2021 年 53 巻 論文ID: e004
2021 年 53 巻 論文ID: e004
Actinophryid and centrohelid heliozoans often share the same habitat; however, field studies have shown that they preferentially exist separately than together, suggesting the existence of a mechanism that does not allow them to co-exist. Therefore, to clarify the interactions between these heliozoans, co-culturing experiments, involving two organisms, an actinophryid and a centrohelid, were performed. For the experiments in the brackish water environment, Actinophrys sol (actinophryid) and Raphidocystis contractilis (centrohelid) were used, and for those in the freshwater environment, a combination of Actinosphaerium eichhornii (actinophryid) and one of three centrohelid species (Choanocystis pantopoda, Raphidocystis ambigua, or Raphidocystis marginata) were used. In all the experiments involving the co-culture environments, the centrohelids grew intensively within the first 3-5 days, after which their cell numbers remained constant. Conversely, the number of actinophryid heliozoans decreased significantly and their disappearance was recorded after 2 days and after 5-11 days for the experiments in the brackish water and freshwater environments, respectively. Further, in some experiments, several centrohelid cells fused together to form a giant multinucleated cell, which jointly, captured and consumed larger organisms, such as Actinophrys sol. Furthermore, Actinosphaerium eichhornii (actinophryid) exhibited an avoidance response against Choanocystis pantopoda (centrohelid) attack. Thus, our experimental results showed that in mixed co-culture environments, centrohelids inhibit the growth of actinophryids. This possibly explains the exclusive mode of existence of these two groups of heliozoans in the natural environment.
Over a long period of time, unicellular heliozoan-like organisms have been classified under the taxon Heliozoa Haeckel, 1866. However, it was later on observed that Heliozoa is a polyphyletic taxon that brings together several evolutionarily unrelated groups belonging to different eukaryotic phyla (Nikolaev et al., 2004). The most widely distributed groups in this regard are centrohelid and actinophryid heliozoans, which inhabit a variety of biotopes, including extreme environments (Amaral Zettler et al., 2003; Weithoff, 2004).
Centrohelid and actinophryid heliozoans are predators. They obtain their food via the action of special cell organelles, such as extrusomes (Hausmann, 2002), and feed on autotrophic and heterotrophic flagellates as well as ciliates. It has also been observed that they can consume organisms larger than themselves, such as diatoms, ciliates, and rotifers (Dragesco, 1964; Weithoff, 2004), when multiple individuals fuse together to form a giant multinucleated cell (Rainer, 1968; Patterson & Hausmann, 1981; Sakaguchi et al., 2002).
Additionally, several centrohelid species co-exist in natural environments (Gaponova, 2008), and it has also been reported that actinophryids and centrohelid heliozoans often occupy the same habitats (Kinoshita et al., 1995; Zimmermann et al., 1996; Sakaguchi et al., 2002), and Rainer (1968) noted that large сentrohelid species such as Acanthocystis turfacea are capable of feeding on both ciliates and other heliozoan species. However, data on the interactions between different heliozoan species is limited.
In this paper, we extend the existing knowledge on the interactions between protozoan species belonging to different taxonomic groups by presenting the first quantitative data on growth and behavior of actinophryid species when they coexisted with centrohelid heliozoans.
The seasonal variations of centrohelid and actinophryid heliozoan species were monitored in an artificial pond (GPS: 50.3308265 30.4684937) located close to Kyiv (Ukraine). Water samples were collected at an interval of month between August 2006 and August 2008. Further, in accordance with the method for collecting centrohelids reported by Mikrjukov (2002), the materials were collected using a 70-µm plankton net. Thereafter, the collected water samples (10 L) were concentrated to 40-200 ml via gravity filtration. The different water samples were prepared in triplicates. The freshly collected samples, without fixation, were investigated in vivo in the laboratory within several hours after sampling. To calculate cell numbers, live counts of these protists were performed. After stirring, 5 ml aliquots were taken from each of the replicate samples and placed in Petri dishes (diameter=55 mm). Thereafter, the number of heliozoans was counted in 50 fields of view using a Carl Zeiss-50 microscope (Carl Zeiss, Inc., NY, USA) followed by the calculation of cell density as previously described (Zhukov, 1975).
For species identification, the living specimens were examined under light microscopes (Carl Zeiss-50 and Nikon Eclipse Ni-U) to determine morphological characteristics, especially the size and shape of the skeletal elements, as previously described (Mikrjukov, 2002; Mikrjukov & Patterson, 2001; Siemensma & Roijackers, 1988; Dürrschmidt, 1987). For detailed species identification, we also examined the exoskeleton (periplast) of the centrohelid heliozoans using a scanning electron microscope (JSM-35C, JEOL) and a transmission microscope (H-7100, Hitachi).
