2025 Volume 120 Issue 1 Article ID: 241127L
2025 Volume 120 Issue 1 Article ID: 241127L
Listvenites and carbonated serpentinites are found in the shear zone of the Median Tectonic Line at the Urayama River, Shikokuchuo City, Ehime Prefecture, Japan. The listvenites are classified into schistose and massive types, and they mainly consist of quartz, magnesite, and dolomite, with minor amounts of Cr-bearing clay minerals. Furthermore, the listvenites contain chromite and small amounts of sulfide minerals, such as millerite and the gersdorffite-cobaltite series. The occurrence of schistose listvenites in the pelitic schist side through the shear zone is a characteristic feature of the listvenites from the Urayama River. The carbonated serpentinites consist of antigorite, magnesite, and quartz, exhibiting a texture in which the lath-shaped antigorite is partially replaced by magnesite. The protoliths of the massive and schistose listvenites are considered to be serpentinite and pelitic schist, respectively, based on their texture, Mg# of magnesite, presence of chromite, and whole-rock chemical compositions. The massive listvenites at the Urayama River are formed from serpentinites that reacted with fluids containing Ca, Sr, K, Na, and CO2. The fluids that reacted and were released from serpentinites, containing Ca and CO2, enriched in Mg, Cr, Ni, and Co, likely interacted with the pelitic schist through the shear zone, producing the schistose listvenite.
Listvenites (also spelled listwanites or listwaenites) are green rocks formed by the metasomatic reaction of an ultramafic protolith, such as serpentinite or peridotite, with CO2-bearing fluids (Halls and Zhao, 1995). It primarily consists of carbonates and quartz and contains minor amounts of sulfide minerals (Halls and Zhao, 1995; Hansen et al., 2005). The carbonates in the listvenites are generally magnesite or dolomite. The Fe content of magnesite varies along the magnesite-siderite solid solution series. In many cases, listvenites contain Cr-bearing clay minerals, such as Cr-bearing muscovite (fuchsite), which imparts their characteristic green color. However, Menzel et al. (2024) treated listvenites as fully carbonated ultramafic rocks in a broad sense and did not consider Cr-bearing muscovite as a required phase in contrast to Halls and Zhao (1995). The number of studies on listvenites in relation to forearc mantle wedges and ophiolites has increased significantly over the last two decades, and its importance in modulating deep carbon cycling has been widely recognized (Hansen et al., 2005; Kelemen et al., 2011; Menzel et al., 2022; 2024; Oyanagi and Okamoto, 2024). In Japan, there are several localities where listvenites are found. The localities are Yukinoura, Nagasaki Prefecture (Mori et al., 2007); Tobe River, Omote River, Shikogawa River, Sekigawa River, and Urayama River near the Median Tectonic Line (MTL); Ochiai and Kogo River near the Kiyomizu Tectonic Line, Ehime Prefecture (Minakawa et al., 2008; Shirose et al., 2022); Izunma, Shizuoka Prefecture (Suzuki et al., 1977); and Itoigawa region, Niigata Prefecture (Ogawara, 2022). We found listvenites with distinct occurrences and properties, coexisting with carbonated serpentinites from the shear zone of the MTL at the Urayama River, Shikokuchuo City, Ehime Prefecture, Japan. In this paper, we report the mineralogical properties of the listvenites and serpentinites and discuss the mobility of elements in CO2-bearing fluids.
