Abstract
Objective: A hyperplastic anterior choroidal artery (AchA) is a rare anomalous vessel that perfuses the posteromedial aspects of the cerebrum in place of the posterior cerebral artery. We describe 3 cases of hyperplastic AchA found among 61 patients with AchA aneurysms who underwent surgical or endovascular treatment at our institution.
Case Presentation: All 3 cases were diagnosed as hyperplastic AchA type 2 according to the Takahashi classification, indicating an anomalous branching temporal artery perfusing the medial temporal lobe. We performed coil embolization for 2 cases and surgical clipping for the third. One embolization case experienced recurrence after 3 years and underwent clipping. All procedures were conducted without complications.
Conclusion: Hyperplastic AchA can be encountered during aneurysm treatment. These cases emphasize the importance of evaluating the vascular anatomy to determine the optimal treatment strategy.
Introduction
The anterior choroidal artery (AchA) is an important structure impacted by distal internal carotid artery (ICA) aneurysms. Understanding the specific AchA anatomy in each case is essential for avoiding complications. The AchA is reported to have rare anomalies of perfusion territory and origin,1,2) which are often misidentified as the posterior communicating artery (PComA) or posterior cerebral artery (PCA). While the incidence of AchA aneurysms in the general population is as low as 0.4%–0.5%, 4%–17% of hyperplastic AchA cases are accompanied by aneurysm,1–4); hyperplastic AchA is therefore considered a risk factor for aneurysm formation. In this study, we report 3 cases of hyperplastic AchA among 61 patients who underwent surgical or endovascular treatment for AchA aneurysms at our institution between January 2015 and June 2024. This study was approved by the ethics committee of the local Institutional Review Board (approval number ONH23021).
