | 0.206 | 0.337 | 0.100 | 0.254 |
| Cr3+ | - | - | - | - | - | - | 0.001 |
| Fe3+ | 0.572 | 0.399 | 0.788 | 0.561 | 0.532 | 0.692 | 0.592 |
| Fe2+ | 0.000 | 0.000 | 0.051 | 0.029 | 0.014 | 0.010 | 0.000 |
| Mn2+ | 0.003 | 0.012 | 0.019 | 0.049 | 0.015 | 0.048 | 0.005 |
| Mg | 0.065 | 0.096 | 0.021 | 0.166 | 0.088 | 0.147 | 0.129 |
| Ca | 0.056 | 0.058 | 0.070 | 0.196 | 0.130 | 0.196 | 0.157 |
| Na | 0.933 | 0.948 | 0.909 | 0.767 | 0.867 | 0.791 | 0.857 |
| K | 0.001 | - | - | - | 0.001 | 0.001 | 0.001 |
| Total | 3.995 | 4.014 | 3.990 | 3.987 | 3.992 | 3.993 | 4.001 |
* Recalculated, see details in text.
** Qz vein contact.
*** Horokanai Ep blueschist.
Blueish sodium amphibole replaces igneous relicts, i.e., augite and hornblende, or it occurs as needle-shaped grains in metabasalts throughout the study area (Sato et al., 2016, Fig. 3f). The Al/(Al + Fe3+) (= YAmp) ratio of sodium amphiboles associated with lawsonite and pumpellyite increased westward, from YAmp = 0.2-0.3 in Zone 1, through YAmp = 0.4-0.7 in the transition zone, to YAmp = 0.8-0.9 in Zone 2. This was accompanied with a slight increase in Mg# [= Mg/(Mg + Fe2+)] from Zone 1 (Mg# ∼ 0.4) to Zone 2 (Mg# ∼ 0.6) (Sato et al., 2016, Fig. 5). This chemical variation in sodium amphibole from riebeckite (Rbk) or magnesioriebeckite (Mrbk) to glaucophane (Gln) also supported westward upgrading.
In the metacherts, sodium amphibole also occurred as acicular grains similar in size to Na pyroxene (Figs. 2g and 2h), however its distribution was limited to the western half of the study area, i.e., the transition zone and Zone 2 of Sato et al. (2016) (Fig. 1b). Almost all the analyzed sodium amphiboles were classified as Rbk or Mrbk (Ca < 0.2 p.f.u.; Fig. 4a; Table 3), and Gln was not found in the metacherts. The estimated Fe3+ content was significantly higher in the metacherts than in the metabasalts. The Mg# ranged from 0.9 to 1.0 in a braunite- and piedmontite-bearing sample (OT10R). In contrast, Ibuki et al. (2010) reported ferro-glaucophane (Mg# = 0.0-0.3) from the Hakoishi metachert. This difference was attributed to the oxidation state (cf. Okay, 1980a; Mottana, 1986; Ubukawa et al., 2007); Mn3+-bearing minerals were common in the former, whereas Mn2+-bearing howieite occurred in the latter.