We found listvenites and carbonated serpentinites from the Urayama River near the MTL, along a shear zone within the pelitic schists, with the metamorphic grade corresponding to the garnet zone in the Sanbagawa metamorphic belt (Figs. 1a and 1b; Aoya et al., 2017). The listvenites are classified into three types based on their occurrences: schistose listvenite, massive listvenite I, and massive listvenite II (Figs. 1c-1f). The schistose listvenite has a light-green color and occur in the pelitic schists along the schistosity without a distinct boundary (Figs. 1 and 2a). The massive listvenites occur as discontinuous layers between the pelitic schists and serpentinites and have a clear boundary with the pelitic schist (Figs. 1e and 1f). The width of the massive listvenite ranged from 10 to 50 cm. Massive listvenite I is dark-green and consists of fragments (Fig. 2b). Massive listvenite II is lime-green and is macroscopically homogeneous (Fig. 2c). Additionally, massive listvenite II occurs in serpentinites as a 10-cm-wide vein. Sulfide minerals, such as millerite, are macroscopically observed in the green parts of each type of listvenite, approximately 0.5 mm in size. The serpentinite body is approximately 3 m in width, and it is carbonatized and silicified (serpentinites I, II, and III). Carbonate veins are abundant in the serpentinites, and a mingling texture of listvenite and serpentinite is observed at the boundary (Fig. 2d). The degree of carbonation in the serpentinites varies with the distance from the listvenites, and the specimen exhibits a dark-green serpentine-enriched part and a highly carbonated lime-green part (Fig. 2e).


The powder X-ray diffraction (XRD) data were collected using a Rigaku Ultima IV with CuKα radiation at 40 kV and 40 mA at Ehime University. Additionally, the oriented and ethylene-glycolated samples of clay minerals were analyzed using XRD after elutriation. The chemical analyses of the minerals were conducted using a JEOL JSM-6510LV scanning electron microscope equipped with an Oxford Instruments X-Max50 energy-dispersive spectrometer at Ehime University. Quantitative analyses were performed at an accelerating voltage of 15 kV, with a beam current of 0.8 nA and 60-s acquisition time. The whole-rock major and trace element compositions of five samples were determined using a Rigaku ZSX Primus II X-ray fluorescence spectrometer at Ehime University. The analytical procedure followed the methods described by Suda and Saito (2018) and Shimooka et al. (2025). For the homogeneous samples, massive listvenite II and serpentinite I, we prepared powders from samples approximately 5 × 2 cm in size and 30-40 g in weight, and 1.5 g of the powdered sample was heated. Thereafter, 0.9 g of the heated powder sample was used to create 1:5 glass beads, and Merck Spectromelt A12 (LiBO2 34%, Li2B4O7 66%) was utilized to prepare the glass beads. For the heterogeneous samples, the schistose listvenite and massive listvenite I, we roughly crushed samples of similar size (5 × 2 cm) and weight (30-40 g); thereafter, we selected the green fragments, excluding large white carbonate veins and chromite-enriched black parts. Powders were created from the green fragments, and 1.5 g of the powdered sample was heated. Subsequently, 0.9 g of the heated powder sample was used to create 1:5 glass beads, and Merck Spectromelt A12 was utilized to prepare the glass beads. The standard materials were measured both before and after the sample measurement to ensure reliability. The relative errors of the major elements (exceeding 0.05 wt%) and the trace elements (exceeding 30 ppm) remained below 10%, excluding those of P2O5 and Th.
The results of the XRD experiments indicated that the listvenites mainly consisted of quartz, magnesite, and dolomite, with minor amounts of clay minerals (Table 1). Furthermore, the listvenites contain small amounts of sulfide minerals, such as millerite, cobaltite, and gersdorffite (schistose listvenite), and chromite (massive listvenite I). Regarding the texture of the listvenites, the schistose listvenite shows a vein-like texture composed of quartz, magnesite, and dolomite (Fig. 3a). Massive listvenite I consists of green aggregates enriched in quartz, black aggregates enriched in chromite, and white veins enriched in magnesite (Figs. 2b and 3b). The quartz and magnesite are cut by dolomite. In contrast to massive listvenite I, massive listvenite II has a homogeneous texture and consists of lath-shaped magnesite and fine-grained quartz (Figs. 2c and 3c). In the green part of the listvenites, Cr-bearing clay minerals, which are the cause of their green color, are observed. The clay minerals are Cr-bearing kaolinite (50-500 µm in length, in the schistose listvenite), Cr-bearing muscovite (approximately 25 µm in length, in massive listvenite I), and Cr-bearing montmorillonite (approximately 5 µm in length, in massive listvenites I and II). The Cr-bearing kaolinite is coarse-grained, whereas Cr-bearing muscovite and montmorillonite are fine-grained and occur in aggregates of fine-grained quartz. In the transition part between massive listvenite II and serpentinite I, the listvenite part contains magnesite, quartz, antigorite, and minor amounts of the Cr-bearing montmorillonite (Fig. 2d; Table 1). The presence of residual antigorite indicates that listvenitization has not yet been completed (Fig. 3d). The serpentinite part contains antigorite, magnesite, quartz, dolomite, and clinochlore (Table 1). The antigorite is mixed with submicron-sized quartz (Fig. 3e).
