2025 年 19 巻 1 号 論文ID: tn.2024-0105
2025 年 19 巻 1 号 論文ID: tn.2024-0105
Objective: LEONIS Mova high-flow type (SB-KAWASUMI LABORATORIES, Kanagawa, Japan) is a steerable microcatheter that enables angle adjustment of the catheter tip using a hand-operated dial, and available as a coaxial microcatheter system with a 1.6-F microcatheter. It was used to navigate an occluded inferior petrosal sinus (IPS) in a patient with cavernous sinus (CS) dural arteriovenous fistula (AVF).
Case Presentation: A man in his 50s presenting with right eye congestion was diagnosed with dural AVF of the CS with bilateral occluded IPSs. The shunted pouch was located in the medial-lateral part of the right CS, with drainage into the superior ophthalmic vein. Transvenous embolization (TVE) from the femoral vein via the occluded IPS was performed. A 6-F guiding catheter was navigated to the right internal jugular vein, and LEONIS Mova high-flow type combined with a 1.6-F Carnelian MARVELS S microcatheter (Tokai Medical Products, Aichi, Japan) was navigated to the occluded IPS. The LEONIS Mova successfully engaged the IPS, and its tip was fixed by adjusting the curve. The microcatheter advanced smoothly into the right CS. After releasing the fixation of the LEONIS Mova, the microcatheter was advanced further into the CS, where the LEONIS Mova was once again fixed. The microcatheter was easily navigated to the shunted pouch, and targeted coil embolization of the shunted pouch, achieving complete occlusion of the dural AVF.
Conclusion: The LEONIS Mova steerable catheter offers flexible angle adjustment and strong support for catheter navigation within an occluded IPS during TVE for CS-dural AVF.
The standard therapeutic strategy for cavernous sinus (CS) dural arteriovenous fistula (AVF) is transvenous embolization (TVE) via the inferior petrosal sinus (IPS), which serves as a primary drainage route in many cases.1) However, in cases of CS-dural AVF, the IPS is frequently occluded. Even in such cases, TVE via the IPS is often the first-line treatment, but an angiographically invisible IPS presents challenges in catheter navigation due to difficulty visualizing its anatomy. Selecting an appropriate catheter for navigation is also difficult, though the penetration of an occluded IPS is feasible, with success rates ranging from 54% to 90%.1–3) It is crucial to support the torque of the guidewire at an appropriate angle corresponding to the course of the IPS to navigate the device through an occluded IPS.
The LEONIS Mova (SB-KAWASUMI LABORATORIES, Kanagawa, Japan) is a steerable catheter that allows for angle adjustments of up to 180° over 15 mm of the catheter tip via a proximal dial operation. The LEONIS Mova comes in 3 types: selective, standard, and high-flow with outer diameters of 2.0F–2.4F, 2.4F–2.6F, and 2.9F–2.9F, respectively. The high-flow type is available as a double coaxial microcatheter system with a 1.6-F microcatheter designed to navigate detachable coils (Fig. 1).4,5) By guiding the LEONIS Mova high-flow through an occluded IPS and adjusting its tip angle for optimal positioning, the catheter can be expected to facilitate microcatheter navigation into the CS. This report presents a case where the LEONIS Mova was effective in the TVE of a CS dural AVF involving an occluded IPS.