Case Presentation
Case 1
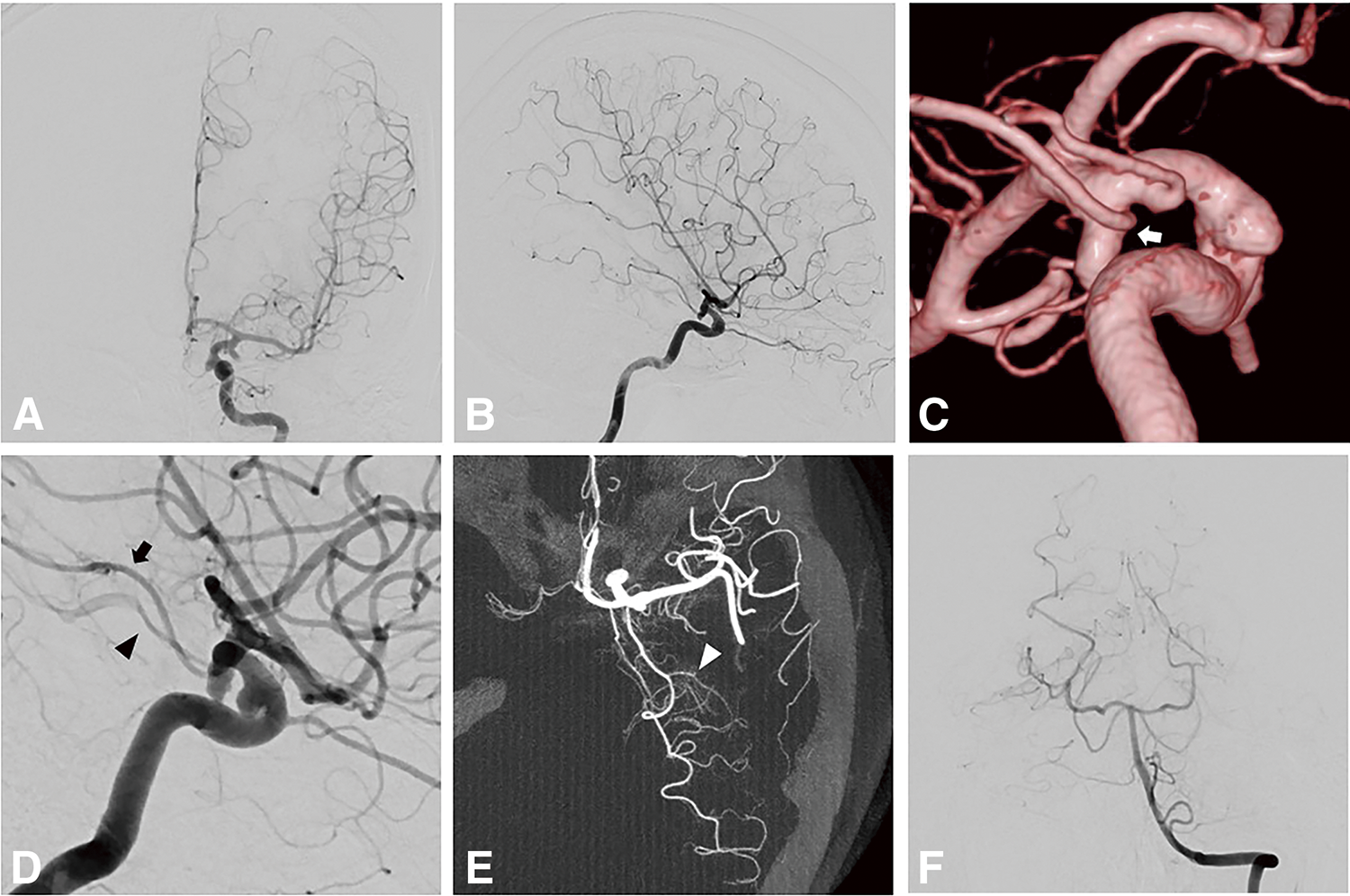
A 37-year-old man was incidentally diagnosed with an AchA aneurysm after presenting with headaches. Left internal carotid angiography revealed a 5.1 mm AchA aneurysm. The diameter of the AchA was 1.3 mm, branching from the aneurysmal neck to supply the medial temporal lobe and choroid plexus (Fig. 1A–1F). Vertebral arteriography revealed that the left PCA did not branch into the anterior and posterior temporal artery; the Alcock test showed that the PComA branched from the ICA proximal to the AchA (Fig. 1G and 1H). Based on these findings, the AchA was diagnosed as hyperplastic with an anomalous temporal artery. Coil embolization was performed under motor-evoked potential (MEP) monitoring. The aneurysm was obliterated with a slight neck remnant, and the AchA was preserved (Fig. 1I). The patient had no postoperative neurological symptoms. A 3-month follow-up MRA showed no recurrence.
Case 2
A 44-year-old man was diagnosed with a left AchA aneurysm at the age of 26. Follow-up indicated that the aneurysm was growing. Left internal carotid angiography showed a 5.4 mm aneurysm with the hyperplastic AchA supplying the medial temporal lobe and choroid plexus (Fig. 2). The diameter of the AchA was 1.1 mm, and the PComA branched from the proximal side of the AchA aneurysm. Surgical clipping was performed under MEP monitoring without any postoperative complications. There was no recurrence at 1-year follow-up.