| Rock name | Type | Constituent minerals | |||||||||
| Qz | Mgs | Dol | Ab | Atg | Kln | Mnt | Ms | Tlc | Chr | ||
| Pelitic schist | ◎ | ○ | ○ | △ | △ | ||||||
| Listvenite | Schistose | ◎ | △ | ○ | △ | ||||||
| Massive I | ○ | ◎ | △ | △ | ○ | ||||||
| Massive II | ◎ | ◎ | △ | ||||||||
| Listvenite with serpentinite | Lime-green part | ○ | ◎ | △ | △ | ||||||
| Dark-green part | ○ | ○ | △ | ◎ | △ | ||||||
| Serpentinite | I | △ | △ | ◎ | |||||||
| II | △ | △ | ◎ | △ | |||||||
| III | ◎ | △ | |||||||||
Qz, quartz; Mgs, magnesite; Dol, dolomite; Ab, albite; Atg, antigorite; Kln, kaolinite; Mnt, montmorillonite; Ms, muscovite; Tlc, talc; Chr, chromite.

Serpentinites I, II, and III primarily consist of antigorite and partially coexist with magnesite, which formed through carbonation. Further, with the carbonation and the decomposition of antigorite, the serpentinites undergo silicification; serpentinites I and II contain quartz, whereas serpentinites II and III contain talc (Table 1). The degree of alteration due to the carbonation and silicification gradually decrease from serpentinite I to III, with significant amounts of antigorite remaining in some areas. In serpentinites located farther from the listvenites, the lath-shaped antigorite is partially replaced by magnesite at the grain boundaries (Fig. 3f). The antigorite in serpentinite I, near the boundary, contains Fe2+ = 0.31-0.32, Al = 0.14-0.21, and Cr = 0.02-0.04 (apfu). In serpentinite II, distant from the listvenite, the composition of the antigorite was Fe2+ = 0.26-0.31, Al = 0.03-0.15, and Cr = 0.01-0.05 (apfu), with the Al content varying significantly. Additionally, the serpentinites contain chromite, millerite, cobaltite, and gersdorffite.
The serpentinites and massive listvenite I contain chromite with a compositionally distinct core-rim texture (Fig. 4). The Mg# [= Mg/(Mg + Fe2+)] of the chromite cores in serpentinite I was 0.2-0.3, with the rims at 0.0-0.1 (Table 2). In massive listvenite II, the core values range from 0.1-0.4, with the rims at 0.0-0.1. The Cr# [= Cr/(Cr + Al)] of the chromite cores in serpentinite I is 0.5-0.6, with the rims at 0.7-0.9. In massive listvenite II, the core values are 0.5-0.6, with the rims at 0.7-0.9. The rims of chromite contain less Mg and Al and more Ti and Fe3+ than the cores. In the serpentinites, the chromite rims are penetrated by antigorite, whereas in massive listvenite I, they are penetrated by quartz and magnesite. The chemical trends of chromite in serpentinite I and massive listvenite I are similar (Fig. 4c). However, these chemical compositions differed from those of Cr-spinel in the serpentinites and dunites from the Sanbagawa Belt (Tasaka et al., 2008; Hattori et al., 2010; Kawahara et al., 2016; Hirauchi et al., 2021). The rim of the chromite was formed during antigorite formation, whereas the core was formed at an earlier stage. The primary chromite, which remained as the core, could have crystallized from more fractionated magmas than those of the other ultramafic bodies in the Sanbagawa Belt.