To conduct the laboratory experiments, two species of actinophryid heliozoans and three species of centrohelid heliozoans were used, as shown in Table 1 and Fig. 1. Actinophrys sol and Raphidocystis contractilis were originally collected from the same brackish water pond in Japan and cultured monoxenically with Chlorogonium capillatum (NIES 3374) as a food source in accordance with Sakaguchi and Suzaki (1999). The other heliozoans (freshwater species) were cultured in Volvic mineral water by adding Chlorogonium capillatum as the sole food source. The heliozoans were then fed three times a week. All the experiments were performed at room temperature (20-25°C).
| Species | Habitat | Collection site | GPS coordinates |
|---|---|---|---|
| Actinophryid heliozoans | |||
| Actinosphaerium eichhornii | Fresh water | Near Khotov in the vicinity of Kyiv, Ukraine | 50.330826 30.468493 |
| Actinophrys sol | Brackish water | Shukkeien, Hiroshima, Japan* | 34.400175, 132.467481 |
| Centrohelid heliozoans | |||
| Choanocystis pantopoda | Fresh water | Kameyama pond, Awara, Fukui, Japan | 36.257717, 136.218027 |
| Raphidocystis ambigua | Fresh water | Shahidullah hall pond, Dhakka, Bangladesh | 23.725806, 90.402444 |
| Raphidocystis contractilis | Brackish water | Shukkeien, Hiroshima, Japan** | 34.400175, 132.467481 |
| Raphidocystis marginata | Fresh water | Near Khotov in the vicinity of Kyiv, Ukraine | 50.330826 30.468493 |
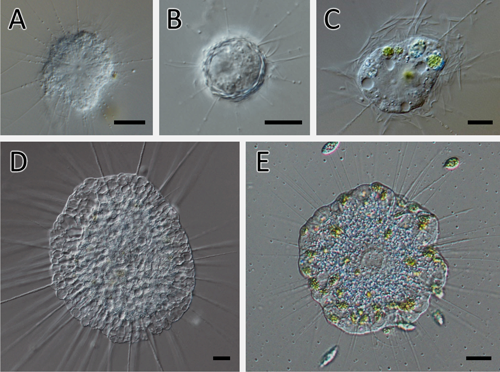
For co-culturing experiments, centrohelid and actinophryid heliozoans were mixed in Petri dishes containing Volvic mineral water (for freshwater species) or 10% artificial seawater (for brackish water species). Petri dishes containing only one species of the heliozoans were used as controls. During the experiments, the cell density of the actinophryid heliozoans was determined by counting the number of cells in a fixed field of view from 5-7 different positions in the Petri dish using a light microscope (CZ-61, Olympus). However, given that it was challenging to accurately measure the cell density of centrohelid heliozoans in the Petri dish owing to their tendency to adhere strongly to the bottom of the Petri dish and form colonies, the approximate percentage of the area occupied by the centrohelid cells at the bottom of the Petri dish was used as an index to estimate the growth of the centrohelid cultures.
To test the possibility of an interaction between actinophryid and centrohelid heliozoans, we monitored the seasonal changes in the population densities of these heliozoans in a pond near Khotov in the vicinity of Kyiv (Ukraine). The species composition of the heliozoans was investigated based on the morphological features observed using light and electron microscopes. Four centrohelid heliozoan species, Choanocystis aculeata (Hertwig & Lesser, 1874), Raphidocystis ambigua (Penard, 1904), Raphidocystis pallida (Schulze, 1874), and Raineriophrys fortesca (Nicholls, 1983) were identified, while one actinophryid heliozoan species (Actinophrys sol) was identified. Additionally, the most abundant species were Choanocystis aculeata and Actinophrys sol.
Considering the two-year observation period of this study, the population density profile of Actinophrys sol showed two distinct peaks, which corresponded to November 2007 and March 2008, while that of centrohelid heliozoans showed three distinct peaks corresponding to December 2006, August 2007, and April 2008 (Fig. 2). These observations indicated that these two heliozoan groups show different seasonal dynamics, i.e., centrohelid and actinophryid heliozoans show seasonal variations that seem mutually exclusive. Further, Choanocystis aculeata was identified as the most abundant species. Its population density profile was characterized by two peaks, one in spring (March-April) and the other in autumn (October or November-December). Furthermore, Raineriophrys fortesca, which was abundant in July-August, showed a single population density peak (in August), while the other centrohelids were less abundant. Furthermore, Raphidocystis ambigua was abundant in November, while Raphidocystis pallida was abundant in August and October.