Case 3
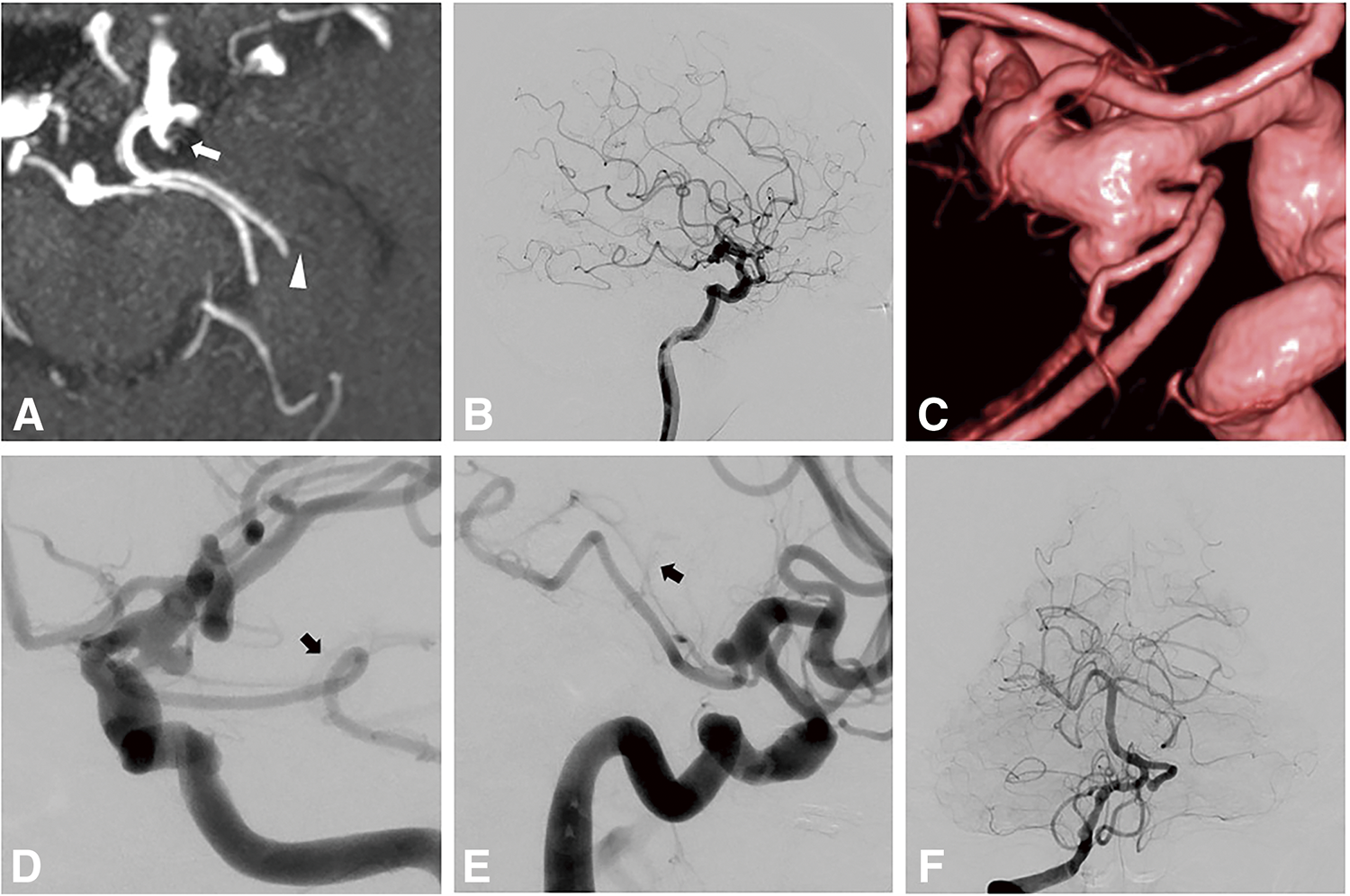
A 77-year-old man was diagnosed with aneurysms of the right middle cerebral artery, anterior communicating artery, and left AchA through MRI. Imaging also revealed 2 arteries branching from the left ICA and perfusing the posteromedial aspect of the left cerebrum (Fig. 3A). Left internal carotid angiography revealed a 3.7 mm AchA aneurysm, with the hyperplastic AchA supplying the medial temporal lobe and choroid plexus (Fig. 3B–3E). Vertebral angiography showed that the left PCA lacked temporal branches (Fig. 3F). The PComA branched from the proximal side of the AchA aneurysm. After surgical clipping of the right middle cerebral and anterior communicating artery aneurysms, coil embolization was performed for the left AchA aneurysm following consultation with the patient. Based on the preoperative angiographic evaluation, the diameter of the AchA was 1.9 mm and the AchA was branched from the aneurysm. It seemed to be difficult to preserve the AchA using a balloon catheter placed in the ICA for remodeling. A balloon catheter (TransForm occlusion balloon catheter; Stryker Neurovascular, Fremont, CA, USA) was inserted into the AchA for remodeling (Fig. 4A–4F) to preserve the AchA. Complete obliteration was achieved without neurological complications, and the AchA was preserved (Fig. 4G). Three years later, recurrence resulting from enlargement of the AchA aneurysm was observed (Fig. 4H). Surgical clipping was performed without complications.