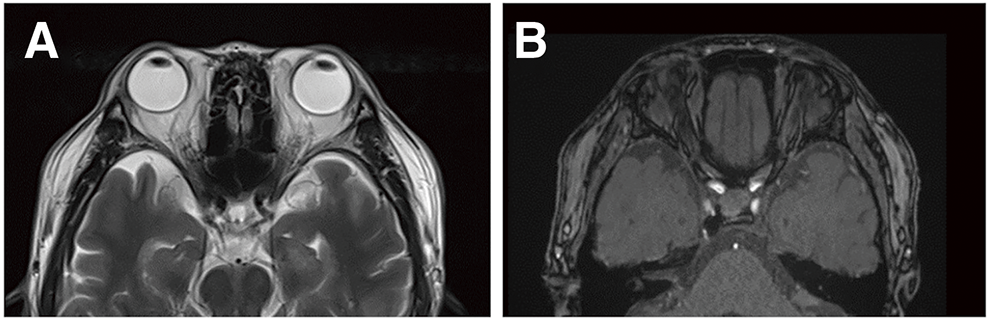
A man in his 50s with well-controlled diabetes presented with right eye congestion that had developed 1 week earlier. Ophthalmological examination revealed a visual acuity of 1.2 in the right eye (1.0 in the left) with no impairment of extraocular movement. The intraocular pressure (IOP) in the right eye was 27 mmHg (left 17 mmHg), and conjunctival congestion was noted in the right eye. MRI of the head showed 22 mm of right eye proptosis, dilatation of the intraorbital veins, and detection of the right CS (Fig. 2A and 2B). Diagnostic cerebral angiography revealed a right CS dural AVF fed by both internal carotid arteries, the right accessory meningeal artery, and the CS branch of the middle meningeal artery. The shunted pouch was located in the medial-lateral portion of the right CS, with drainage into the superior ophthalmic vein (SOV) (Fig. 2C–2F). Neither IPS was contrasted. Endovascular TVE via both IPSs was planned.

Under general anesthesia, heparin was administered to maintain an activated clotting time of 200 seconds. A 4-F sheath was deployed in the right femoral artery, and a 4-F catheter was navigated for angiography. Two 6-F Fubuki guiding sheaths (Ashahi Intecc., Aichi, Japan) were deployed into the bilateral femoral veins, navigating both internal jugular veins (IJV). Initially, a 0.035-inch Radifocus guidewire (Terumo, Tokyo, Japan) and a 0.035-inch Silverway guidewire (Asahi Intecc.) were used as part of the frontier-wire technique to attempt navigation of the right IPS via the right IJV using a 3.2-F Tactics Plus intermediate catheter (120 cm; Technocrat, Aichi, Japan), but neither wire advanced into the IPS. Subsequently, the same devices were used via the left IJV to attempt advancement into the left IPS, but this also failed.
Next, a 2.9-F LEONIS Mova (high-flow type; SB-KAWASUMI LABORATORIES) steering catheter (130 cm) combined with a 1.6-F Carnelian MARVELS S (155 cm; Tokai Medical Products, Aichi, Japan) and a 0.014-inch CHIKAI black 14 soft tip (Ashahi Intecc.) was guided from the right IJV into the IPS. The guidewire and microcatheter advanced into the occluded IPS, followed by the LEONIS Mova, which was navigated into the IPS by manually adjusting the angle of its tip, and its tip was fixed by adjusting the curve (Fig. 3A). The guidewire and microcatheter advanced smoothly into the right CS. After releasing the fixation of the LEONIS Mova, the microcatheter was advanced further into the CS, where the LEONIS Mova was once again fixed (Fig. 3B). The guidewire and microcatheter were advanced past the common ophthalmic vein, maneuvered into the shunt pouch. A video of the selective navigation into the IPS is included as Supplementary Video. 3D angiography confirmed the LEONIS Mova's position just proximal to the CS, while the microcatheter tip was located within the shunt pouch (Fig. 3C and 3D). Eight coils, including a 0.014-inch Optimax Complex Soft (5 mm–10 cm; Balt, Montmorency, France) and seven 0.012-inch i-ED Complex Infini coils (Kaneka Medics, Osaka, Japan; total length 46 cm), were embolized into the shunt pouch (Fig. 3E). Angiography revealed no remaining arteriovenous shunt, and the reflux of the SOV was not detected (Fig. 3F).

Post-operatively, the patient’s proptosis and conjunctival congestion completely resolved within 1 week. At 6 months post-procedure, the IOP had normalized, and no recurrence was observed. MRI at 6 months post-procedure showed no signs of proptosis (Fig. 4).