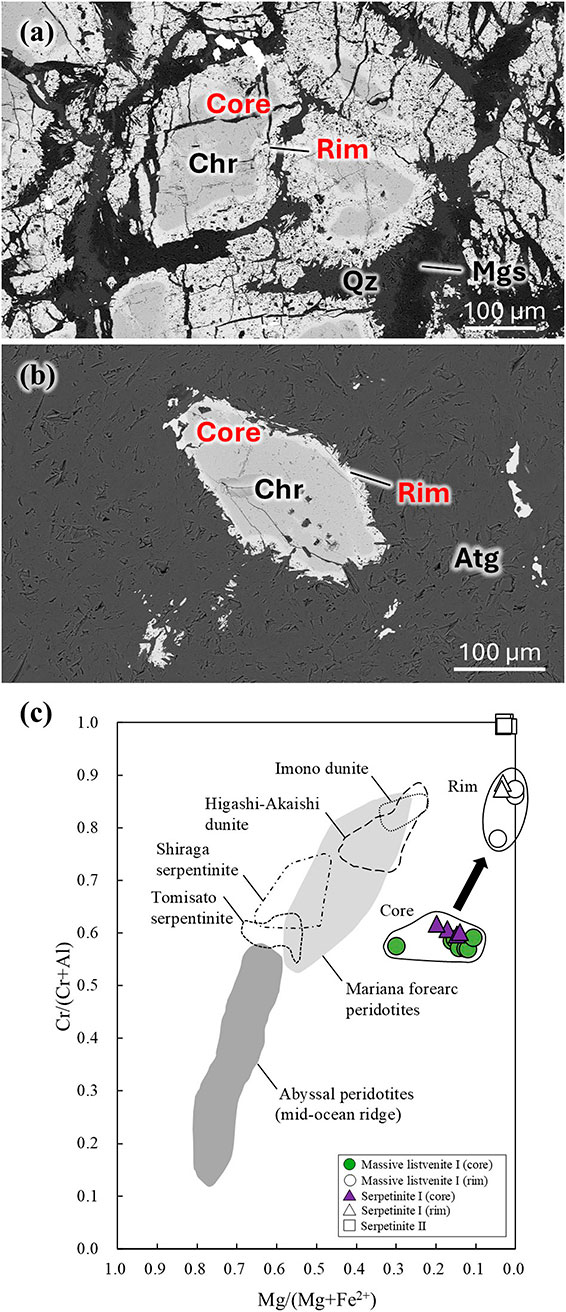
| Rock name | Massive listvenite I | Serpentinite I | ||
| Mineral | Chromite | Chromite | ||
| Core | Rim | Core | Rim | |
| n = 8 | n = 5 | n = 4 | n = 2 | |
| TiO2 | 0.40 | 3.26 | 0.05 | 3.27 |
| Cr2O3 | 39.10 | 36.31 | 42.41 | 38.21 |
| Al2O3 | 19.22 | 4.44 | 18.57 | 3.53 |
| Fe2O3* | 7.54 | 27.70 | 6.38 | 27.27 |
| FeO* | 27.91 | 24.58 | 27.56 | 23.51 |
| MgO | 2.91 | 0.14 | 3.06 | 0.46 |
| MnO | 0.94 | 1.55 | 0.80 | 1.41 |
| ZnO | 0.73 | 0.58 | 1.22 | 1.16 |
| Total | 98.75 | 98.56 | 100.61 | 98.75 |
| O = 4 | O = 4 | |||
| Fe2+ | 0.79 | 0.75 | 0.77 | 0.72 |
| Mg | 0.15 | 0.01 | 0.15 | 0.02 |
| Mn | 0.03 | 0.05 | 0.02 | 0.04 |
| Zn | 0.02 | 0.02 | 0.03 | 0.03 |
| Ti | 0.01 | 0.09 | 0.02 | 0.09 |
| ΣA | 1.01 | 1.01 | 1.01 | 0.99 |
| Cr | 1.04 | 1.05 | 1.11 | 1.10 |
| Al | 0.76 | 0.19 | 0.73 | 0.15 |
| Fe3+ | 0.19 | 0.76 | 0.16 | 0.75 |
| ΣB | 1.99 | 2.00 | 2.00 | 2.00 |
| Mg/(Mg + Fe2+) | 0.16 | 0.01 | 0.16 | 0.03 |
| Cr/(Cr + Al) | 0.58 | 0.85 | 0.60 | 0.88 |
* FeO and Fe2O3 were calculated by stoichiometry.