| # | OD209 | KY1822 | KY1815a | OD311 | KHK31** |
| Coexistence | Ab | Napx | Napx | Napx Ab | |
| Oxide wt% | |||||
| SiO2 | 56.32 | 55.41 | 53.81 | 56.69 | 54.59 |
| TiO2 | 0.10 | n.d. | n.d. | n.d. | 0.06 |
| Al2O3 | 3.31 | 1.70 | 2.21 | 7.47 | 3.63 |
| Fe2O3* | 12.49 | 15.76 | 13.52 | 6.76 | 13.74 |
| FeO* | 7.20 | 3.83 | 14.17 | 12.17 | 11.47 |
| MnO | 1.07 | 2.92 | 2.43 | n.d. | 0.27 |
| MgO | 9.52 | 9.79 | 4.65 | 7.61 | 6.41 |
| CaO | 0.23 | 0.57 | 0.72 | 0.73 | 0.72 |
| Na2O | 7.34 | 7.13 | 6.57 | 7.09 | 7.28 |
| K2O | n.d. | n.d. | 0.07 | n.d. | 0.04 |
| Total | 97.58 | 97.11 | 98.15 | 98.52 | 98.20 |
| Cations for O = 23 | |||||
| Si | 8.011 | 7.995 | 7.994 | 7.980 | 7.926 |
| Ti | 0.011 | - | - | - | 0.006 |
| Al | 0.555 | 0.289 | 0.387 | 1.239 | 0.621 |
| Fe3+ | 1.470 | 1.711 | 1.511 | 0.716 | 1.501 |
| Fe2+ | 0.724 | 0.462 | 1.761 | 1.433 | 1.392 |
| Mn2+ | 0.129 | 0.357 | 0.306 | - | 0.033 |
| Mg | 2.019 | 2.106 | 1.030 | 1.597 | 1.388 |
| Ca | 0.035 | 0.088 | 0.115 | 0.110 | 0.112 |
| Na | 2.024 | 1.995 | 1.892 | 1.935 | 2.048 |
| K | - | - | 0.013 | - | 0.008 |
| Total | 14.978 | 15.003 | 15.010 | 15.010 | 15.035 |
* Recalculated, see details in text.
** Horokanai Ep blueschist.
Figure 5a illustrates the Al/Fe3+ ratios and distribution coefficient [KD = (Al/Fe3+)Namp/(Al/Fe3+)Napx] of coexisting Na pyroxenes and sodium amphiboles from the Hakoishi metacherts and metabasalts. KD equals 1.0 on average (0.5-2.0) in the metacherts and 4.0 (1.0-8.0) in the metabasalts. One exceptional metabasalt sample (OD112) contained pairs that displayed an equal distribution (KD ∼ 1.0). In the samples, Na pyroxene and sodium amphibole occurred in the veinlets, suggesting simultaneous nucleation and growth from fluids. This is unlike other metabasalts (Na pyroxene replaces igneous augite) and similar to metacherts.

The Fe2+/Mg distribution between Na pyroxene and chlorite (Fig. 6a) and between sodium amphibole and chlorite (Fig. 6b) in metabasalts of the Hakoishi subunit are examined. Despite intra-sample variability in the Fe2+/Mg ratio of Na pyroxene, likely due to low Fe2+ and Mg contents, Na pyroxene apparently exhibits a slight preference for Mg. In contrast, the nearly equal partitioning is observed in Fe2+/Mg ratios of chlorite and sodium amphibole.

Figures 5 and 6 present estimation errors (error bars) for microprobe analysis and two estimation methods discussed in the previous chapter. Droop’s method is inappropriate for high-grade sodium amphiboles with significant ANa contents, and therefore, was not included in Figures 5c and 5d.
Generally, XJd is controlled by metamorphic pressure through the equilibrium reaction Ab = Jd + Qz. In the Hakoishi rocks, the abundance of albite and quartz suggests that the albite association is a primary factor determining the upper limit of XJd. Maximum XJd values in each sample are comparable to those in relevant grade metabasalts and metacherts, and exhibit a general westward increase (Figs. 3b and 3d). Local variations in XJd within individual samples, in which XJd should be constant, may be attributed to differences in Al availability or effective variance.
While albite veins are abundant in metabasalts, they are less common in metacherts, and some metacherts even lack matrix albite, resulting in lower XJd. Comparing isophysical samples, the range of XJd is narrower in more-phased (lower variance) sample (KY1822; Qz + Ab + Lws + Napx + Hem) than in less-phased one (KY1819; Qz + Lws + Napx + Hem; Fig. 3b).
Impact of associated phases and igneous relics on XJdHowever, a significant decrease in XJd in sodium amphibole-bearing rocks compared to that in sodium amphibole-free rocks was observed in both metabasalts and metacherts (Fig. 3). In the equivalent grade, XJd was restricted to a lower and narrower range when sodium amphibole coexisted. However, westward upgrading in XJd was observed in sodium amphibole-bearing and sodium amphibole-free samples, respectively (Fig. 3). Similarly, the Al content in the sodium amphibole was slightly lower when Na pyroxene coexisted in both lithologies (Fig. 4).