Discussion
Hyperplastic AchA is classified into 4 types by Takahashi et al. according to its perfusion area: anomalous anterior temporal artery (type 1), anomalous temporal artery (type 2), anomalous occipito-parietal artery (type 3), and anomalous temporo-occipito-parietal artery (type 4); the frequencies of these types are 20%, 60%, 8%, and 12%, respectively.1) The presented cases each had anomalous temporal arteries that supplied the medial temporal lobe and were thus classified as type 2, the most common form of hyperplastic AchA.
During embryonic development, the AchA perfuses the posteromedial cerebral hemisphere before regressing and being replaced by the development of the PComA and posterior circulatory system.5) Hyperplastic AchA is a remnant of this cortical branch and shows a similar distribution to the PCA; it is often misidentified as a posterior anomaly, such as a fetal-type or duplicated PComA. However, hyperplastic AchA has a choroidal branch and must be distinguished from the PCA.
The frequency of hyperplastic AchA on MRI or cerebral angiography is reported to be 0.55%–2.3%,1,4,6) and 4%–17% of these cases are associated with aneurysms. Kang et al. observed hyperplastic AchA in 7% of AchA aneurysms treated with coil embolization.7) In this study, we include cases treated with surgical clipping as well as coil embolization. Hyperplastic AchA was found in 3 (4.9%) of 61 cases of AchA aneurysm. This result is consistent with former reports and suggests that hyperplastic AchA is more likely to be present in patients with AchA aneurysms than in the general population. However, there have only been 17 reports of hyperplastic AchA with aneurysm (Table 1)1,4,5,8–17) and treatment strategies for associated aneurysms have not yet been discussed in the literature. In this study, coil embolization was performed in 2 patients, 1 of whom had a recurrence despite complete occlusion being achieved with a balloon-assisted technique. Youn et al. also reported recurrence after coil embolization of a hyperplastic AchA aneurysm.15) Insufficient embolization due to concerns about ischemic complications may increase the risk of recurrence. In addition, the large vessel diameters typical of hyperplastic AchA may be a risk factor for recurrence, as is the case with fetal-type PComA aneurysms.1,18)
Table 1 Cases of hyperplastic AchA aneurysms
| Case no. |
Author (Year) |
Age |
Sex |
Takahashi type |
Ruptured/Unruptured |
Treatment |
Complication |
Outcome (GOS) |
| 1 |
Takahashi M (1980)4) |
43 |
Female |
2 |
N/A |
N/A |
N/A |
N/A |
| 2 |
Takahashi S (1990)1) |
N/A |
N/A |
2 |
Ruptured |
Clipping |
Asymptomatic AchA occlusion |
GR |
| 3 |
Abrahams JM (1999)5) |
37 |
Female |
3 |
Ruptured |
Clipping |
None |
GR |
| 4 |
Matsumoto K (2000)8) |
55 |
Female |
3 |
Unruptured |
Clipping |
None |
GR |
| 5 |
Shioya H (2005)9) |
64 |
Female |
2 |
Unruptured |
Clipping |
None |
GR |
| 6 |
Okazaki T (2011)10) |
18 |
Male |
3 |
Ruptured |
Coiling |
None |
GR |
| 7 |
Aoki Y (2013)11) |
56 |
Male |
2 |
Unruptured |
Clipping |
None |
GR |
| 8 |
Hyun DK (2014)12) |
35 |
Male |
2 |
Ruptured |
Coiling |
Cerebral infarction |
MD |
| 9 |
Doi K (2018)13) |
N/A |
N/A |
3 |
N/A |
N/A |
N/A |
N/A |
| 10 |
Mitsuhashi T (2023)14) |
48 |
Male |
3 |
Ruptured |
Coiling |
None |
GR |
| 11 |
Youn S (2024)15) |
38 |
Male |
2 |
Ruptured |
Coiling → Clipping |
None |
GR |
| 12 |
Tatsuta Y (2024)16) |
60 |
Female |
2 |