The average Mg# of the antigorite in the serpentinites (0.90) is comparable to that of magnesite, with values of 0.87 in the serpentinites, 0.89 in massive listvenite I, and 0.87 in massive listvenite II (Table 3). In contrast, the magnesite in the schistose listvenite exhibits a wide range and a relatively low average value (0.66) of Mg#, with some analytical points corresponding to siderite compositions. These results indicates that the magnesite in the massive listvenites directly derive from the antigorite in the protolith serpentinite. This is consistent with the presence of chromite in massive listvenite I, which exhibited chemical zoning similar to that of chromite in serpentinite I. The textures of the lath-shaped magnesite and fine-grained quartz in massive listvenite II derive from the replacement of the lath-shaped antigorite in the serpentinite. The protolith of the schistose listvenite is interpreted as a pelitic schist based on its schistose texture and the difference in the Mg# of magnesite compared with that in the serpentinites.
| Rock name | Schistose listvenite |
Massive listvenite I |
Massive listvenite II |
Serpentinite I | Serpentinite II | |||
| Mineral | Magnesite | Magnesite | Magnesite | Antigorite | Magnesite | Antigorite | Magnesite | |
| Core | Rim | |||||||
| n = 3 | n = 21 | n = 14 | n = 19 | n = 27 | n = 6 | n = 18 | n = 6 | |
| SiO2 | - | 0.46 | 0.62 | 0.41 | 42.91 | 7.60 | 43.63 | 1.60 |
| TiO2 | - | 0.03 | - | - | - | - | - | - |
| Cr2O3 | - | 0.10 | 0.03 | 0.04 | 0.41 | 0.18 | 1.13 | - |
| Al2O3 | - | 0.35 | 0.02 | 0.07 | 1.77 | 0.36 | 0.68 | 0.09 |
| MgO | 37.45 | 29.50 | 37.14 | 36.79 | 35.13 | 36.01 | 34.75 | 37.22 |
| FeO | 10.46 | 20.58 | 8.09 | 9.58 | 7.13 | 9.95 | 7.03 | 9.97 |
| CaO | 0.29 | 0.47 | 0.35 | 0.80 | 0.02 | 0.26 | - | 0.24 |
| NiO | - | 0.04 | - | - | - | - | 0.17 | 0.09 |
| CO2* | 47.53 | 46.59 | 46.74 | 47.40 | - | 57.38 | - | 49.44 |
| H2O* | - | - | - | - | 12.66 | - | 12.67 | - |
| Total | 95.74 | 98.37 | 92.99 | 95.13 | 100.03 | 111.74** | 100.06 | 98.64 |
| O = 1 | O = 1 | O = 1 | O = 7 | O = 1 | O = 7 | O = 1 | ||
| Si | - | 0.01 | 0.01 | 0.01 | 2.03 | 0.10 | 2.06 | 0.02 |
| Ti | - | 0.00 | - | - | - | - | - | |
| Cr | - | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.03 | - |
| Al | - | 0.01 | 0.00 | 0.00 | 0.10 | 0.01 | 0.06 | 0.00 |
| Mg | 0.86 | 0.69 | 0.87 | 0.85 | 2.48 | 0.69 | 2.45 | 0.82 |
| Fe2+ | 0.13 | 0.28 | 0.11 | 0.12 | 0.28 | 0.11 | 0.28 | 0.12 |
| Ca | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | - | 0.00 |
| Ni | - | 0.00 | - | - | - | - | 0.01 | 0.00 |
| CO2* | 1.00 | 1.00 | 1.00 | 1.00 | - | 1.00 | - | 1.00 |
| OH* | - | - | - | - | 4.00 | - | 4.00 | - |
| Mg/(Mg + Fe2+) | 0.87 | 0.71 | 0.89 | 0.87 | 0.90 | 0.87 | 0.90 | 0.87 |
* CO2 and H2O were calculated by stoichiometry; CO2 = 1 apfu and OH = 4 apfu.