The steerable microcatheter has a flexible, manually controlled tip and 2 steering wires arranged within the catheter wall, running from the handle to the distal tip. This type of catheter has been used in abdominal interventions since 2014 to deliver or administer embolic materials, drugs, or contrast agents into peripheral vessels.6,7) Tokuyama et al. reported the use of the SwiftNINJA steerable microcatheter (Sumitomo Bakelite, Tokyo, Japan; the same product as LEONIS Mova) in 13 cases of dural AVF, employing a high-flow type microcatheter with a coaxial microcatheter (Carnelian MARVELS S; Tokai Medical Products) in 12 procedures. Three of these were cases of CS dural AVF treated with TVE, all of which were successfully treated.4) The steerable microcatheter received government approval and reimbursement for neuroendovascular procedures in Japan in November 2022. Suzuki et al. reported the successful use of the LEONIS Mova selective (SB-KAWASUMI LABORATORIES) in cases of giant internal carotid aneurysms with severe curves to navigate into the middle cerebral artery and deploy flow diverters.5)
In the field of neurointervention, the Bendit21 catheter (Bendit Technologies, Petach Tikva, Israel) has been used internationally as a steerable catheter.8–10) The Bendit21 catheter has an outer diameter of 3.1 Fr (1.03 mm, 0.041 inch) and an inner diameter of 0.53 mm (0.021 inch), and can be used without a guidewire and allows for the passage of stents, flow diverter stents, and coils. In a prospective, multicenter clinical study conducted by Killer-Oberpfalzer et al., the Bendit21 was used in 25 patients, including 14 with aneurysms, 2 with arteriovenous malformations/fistulas, 1 with a stroke, 4 with intracranial stenosis, and 4 with other conditions. Bendit21 was used without a guidewire in 12 out of 25 (48.0%) procedures. Therapeutic devices were successfully delivered as intended with the Bendit21 in 14 out of 18 cases (77.8%); however, 4 deficiencies occurred in 3 patients with aneurysms where coils, an intrasaccular device, or a flow diverter were attempted. There were no device-related safety events or mortalities.8) Qiao et al. retrospectively reviewed the use of Bendit21 in 10 neurointerventions (2 aneurysms, 4 acute ischemic strokes, 1 intracranial stenosis, 1 trauma, 1 venous thrombosis, and 1 arteriovenous malformation) and reported no complications. They concluded that the high torque transmission and acute angulation of the microcatheter might improve access to difficult proximal curves and the anatomy of the distal clinoidal/ophthalmic segment.9) However, reports of the use of steerable catheters in dural AVFs are still rare.4)
In the treatment of CS-dural AVF, the IPS is the standard access route to the CS for TVE. However, in 41%–56% of CS-dural AVF cases, the IPS is angiographically occluded.1–3) In cases where the IPS is angiographically occult, access to the CS via the IPS is attempted using a 0.035-inch guidewire as part of the frontier-wire technique, known as the “kurukuru method” in Japan. The guidewire is then traced into the opened IPS. Additionally, the microwire looping technique, which breaches the occluded IPS with support from a guiding system, is also helpful.11) In this method, the intermediate catheter is fixed at the IPS outlet to provide stable support.
However, the IPS has many anatomical variations,12–15) and in cases where the IPS is angiographically invisible, it is difficult to predict the IPS anatomy. As the wire and catheter are guided from the internal jugular vein side into the IPS, the anatomy of the IPS becomes clearer. However, if the catheter angle is incorrect while guiding into the CS, the torque from the wire and catheter may not transmit effectively, making it difficult to enter the CS. When using the LEONIS Mova high-flow catheter together with the Carnelian MARVELS S catheter and wire, it is possible to adjust and fix the angle of the LEONIS Mova to the ideal position while advancing through the IPS. As the Carnelian MARVELS S advances, the angle of the LEONIS Mova can be released temporarily, allowing it to follow distally, and then adjusted and fixed again. This process stabilizes the control of the Carnelian MARVELS S, the wire, microcatheter navigation, and coil deployment. In this case, the catheter was easily guided to the shunted pouch, and targeted TVE was achieved.
This study is a single case report, and there are few reports on the safety and efficacy of the LEONIS Mova in TVE for dural AVFs, highlighting the need for larger scale prospective studies.
The LEONIS Mova steerable catheter (SB-KAWASUMI LABORATORIES) offers flexible angle adjustments and strong support for guiding catheters through the IPS in cases of CS-dural AVF with an occluded IPS during trans-IPS TVE. This facilitates precise catheter control within the CS and enables delicate coil embolization.
The authors declare that they have no conflicts of interest.
A video of the selective navigation into the IPS.