Unruptured |
Wrapping |
None |
GR |
| 13 |
Tatsuta Y (2024)16) |
78 |
Female |
3 |
Unruptured |
Coiling |
None |
GR |
| 14 |
Saito K (2023)17) |
80 |
Female |
1 |
Ruptured |
Coiling |
None |
SD |
| 15 |
Present case 1 |
36 |
Male |
2 |
Unruptured |
Coiling |
None |
GR |
| 16 |
Present case 2 |
44 |
Male |
2 |
Unruptured |
Clipping |
None |
GR |
| 17 |
Present case 3 |
77 |
Male |
2 |
Unruptured |
Coiling → Clipping |
None |
GR |
AchA, anterior choroidal artery; GOS, Glasgow Outcome Scale; GR, good recovery; MD, moderate disability; N/A, not available; SD, severe disability
On the other hand, the large diameters of the hyperplastic AchA enabled us to use various adjunctive techniques. Because the average diameter of a normal AchA is 0.75 mm,1) placing a catheter can induce ischemic complications. However, in case 3, for example, the 1.9 mm diameter of the AchA and the position of the aneurysmal neck allowed the balloon catheter to be inserted for remodeling without ischemic complications. There have been reports of PcomA aneurysms where balloon catheters were inserted into the PcomA for neck remodeling, successfully preserving the incorporated branch.19,20) This technique has the possibility of the injury of the vessel or its perforator due to the advancement of the balloon catheter and inflation of the balloon.19) But no complications related to the procedure have been reported. Since the AchA has important perforating branches, we paid considerable caution to manipulation of the balloon catheter and ensured that blood flow was maintained after insertion of the balloon catheter. The treatment resulted in successful preservation of the AchA and achieved complete occlusion of the aneurysm.
In our cases, the average diameter of the hyperplastic AchA was 1.4 mm, ranging from 1.1–1.9 mm. Previous studies have reported the safety of coil embolization with stents in small vessels,21) suggesting the diameter of the hyperplastic AchA may be large enough to accommodate a low-profile stent. Stent-assisted coil embolization may therefore be a viable option for the treatment of hyperplastic AchA aneurysm. However, there are no previous comparable reports on the diameters of hyperplastic AchAs. Measurement of the diameter of the AchA is important to determine the treatment strategy, including adjunctive techniques.
Conclusion
Although AchA anomalies are rare, hyperplastic AchA is encountered with a higher relative incidence during the treatment of AchA aneurysms. The recognition of this anomaly is important in determining treatment strategies for AchA aneurysms. Hyperplastic AchA may be a risk factor for recurrence after coil embolization for AchA aneurysms, but various adjunctive techniques may be applicable depending on the diameter of the hyperplastic AchA.
Acknowledgments
We thank Wilf Gardner, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- 1) Takahashi S, Suga T, Kawata Y, et al. Anterior choroidal artery: angiographic analysis of variations and anomalies. AJNR Am J Neuroradiol 1990; 11: 719–729.
- 2) Otomo E. The anterior choroidal artery. Arch Neurol 1965; 13: 656–658.
- 3) Nakagawa T, Hashi K. The incidence and treatment of asymptomatic, unruptured cerebral aneurysms. J Neurosurg 1994; 80: 217–223.
- 4) Takahashi M, Arii H, Tamakawa Y. Anomalous arterial supply of temporal and occipital lobes by anterior choroidal artery: angiographic study. AJNR Am J Neuroradiol 1980; 1: 537–540.