** The total value exceeds 100 because the magnesite partially contains quartz.
The mass transfer during the listvenite formation was simulated by the isocon method (Grant, 1986) using the whole-rock compositions of the listvenites, pelitic schist, and serpentinite (Fig. 5; Table 4). The chemical compositions of the pelitic schist and serpentinite I (CO) were used as protoliths, whereas those of the listvenites were treated as altered rocks (CA). Al2O3 was assumed to be an immobile element, as considered in prior studies (Mori et al., 2007; Godard et al., 2021). This assumption is consistent with the reaction in which Cr-bearing clay minerals were formed through the decomposition of Al- and Cr-bearing antigorite. The 1:1 reference line on the isocon diagrams represents zero mass change during alteration. The isocons defined by Al2O3 are below the reference line, indicating the overall mass gain during the alteration. The elements plotted above and below the isocons are enriched and depleted, respectively, in the altered rock compared with those of the protolith. Compared with the pelitic schist, the schistose listvenite shows an increase in MgO, CaO, Cr, Ni, Co, and loss on ignition (LOI) and a decrease in K2O and Na2O. Compared with serpentinite I, massive listvenite I shows an increase in CaO, K2O, Na2O, and Sr, whereas massive listvenite II shows an increase in CaO, Na2O, and Sr. For the LOI of the massive listvenites, the amount of CO2 increase, whereas that of H2O decrease because of the dehydration of the antigorite and the formation of magnesite. The listvenitization is generally characterized by changes in major elements and the enrichment of fluid-mobile trace elements such as K, Li, Ba, Cs, Sr, Pb, As, and Sb (Godard et al., 2021; Menzel et al., 2024). These fluid-mobile trace elements were incorporated in the massive listvenites.
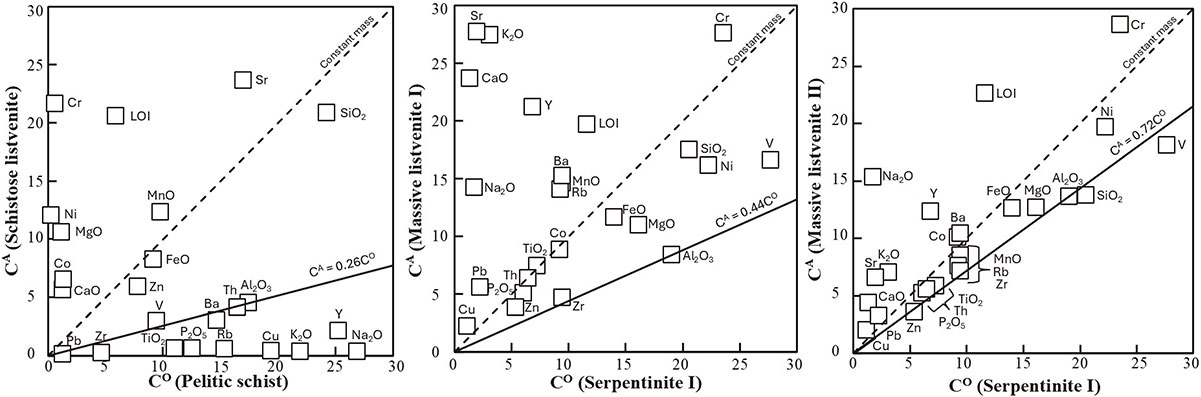
| Pelitic schist | Schistose listvenite | Massive listvenite I |
Massive listvenite II |
Serpentinite I | |
| Major element (wt%) | |||||
| SiO2 | 60.65 | 52.20 | 35.00 | 27.51 | 41.08 |
| TiO2 | 0.74 | 0.04 | 0.02 | 0.02 | 0.02 |
| Al2O3 | 17.43 | 4.51 | 0.84 | 1.37 | 1.90 |
| FeO** | 4.58 | 4.12 | 5.82 | 6.33 | 6.99 |
| MnO | 0.10 | 0.12 | 0.15 | 0.09 | 0.09 |
| MgO | 1.17 | 10.60 | 21.94 | 25.43 | 32.30 |
| CaO | 1.25 | 5.65 | 5.93 | 1.10 | 0.34 |
| Na2O | 2.69 | 0.03 | 0.03 | 0.03 | 0.00 |
| K2O | 3.65 | 0.06 | 0.07 | 0.02 | 0.01 |
| P2O5 | 0.13 | 0.01 | 0.01 | 0.01 | 0.01 |
| LOI | 5.89 | 20.58 | 27.04 | 34.58 | 13.41 |
| Total | 98.29 | 97.93 | 96.84 | 96.47 | 96.16 |
| Trace element (ppm) | |||||
