2025 年 19 巻 1 号 論文ID: cr.2024-0088
2025 年 19 巻 1 号 論文ID: cr.2024-0088
Objective: There are only a few reports about flow diverter (FD) placement for large thrombosed aneurysms of the middle cerebral artery (MCA). We present a case of FD placement for a recurrent large thrombosed aneurysm of MCA at our hospital.
Case Presentation: A 72-year-old man with transient visual field disturbance underwent craniotomy for a large aneurysm in the left MCA; dome clipping was performed because of severe arteriosclerosis. Over several years, the residual aneurysm gradually increased in size, and despite antiplatelet therapy, the patient experienced repeated cerebral infarctions due to intra-aneurysmal thrombosis. A closer examination revealed that the M2 superior trunk was occluded. Thus, we performed FD placement, without further complications. After 6 months, the aneurysm was confirmed to be occluded with an O’Kelly–Marotta grading scale (OKM grade) of D. There was no enlargement of the thrombus inside the aneurysm, and the patient is currently under follow-up observation.
Conclusion: FD placement may be an option for large thrombosed aneurysms of MCA that are difficult to treat with conventional methods.
Although the indications for flow diverter (FD) placement are gradually expanding and becoming more widespread across Japan, FD placement is less commonly performed for middle cerebral artery (MCA) aneurysms due to their often complex anatomy and the need for more peripheral manipulation. Additionally, 1 reason for this is that surgical clipping is relatively easier. There are only a few reports on FD placement for large thrombosed aneurysms of MCA worldwide.1) We present a case of FD placement for a recurrent large thrombosed aneurysm of MCA at our hospital.
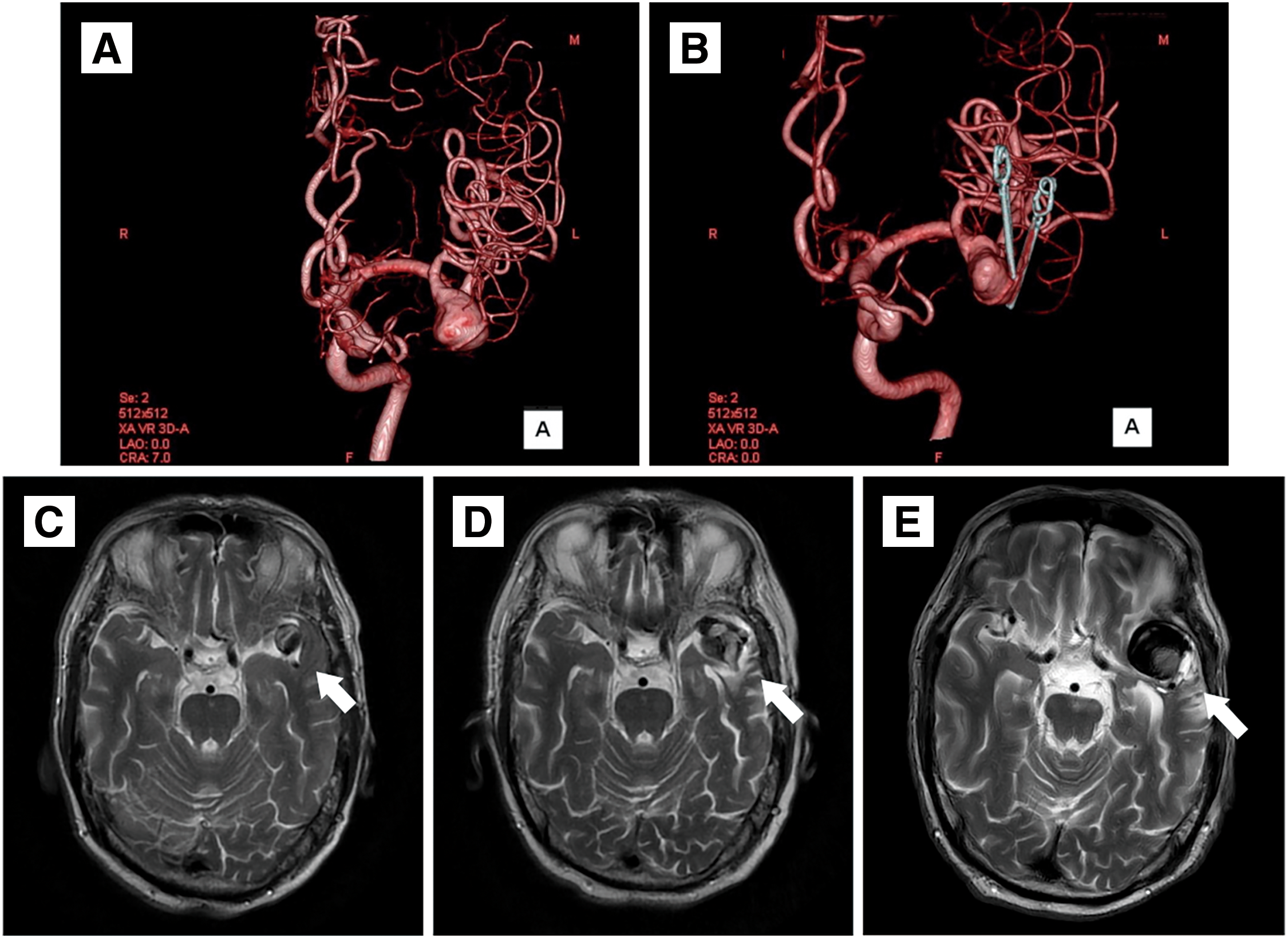