- 5) Abrahams JM, Hurst RW, Bagley LJ, et al. Anterior choroidal artery supply to the posterior cerebral artery distribution: embryological basis and clinical implications. Neurosurgery 1999; 44: 1308–1314.
- 6) Uchino A, Saito N, Takahashi M, et al. Variations of the posterior cerebral artery diagnosed by MR angiography at 3 tesla. Neuroradiology 2016; 58: 141–146.
- 7) Kang HS, Kwon BJ, Kwon OK, et al. Endovascular coil embolization of anterior choroidal artery aneurysms. Clinical article. J Neurosurg 2009; 111: 963–969.
- 8) Matsumoto K, Akagi K, Abekura M, et al. Cerebral aneurysm associated with an anomalous hyperplastic anterior choroidal artery. Acta Neurochir (Wien) 2000; 142: 347–350.
- 9) Shioya H, Kikuchi K, Suda Y, et al. Ruptured internal carotid -posterior communicating artery aneurysm associated with an anomalous hyperplastic anterior choroidal artery and aneurysm: case report. No Shinkei Geka 2005; 33: 155–162.
- 10) Okazaki T, Shibukawa M, Kiura Y, et al. Endovascular coil embolization for ruptured aneurysm associated with persistent primitive anterior choroidal artery—case report—. Neurol Med Chir (Tokyo) 2011; 51: 127–129.
- 11) Aoki Y, Endo H, Niizuma K, et al. Efficacy of fusion image for the preoperative assessment of anatomical variation of the anterior choroidal artery. No Shinkei Geka 2013; 41: 1075–1080.
- 12) Hyun DK, Shim YS, Park HS, et al. Thromboembolic complication following neurointervention in ruptured anomalous hyperplastic anterior choroidal artery aneurysm. Neuroradiol J 2014; 27: 103–107.
- 13) Doi K, Mizuno T, Shigematsu Y, et al. A new type of hyperplastic anterior choroidal artery. J Clin Neurosci 2018; 51: 72–74.
- 14) Mitsuhashi T, Oishi H, Teranishi K, et al. Ruptured anomalous hyperplastic anterior choroidal artery aneurysm: a case report. Br J Neurosurg 2023; 37: 296–297.
- 15) Youn S, Park SK, Kim MJ. Coil embolization and recurrence of ruptured aneurysm originating from hyperplastic anterior choroidal artery. J Cerebrovasc Endovasc Neurosurg 2024; 26: 181–186.
- 16) Tatsuta Y, Endo H, Ogino T, et al. Internal carotid artery-persistent primitive anterior choroidal artery aneurysms: report of two cases and literature review. Acta Neurochir (Wien) 2024; 166: 94.
- 17) Saito K, Nogawa H, Shibao S, et al. Ruptured cerebral aneurysm related to variants of the origin and perfusion area of the anterior choroidal artery: a case report. Nosotchu No Geka 2023; 51: 161–166 (in Japanese).
- 18) Choi HH, Cho YD, Yoo DH, et al. Impact of fetal-type posterior cerebral artery on recanalization of posterior communicating artery aneurysms after coil embolization: matched-pair case-control study. J Neurointerv Surg 2020; 12: 783–787.
- 19) Nariai Y, Takigawa T, Hyodo A, et al. Double-balloon-assisted coiling for wide-necked posterior communicating artery aneurysms with a fetal-type variant of the posterior cerebral artery: a case series. Neurointervention 2022; 17: 183–189.
- 20) Chen Z, Niu Y, Tang J, et al. Endovascular treatment of posterior communicating artery aneurysms in the presence of the fetal variant of posterior cerebral artery. Interv Neuroradiol 2015; 21: 456–461.
- 21) Ozaki T, Fujinaka T, Kidani T, et al. Coil embolization of unruptured cerebral aneurysms using stents in small arteries less than 2 mm in diameter. Neurosurgery 2022; 90: 538–546.