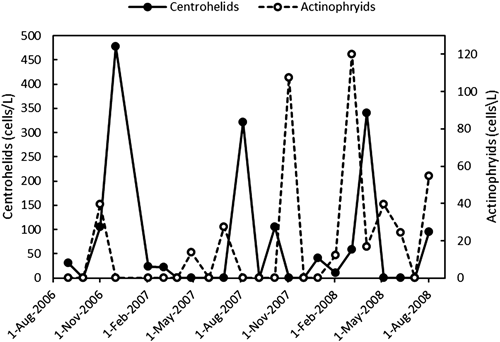
For centrohelids, the vertical axis of the graph shows the total cell density of the four centrohelid heliozoan species as described in the text.
Our data demonstrated that the seasonal dynamics of centrohelids and actinophryids are characterized by a rapid increase in cell density, followed by a rapid decrease and then complete disappearance, possibly owing to transformation into cysts. The seasonal cycles of these two heliozoan taxa showed three abundance peaks in March-April, August, and December. In most cases, the maxima of actinophryids preceded the abundance peaks of centrohelids, suggesting that the centrohelid heliozoans exert an inhibitory effect on the growth of actinophryids.
Interaction between centrohelid and actinophryid heliozoans during co-culturingCo-culturing experiments were performed using pairs of freshwater actinophryid and centrohelid heliozoan species (Fig. 3A and B). Specifically, Actinosphaerium eichhornii (an actinophryid species from a pond close to Khotov, Ukraine) and Choanocystis pantopoda (a centrohelid species from Kameyama pond, Japan) were mixed and co-cultured for 2 wk (Fig. 3A). Thus, it was observed that the cell numbers of both A. eichhornii and C. pantopoda increased up to Day 3. On the one hand, the density of C. pantopoda reached its maximum level on Day 3 and remained stable thereafter for 14 days. On the other hand, the cell number of A. eichhornii started to decrease from Day 4, an observation that was in contrast to C. pantopoda. A similar co-culture experiment with A. eichhornii was conducted using another centrohelid species, Raphidocystis ambigua (collected from SH pond in Bangladesh). The results obtained are presented in Fig. 3B, which shows an overall trend similar to that shown in Fig. 3A, with the centrohelid cell number increasing, while that of the actinophryids was decreasing. In this experiment, A. eichhornii disappeared after 12 days. As in the previous experiment, the number of centrohelid (R. ambigua) cells reached a maximum level on Day 5 and thereafter, remained stable. Considering Fig. 3C, which shows the results of the control experiment involving the growth of A. eichhornii only, it became evident its growth was inhibited when it coexisted with centrohelid heliozoans.
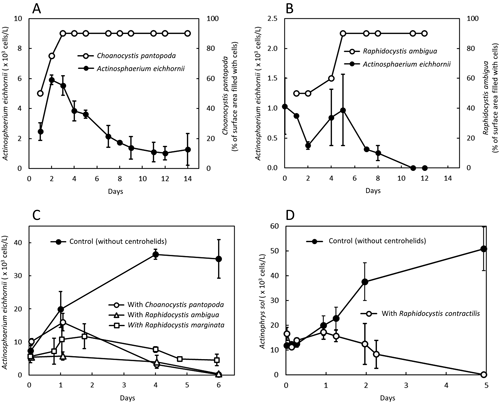
The upper and lower points of the error bars represent the two sets of data obtained.
Regarding the experiment in which brackish water was used for the culturing of heliozoan species (Fig. 3D), we used Actinophrys sol (actinophryid species) and Raphidocystis contractilis (a centrohelid species) collected from the same pond in Hiroshima (Japan). The monitoring of their cell densities in the presence and absence of one another showed that in the absence of R. contractilis, the number of A. sol cells showed a constant increase. Conversely, in the presence of R. contractilis, the number of A. sol cells decreased and finally disappeared by Day 5. Even though not shown in this graph, R. contractilis, like the freshwater centrohelids, proliferated regardless of the presence of A. sol, spreading and increasing at the bottom of the Petri dishes within a few days of the experiment.
Actinophryid and centrohelid heliozoan cell-to-cell interactionRaphidocystis contractilis attacked and preyed on Actinophrys sol. As shown in Fig. 4, multiple R. contractilis individuals gathered around a single A. sol, fused with each other, and ingested the A. sol cell into their common food vacuole, i.e., during the process of capturing A. sol, several R. contractilis individuals (up to 10 cells) gathered around a single A. sol. At the same time, the retraction of the axopodia of A. sol occurred, and thereafter, all axopodia disappeared completely. Thus, A. sol could not escape the attack by R. contractilis. Subsequently, R. contractilis gradually fused with neighboring individuals, and the resulting internal space became a common food vacuole containing A. sol. In the phagosome, A. sol was digested and absorbed, and after a few hours, the fused R. contractilis cells separated again and existed as single cells.