A 72-year-old man was referred to our hospital for a left MCA large aneurysm on MRI, which was associated with transient left visual field disorder. Angiography revealed a 15-mm diameter aneurysm (Fig. 1A). The MCA aneurysm was clipped partially, due to the presence of significant arteriosclerosis (Fig. 1B). The residual aneurysm gradually increased in size (Fig. 1C–1E). Two years after the clipping, he started taking 100 mg/day aspirin because of amaurosis fugax on the left side. Four years after the clipping, he developed cerebral infarction in the left MCA territory and was treated at a different hospital (Fig. 2A and 2B). A closer MRI revealed thrombosis within the aneurysm, leading to a diagnosis of cerebral embolism due to thrombus in the aneurysm. The patient was also added with clopidogrel 75 mg/day. Eight years after the clipping, another stroke occurred in the left MCA territory, resulting in dysarthria and motor aphasia (Fig. 2C and 2D). Because the aneurysm continued to gradually increase in size, the patient was referred to our hospital 8 years after the clipping. Cerebral angiography revealed the large left MCA aneurysm with a maximum dome diameter of 13 mm (Fig. 3A and 3B), and the left M2 superior trunk was occluded. The maximum dome diameter of the aneurysmal measured on MRI was 27 mm, and the majority of the aneurysm was thought to be thrombosed (Fig. 3C). Although the patient did not show symptoms related to the mass effect of the thrombosed aneurysm, repeated cerebral infarctions developed due to intra-aneurysmal thrombosis, despite dual antiplatelet therapy (DAPT), indicating the need for surgical intervention. The left M2 had only 1 inferior trunk due to occlusion and had developed into a sidewall aneurysm, which made FD placement more favorable. Therefore, FD placement was performed in the same year.

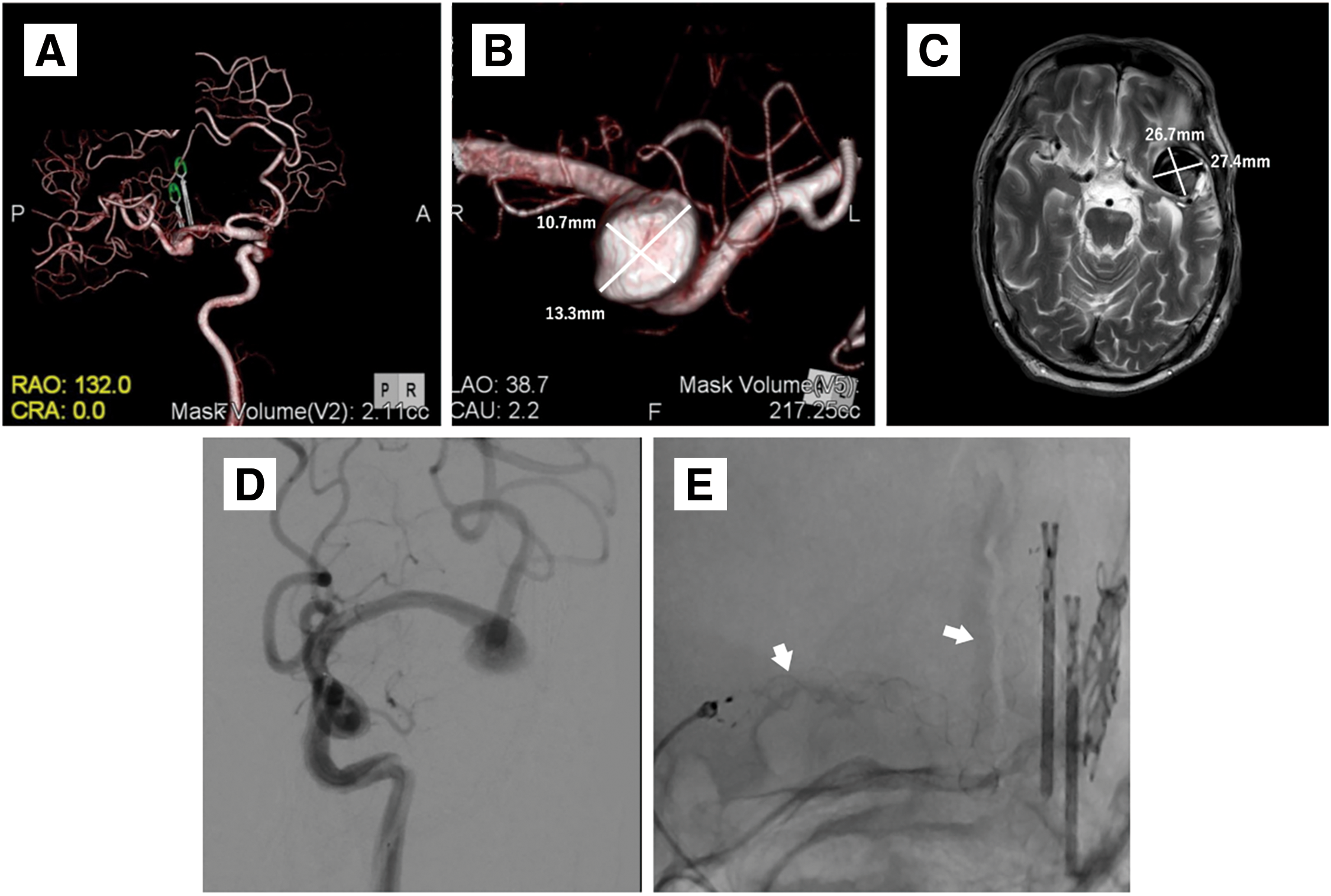