| V | 94.5 | 29.6 | 16.6 | 18.1 | 27.7 |
| Cr | 58.5 | 2163.5 | 2761.1 | 2861.1 | 2357.1 |
| Co | 13.5 | 65.2 | 88.3 | 101.0 | 92.1 |
| Ni | 27.8 | 1204.2 | 1616.0 | 1968.9 | 2224.7 |
| Cu | 38.8 | 0.8 | 5.6 | 5.1 | 2.9 |
| Zn | 78.0 | 59.0 | 38.7 | 36.1 | 54.0 |
| Rb | 153.6 | 5.7 | 7.0 | 3.8 | 4.6 |
| Sr | 169.9 | 236.5 | 277.5 | 65.9 | 19.8 |
| Y | 25.3 | 2.1 | 2.1 | 1.2 | 0.7 |
| Zr | 187.2 | 8.9 | 4.7 | 7.2 | 9.4 |
| Nb | 13.8 | 0.0 | 0.0 | 0.0 | 0.2 |
| Ba | 586.3 | 120.7 | 152.5 | 104.7 | 94.4 |
| Pb | 13.1 | 1.4 | 1.4 | 0.8 | 0.6 |
| Th | 16.5 | 4.1 | 6.4 | 5.6 | 6.5 |
* LOI, loss on ignition.
** Total iron as FeO.
All types of listvenites and serpentinites from the Urayama River are characterized by the presence of the cobaltite-gersdorffite series, which sometimes overgrows chromite. The chemical compositions of gersdorffite and cobaltite in the three types of listvenites and serpentinites are plotted on an Fe-Co-Ni triangular diagram to estimate the formation temperatures, incorporating isothermal lines based on Klemm (1965) (Fig. 6; Supplementary Table S1; Table S1 is available online from https://doi.org/10.2465/jmps.241127L). Resultantly, the cobaltite in the massive listvenites and serpentinites exhibits similar chemical compositions, indicating their formation at 500-600 °C. These temperatures corresponded to those of the serpentinization process for forming antigorite. However, the cobaltite-gersdorffite series in the schistose listvenite and gersdorffite in serpentinite I exhibits a wide range of Co-Ni substitutions. These trends may correspond to a Co-Ni continuous solid solution without an immiscibility gap above 500 °C. Alternatively, the occurrence of gersdorffite near the end-member composition may imply crystallization at low temperatures below 300 °C and Ni enrichment (relative to Co) in the fluid, based on the absence of cobaltite near the end-member and the frequent occurrence of millerite during late-stage processes.

Furthermore, the listvenites from the Urayama River are characterized by the presence of Cr-bearing clay minerals. They occur primarily as Cr-bearing montmorillonites in the massive listvenites (or, in specific cases, Cr-bearing muscovite in massive listvenite I) within fine-grained quartz aggregates and as Cr-bearing kaolinites in the schistose listvenite. The reactions of the listvenite were completed at 100-150 °C for the massive listvenites (at 300 °C for a restricted area of massive listvenite I) and at 100-200 °C for the schistose listvenite. This is based on the formation temperatures of the clay minerals (Hedenquist and Arribas, 2022).