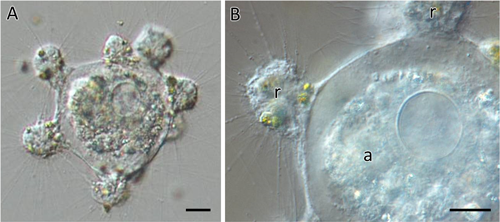
(A) Low magnification picture of several R. contractilis individuals forming a common food vacuole. (B) Higher magnification picture of the common food vacuole and the surrounding R. contractilis cells (r) with an A. sol cell (a) being digested (Scale bars=10 µm).
Another interesting cellular behavior was observed between the freshwater species Choanocystis pantopoda (centrohelid) and Actinosphaerium eichhornii (actinophryid), as shown in the series of micrographs in Fig. 5. The first photo (Fig. 5A) shows the two heliozoan species just after they came into contact with each other, with their axopodia closely entwined. In the subsequent photographs (Fig. 5B-F), the position of C. pantopoda remained unchanged (note the immobile markers in the white ovals); however, A. eichhornii gradually moved away from the smaller centrohelid heliozoan. This escape response was always observed when A. eichhornii came into contact with C. pantopoda, suggesting that A. eichhornii recognized C. pantopoda as a predator and actively avoided contact with it. Reportedly, in actinophryids, the induction of cell motility is mediated by cell surface receptors (Grębecki & Hausmann, 1992). Therefore, it is possible that the actinophryid heliozoans perceived some signal on the axopodial surface of the centrohelid when they come into contact with the attacking centrohelid and responded by changing their axopodial movement.
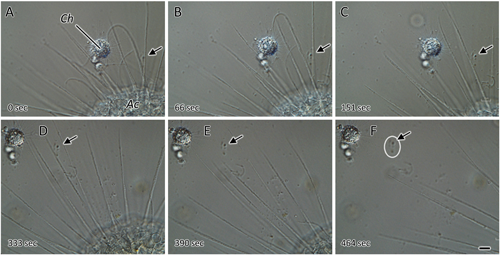
The elapsed time from Fig. 5A is shown in the lower left corner of each figure. The objects indicated using the arrows represent debris on the coverslip, showing a fixed reference point. A. eichhornii fled after encountering C. pantopoda. (Scale bars=10 µm).
Further, regarding actinophryid heliozoans, it is well known that the captured prey swims around in the food vacuole for a long time without being paralyzed (Dragesco, 1964; Rainer, 1968; Suzaki et al., 1980; Patterson & Hausmann, 1981). Conversely, our results are consistent with previous observations that in the process of prey capturing, Raphidocystis contractilis possibly releases a specific substance that paralyzes the prey and rapidly weakens its cells (Bardele, 1969; Sakaguchi et al., 2002). This possibly gives the centrohelid heliozoans an advantage in the race for survival, making it impossible for even the much larger actinophryid heliozoans to prey on them.
Actinophryid and centrohelid heliozoans, which are both passive predators that sit still at the bottom of water bodies, waiting for prey organisms to swim by, share the same habitat, even though their phylogenetic positions are very far apart. The organisms they capture as prey are also common, mainly protists and small multicellular animals. Additionally, they also capture their prey organisms via a common method, i.e., they both use long and contractile axopodia to capture prey and display similar mechanisms for the recognition of β-1,3 glucan molecules on the surface of prey organisms (Sakaguchi et al., 2002; Bhadra et al., 2017). Thus, actinophryid and centrohelid heliozoans are thought to compete with each other in the same niche.
In this study, we observed that actinophryid and centrohelid heliozoans show exclusive increases or decreases in cell numbers under natural conditions. This suggests that population fluctuations may occur due to prey-predator interactions, as it is well known for Paramecium and Didinium (Veilleux, 1979). In this study, our experiments, conducted under laboratory conditions, showed that centrohelid heliozoans inhibit the growth of actinophryid heliozoans. Further, the observed actinophryid predation by centrohelid may not be due to direct predation only, but may also be the result of some toxic substances released by centrohelids. Other possible reasons include: (1) actinophryids showed escape behavior when they came in contact with centrohelids as demonstrated in this study, (2) the extrosome of centrohelids may contain some toxic/anesthetic substances (Sakaguchi et al., 2002), and (3) some centrohelid species have been noted as toxic to rotifers (Cheng et al., 1997). Whatever the case, it can be considered that centrohelids proliferate by effectively eliminating other heliozoans that share their niches.
The centrohelid heliozoans showed a sharp decline in population size as soon as they reached their proliferation peak in the natural environment. However, this proliferation was independent of the season (Fig. 2). Further, centrohelids, like other protists, do not like extreme cold or hot environments. Under such conditions, they often form cysts and endure adverse conditions, and given that seasonal factors did not influence the observed centrohelid population fluctuation, it can be suggested that some other organisms that prey on them may also be involved.
This work was partly supported by the Japan Society for the Promotion of Science (Invitational Fellowship for Research in Japan). We would like to thank Editage (www.editage.com) for English language editing.