Endovascular treatment: Clopidogrel (75 mg/day) was replaced with prasugrel (3.75 mg/day) 2 weeks before endovascular treatment. Under general anesthesia, a 5 Fr guiding sheath (Fubuki; Asahi Intecc, Aichi, Japan) was introduced after puncturing the right radial artery. Intravenous heparin sodium of 4000 units was administered, and the activated clotting time was controlled to more than 200 seconds. A combination of a 5F SOFIA (Terumo, Tokyo, Japan), Headway27 (Terumo), and Synchro select soft (Stryker, Kalamazoo, MI, USA) was used to navigate from the left internal carotid artery (ICA) to the left M2. A FRED flow diverter (Terumo) 4.0–44 mm was deployed from the left M2 inferior trunk. The FRED was anchored at the M2 segment, and the system pull technique was used to obtain the shortest possible course within the mass and deployed at the proximal M1 segment to the aneurysm (Fig. 3D and 3E). After deployment of the FD, cone beam CT was performed using a 5-fold diluted contrast medium to evaluate the apposition of the FD. The endovascular procedure was terminated after confirming good FD apposition. The patient was discharged on postoperative day (POD) 4 without any complications.
Post-discharge course: MRI was performed 1 month and 1 year after discharge, but no significant change was observed, including in the size of the thrombosed aneurysm (Fig. 4A and 4B). Angiography was performed 6 months after the surgery, and complete occlusion of the aneurysm O’Kelly–Marotta grading scale (OKM) Grade D was confirmed (Fig. 4C and 4D). Comparing the vascular lumen diameter inside the FD immediately after placement and 6 months later, the lumen was reduced in diameter in the proximal, mid, and distal portions of the FD, suggesting endothelialization of the device (Fig. 5A and 5B). Thus, the antiplatelet agent was reduced to a single dose of prasugrel 3.75 mg/day alone. The patient will be followed up periodically by MRI every 3 months.