Conversely, the assemblages of other metamorphic minerals, such as pumpellyite, lawsonite and epidote, or their Al/Fe3+ ratios, displayed no clear correlation with XJd. In addition, the oxidation states did not appear to be a prime factor owing to the Al contents in Na pyroxene and sodium amphibole being similar in the highly oxidized (OT10R, piemontite-bearing) and moderately oxidized samples (KY1815, howieite-bearing). This implies that the Al/Fe3+ partitioning between Na pyroxene and sodium amphibole primarily determines XJd.
Regarding Na pyroxene, the Hakoishi metacherts were characterized by the absence of igneous augite. This indicated that the Na pyroxene developed through nucleation and growth, in contrast to the replacement of igneous pyroxene in metabasalts. The higher content and westward decrease of Ca of Na pyroxenes in the metabasalts may be regarded as snapshots of the CaMg-NaAl substitution from the igneous precursor to various extents (Figs. 3a and 3c).
Al/Fe3+ partitioning between Na pyroxene and sodium amphiboleThe effect of the sodium amphibole coexistence on XJd is described as the result of competition between the two minerals for a limited amount of Al in the local effective bulk. The loss of one or two may have resulted in a lower Al content under coexistence. This rivalry is characterized by an Al/Fe3+ partition.
Onuki and Ernst (1969) introduced it into metasiliceous and metaclastic rocks of the blueschist and eclogite facies to obtain KD = 1.2. This indicates that the ion-to-ion exchange between the M2 site of sodium amphibole and the M1 site of Na pyroxene has only weak cation-preference. Okay (1980b) reported KD ∼ 2.1 from low-grade mafic and siliceous rocks (Tavşanlı Region, Northwest Turkey), with Al rather concentrated in sodium amphibole.
By definition (equation 2 in Onuki and Ernst, 1969), the distribution coefficient is determined by the equilibrium temperature. However, two different trends in KD were recognized within a single metamorphic unit. In the topotaxial growth texture, Al was concentrated in sodium amphibole, whereas nearly equal partitioning were observed in the modes where nucleation and growth were dominant. This demonstrates that the mineral growth process, rather than lithotypes or bulk composition, determines elemental partitioning.
These trends have also been observed in other blueschist regions. For instance, the Horokanai lawsonite/epidote-bearing blueschists (zone II/III of Shibakusa, 1989) in the Kamuikotan belt, Hokkaido, Japan, suffered a metamorphic temperature slightly higher (280-300 °C; Naemura et al., 2022) than that of the Hakoishi lawsonite blueschist. Despite the comparable peak metamorphic temperatures, KD was mostly equal to 1 (Fig. 5b). This can be attributed to the different rock fabrics. The Horokanai schists display penetrative schistosity and an uncommon occurrence of relict igneous pyroxene.
Furthermore, the documented coexistence of Na pyroxene (aegirine, jadeite, and omphacite of Morimoto et al., 1988) and sodium amphibole (BNa >1.75, Si >7.5 p.f.u.) was investigated. To exclude errors in the calculation method, Fe3+/Fetot was recalculated, as mentioned in the text, regardless of the estimates in each document.
Figure 5c compares the KD in the metamorphic rocks of the blueschist and eclogite facies from the Franciscan Complex. Significantly higher values (KD ∼ 4) were calculated for rocks in which Na pyroxene replaced relict augite (Brown and Ghent, 1983; Liou and Maruyama, 1987; Maruyama and Liou, 1987; Maruyama and Liou, 1988). Other blueschist facies rocks (Brown and Bradshaw, 1979; Patrick and Day, 1989) and eclogites (other references in Fig. 5c) display KD values close to 1.