Formation processes and origins of the listvenitesWe conclude that the massive listvenites at the Urayama River were formed from the serpentinites that reacted with a fluid containing Ca, Sr, K, Na, and CO2. In particular, massive listvenite II has the texture of lath-shaped magnesite with quartz and a similar whole-rock chemical composition to the serpentinite, excluding Ca, Sr, K, Na, and CO2. Therefore, magnesite with an Mg# similar to that of the serpentinite and quartz was formed by the dehydration of antigorite, as shown in the following chemical equation: 2 Mg48Si34O85(OH)62 [Antigorite] + 96 CO2 = 96 MgCO3 [Magnesite] + 68 SiO2 [Quartz] + 62 H2O. This reaction involved high CO2 concentrations by fast fluid replenishment, resulting in the direct formation of magnesite and quartz from serpentine, rather than the formation of talc (Menzel et al., 2024). Fine-grained Cr-bearing clay minerals, such as Cr-bearing muscovite and montmorillonite, were derived from the decomposition of the Fe-, Al-, and Cr-bearing antigorite. The fluids reacted and were released from the antigorite, containing Ca and CO2, enriched in Mg, Cr, Ni, and Co, and interacted with the pelitic schist through the shear zone. The Cr-bearing kaolinite was formed through the alteration of albite and muscovite in the pelitic schist. The presence of sulfarsenides and clay minerals suggests that the reaction that produced the listvenites from the Urayama River started at 500-600 °C, and it was completed at 100-150 °C. For the carbonation of serpentinite in the Sanbagawa metamorphic belt, an ophicarbonate (carbonate-bearing serpentinite) associated with the pelitic schist, near the boundary between the garnet and chlorite zones, has been reported from the Higuchi serpentinite body (Okamoto et al., 2021). The carbonation of the serpentinite occurred at approximately 400 °C, facilitated by the CO2-rich fluid derived from metasediments. Similarly, from the Nishisonogi metamorphic belt, Mori et al. (2007) reported the formation of the massive listvenite resulting from a reaction between the serpentinite and pelitic schist, which is comparable to that of the garnet zone of the Sanbagawa metamorphic belt, at temperatures exceeding 350 °C. However, unlike these rocks, the reaction zones consisting of chlorite and talc are not observed between the serpentinite and the pelitic schist at the Urayama River. The occurrence of schistose listvenites in the pelitic schist side through the shear zone is a characteristic feature of the listvenites from the Urayama River, and this is contrary to previous reports. The reports of the listvenite in the pelitic schist are restricted to localities near the MTL (Suzuki et al., 1977; Minakawa et al., 2008; Shirose et al., 2022). The massive listvenite from Izunma contains Cr-bearing smectites formed at low temperatures (Suzuki et al., 1977). Additionally, the schistose listvenite with Cr-bearing kaolinites, associated with carbonated serpentinite, has been reported from the Omotegawa River (Shirose et al., 2022). The large-scale listvenites in Japan occur in association with a small serpentinite body embedded in the pelitic schist, with the metamorphic grade corresponding to the garnet zone. In contrast, near the MTL, the schistose listvenite is widely observed in the pelitic schist corresponding to low metamorphic grades, the chlorite zone, and does not coexist with a substantial serpentinite body. The widespread distribution of the listvenite in the Sanbagawa Belt indicates that the serpentinite blocks within pelitic schists, derived from a shallow mantle wedge, reacted with CO2-rich fluids. The fluid was released from the carbonated serpentinite or the rock body migrated along the shear zone and reacted with pelitic schists in relatively shallow parts. The structural and chemical variations of the Cr-bearing clay minerals and Co-Ni sulfides indicate that these reactions span a wide P-T range. The origin and reaction mechanism of the CO2-rich fluids remain to be determined. Further investigations into the distribution and characterization of the listvenites around the MTL, as well as an analysis of the composition and origin of the fluids using fluid inclusions and isotope analysis, are required.
We thank Y. Ichiyama for editorial handling and for providing constructive comments. We also thank the anonymous reviewers for their thoughtful and constructive reviews. We are grateful to S. Saito, Department of Earth Sciences, Graduate School of Science and Engineering, Ehime University, and K. Shimooka, Department of Applied Chemistry for Environment, School of Science and Technology, Kwansei Gakuin University, for their support in conducting the whole-rock chemical analyses using XRF. We also thank S. Enju, Department of Earth Sciences, Graduate School of Science and Engineering, Ehime University, for the valuable discussions.
Supplementary Table S1 is available online from https://doi.org/10.2465/jmps.241127L.