Although the indications for FD placement have gradually expanded and FD placement is already a standard treatment for ICA aneurysms,2) aggressive FD placement is rarely performed for MCA aneurysms because they are often anatomically complex and require more peripheral manipulation. Additionally, the relative ease of surgical clipping is one reason for this. According to a meta-analysis of FD placement for aneurysms in the peripheral anterior circulation,3) distal anterior cerebral artery (dACA) aneurysms, anterior communication artery (Acom) aneurysms, and MCA aneurysms with OKM grade C and D occlusion in dACA, Acom, and MCA were 82%, 88%, and 78%, respectively, and the complication rates were 9%, 8%, and 18%, respectively, indicating significantly worse outcomes in MCA aneurysms than dACA and Acom aneurysms. According to a meta-analysis focused on FD placement in MCAs,4) the rate of OKM grade C or D occlusion was 78%, while the rate of procedure-related complication was up to 20.7%. The most common complications were ischemic (16.3%), perianeurysmal inflammation (2.6%), hemorrhage (2%), and arterial dissection/perforation (1.8%). Because 10% of patients had permanent residual symptoms, they concluded that FD placement for MCA aneurysms should be considered only when conventional treatment is not possible because of the high risk of complications. Some reports also suggest that FD placement is not appropriate for MCA aneurysms because of the high number of complications and low occlusion rate.5) By contrast, some articles suggest that FD placement for MCA aneurysms is a safe treatment with satisfactory outcomes when limited to the M1 segment and small aneurysms.6–8) There are few reports of FD placement for large MCA aneurysms and even fewer cases where FD placement alone was used to treat thrombosed aneurysms worldwide. In endovascular treatment, even after complete thrombosis of the aneurysm, blood flow from the vasa vasorum to the arterial wall persists, allowing the thrombosed aneurysm to continue growing. Endovascular treatment alone is challenging because blocking blood flow into the aneurysm lumen may not be sufficient to halt its growth.9) There is a report that 77% of patients treated with coil embolization alone for partially thrombosed aneurysms had a recurrence.10) According to reports on the use of FD placement for thrombosed aneurysms, the complete occlusion rate was 77% and aneurysm enlargement was observed in 6.3% of cases, concluding that FD placement is effective for thrombosed aneurysms.11) However, aneurysms with more than 50% intraluminal thrombus prior to treatment have lower rates of complete occlusion, potentially due to the inflammatory response induced by the thrombus, which delays endothelialization of the FD. In the present case as well, the aneurysm before treatment was mostly thrombosed, suggesting that achieving complete occlusion would be difficult; however, complete occlusion was ultimately obtained over 6 months. Although the use of FD placement has been rapidly increasing in recent years, we could not find any reports on FD placement for thrombosed MCA aneurysms except those performed in conjunction with bypass-assisted surgery.1) In the reported case, a side-to-side anastomosis was performed to the peripheral MCA, followed by occlusion of 1 of the 2 M2 branches, converting the bifurcation aneurysm into a sidewall aneurysm. In our case, the M2 superior trunk was occluded spontaneously due to cerebral infarction and had already transformed into sidewall aneurysm, which is considered one of the factors contributing to the favorable thrombosis observed.
According to the literature on endothelialization following FD placement, the diameter of the remodeled artery showed a 15% reduction compared to its size immediately after the placement.12) Angiography before and after the placement showed reduced vessel diameter in all areas: the central, aneurysm neck, and distal parts of the FD placement site. This suggests that the endothelialization of the FD occurred, potentially contributing to the favorable thrombosis achieved after FD placement, despite the presence of a large thrombus in the aneurysm. Although FD placement under special circumstances requires careful monitoring of patient progress, FD placement for large thrombosed aneurysms of the MCA may be a treatment option, depending on the individual case.
We reported a case of a large thrombosed MCA aneurysm successfully treated by FD placement, which suggests that FD placement may be a good option for large thrombosed MCA aneurysms in limited cases.
Conceived and designed the analysis: Yusuke Tanaka, Naoyuki Noda, Noriaki Sekiguchi, Raisa Funatsuya, and Masato Tsuchimochi. Data collection: Yusuke Tanaka and Koji Suzuki. Contributed data/analysis tools: Yusuke Tanaka, Yasuhiro Uriu and Shin Tanino. Performed the analysis: Yusuke Tanaka and Kosuke Miyahara. Wrote the paper: Yusuke Tanaka.
Data availabilityThe datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical approvalNot applicable.
Consent for participationInformed consent for participation was obtained from the patient.
Consent for publicationWritten informed consent was obtained from the patient to publish this case report.
Disclosure statementThe authors declare that they have no conflicts of interest.