The coexistence with other reports is shown in Figure 5d. Similarly, pairs of blueschist facies overgrown igneous augite or hornblende (Queyras, western Alps, Pognante and Kienast, 1987; Mikabu belt in Kanto mountains, Japan, Hirajima, 1985) show higher KD (∼ 4), compared to other rocks without such texture (∼ 1) (chlorite zone of the Sanbagawa belt in Shikoku, Sakaguchi and Ishizuka, 2008; Mn nodule from garnet zone in the Sanbagawa belt in Kanto Mountains, Hirajima, 1989; garnet-bearing epidote blueschist in Osayama mélange in Chugoku Mountains, Tsujimori and Liou, 2005). Eclogite facies rocks from Oman (El-Shazly et al., 1990; Searle et al., 1994) and the western Alps (Koons, 1986; Matsumoto and Hirajima, 2005) yielded a KD of ∼ 1.
Kinetic control on Na pyroxene compositionsIn summary, the XJd of Na pyroxene in a low-grade environment is restricted by Al availability in the effective bulk compositions. When sodium amphibole is present, competition lowers the Al content of both minerals. However, the outcome of this competition is influenced by growth mechanisms. Topotaxial growth leads to Al enrichment in sodium amphibole, while nucleation and growth processes result in more equitable Al distribution.
Thus, there is a kinetic preference for these elements. Morata et al. (1994) explained the different extents of topotaxial replacement based on the structural affinity between igneous and metamorphic pyroxenes. Ti-rich augite in transitional-alkaline metabasite is structurally closer to Na pyroxene than Ti-poor augite in tholeiitic metabasites and is extensively replaced. Analogously, the replacement of the metamorphic phase of Al-endmembers (Jd, Gln) and Fe3+-endmembers (Aeg, Mrbk) with igneous ones (Aug, Mhbl) is considered.
For simplicity, the unit cell parameters for the relevant members of the pyroxene and amphibole groups (data from Anthony et al., 1995) are considered. Comparing the Al- and Fe3+- endmembers, Aeg (unit cell volume: 429.1 Å3) is closer to Aug (432.5 Å3) in relation to Jd (401.2 Å3); conversely, Mrbk (895.9 Å3) and Gln (880.7 Å3) are structurally close to Mhbl (950.1 Å3) to a similar extent. A larger departure from the unit cells of the igneous phase translates into a larger distortion produced by topotaxial growth. Therefore, elemental partitioning occurred in the replacing fabric, whereas such constraints were not present during nucleation and growth. This could explain the strong Fe3+ preference of Na pyroxene for replacing igneous augite as opposed to the coexisting amphibole.
Fe2+-Mg partitioning between Na pyroxene and coexisting minerals in metabasalts of the Hakoishi subunit reveals a slight Mg-enrichment of Na pyroxene relative to coexisting chlorite (Fig. 6a), contrasting with the near-equal partitioning between chlorite and sodium amphibole (Fig. 6b). This trend is consistent with observations from lawsonite-bearing metabasites in Cazadero, California (Maruyama and Liou, 1988) and in the Chugoku Mountains (Tsujimori and Liou, 2007). Given the Mg-rich composition of the relic igneous pyroxenes, this implies a non-equilibrium state in the Fe2+-Mg partition in Na pyroxene replacing igneous relic phases.
All these parameters are in the standard state; thus, these kinetic effects are expected to be less pronounced at higher temperatures, where better equilibration is expected. Given the observed equal element partition (KD ∼ 1) seen in documented higher-grade rocks, the Al preference of replacing phases should have a high temperature limit near 300 °C.
Below this temperature, Na pyroxene developed by topotaxial growth may represent a metastable state by sluggish kinetics. In the quest for equilibrium in a clinopyroxene system, such a texture should be excluded. Consequently, the Na pyroxenes in the metachert samples provide promising examples for constructing a ternary phase diagram of Na pyroxene as they are virtually free of the influence of igneous pyroxenes.
We express our sincere gratitude to the reviewers, Dr. S. Endo and Prof. T. Tsujimori, for their constructive comments. We also thank Prof. S. Kumar for his helpful handling of this manuscript. Finally, we gratefully acknowledge the work of past members of our laboratory. This work was partly supported by Grant-in-Aid for Scientific Research (No. JP19H01999) and Bai Xian Asia Institute.