2024 Volume 66 Issue 1 Pages 60-65
2024 Volume 66 Issue 1 Pages 60-65
Purpose: To investigate the surface topography and nickel content of nickel-titanium (NiTi) archwires exposed to either routine oral hygiene or a prophylactic regimen with casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) during orthodontic treatment.
Methods: This in vivo study involved 40 orthodontic patients with fixed appliances, who were randomly assigned to either a routine oral hygiene group or a CPP-ACP supplementary regimen group. Twenty new NiTi archwires served as controls. All archwires underwent scanning electron microscopy and energy-dispersive spectroscopy to evaluate their surface topography and elemental composition. The nickel content was quantified as a percentage of total weight and the Ni/Ti ratio, and statistical comparisons were made using pairwise tests.
Results: Wires exposed to fluoride toothpaste showed signs of pitting corrosion, deep grooves, and corrosion debris. In contrast, wires exposed to supplementary CPP-ACP exhibited smooth surface areas interspersed with microdefects and deposits. Statistically significant differences in nickel content were found between the new and retrieved archwires, as well as between wires exposed to routine oral hygiene and CPP-ACP (P < 0.001). The archwires exposed to CPP-ACP had the lowest nickel content (P < 0.001).
Conclusion: The use of CPP-ACP holds promise for application as a safe anticariogenic agent with possible protective properties during orthodontic treatment.
In contemporary orthodontic practice, wires made of nickel-titanium (NiTi) alloys are often exposed to harsh conditions in everyday applications. Fouling and degradation of wires result from a combination of environmental and mechanical factors. Stress, strain, friction, abrasion, erosion, and wear, in combination with the corrosive environment (saliva, gastric acid, oral flora, and byproducts), can cause irreversible damage to the surface morphology, leading to degradation, and consequently, release of nickel [1,2]. Such a continuous process is not the simple sum of all factors but rather their interaction [3]. In addition, variations in pH and temperature, bacteria, medications, acid drinks, and chemoprophylactic agents, such as fluoride, make the degradation of NiTi wires unpredictable. When NiTi archwire is exposed to an environment containing fluoride as an anticariogenic agent, there is a significant risk of wire deterioration, corrosion, and fracture [4].
Orthodontic treatment with fixed appliances is typically applied for adolescents between 12 and 16 years of age. The quality, duration, and esthetic outcome of orthodontic treatment is adversely affected by poor oral hygiene and accumulation of dental plaque around orthodontic brackets, on the archwires, and between the brackets and the gingival margins [5]. Adolescents are at higher risk of developing cavitated caries and white spot lesions (WSL) due to relatively poor compliance with oral hygiene regimen, and dietary choices (soft drinks, sugary food and snacks) [6,7]. These factors compromise oral health, especially in young orthodontic patients [8]. Thus, chemoprophylactic agents are frequently recommended to help balance their poor oral health.
Daily use of sodium fluoride (NaF) toothpaste (5,000 ppm F) or mouth rinse (0.2% NaF) combined with regular toothpaste is effective for preventing dental caries and WSL in orthodontic patients [9]. However, some limiting factors can influence routine application of fluoride. Before enamel can remineralize, there must be a sufficient presence of calcium (Ca) and phosphate (P) ions [10].
The availability of Ca and P ions can be a restrictive factor for remineralization after topical application of fluoride, because for every two fluoride ions, ten Ca and six P ions are required to form a unit cell of fluoroapatite [11,12]. In addition, NaF reacts with bacterial products from dental plaque forming hydrofluoric acid (HF), which weakens the surface passive oxide layer of orthodontic wires, increases their surface roughness, and decreases NiTi corrosion resistance [4,13,14].
Such poor corrosion resistance might also result in toxic and allergic reactions due to nickel release. Thus, corrosion of orthodontic wires in patients exposed to topical fluoride is a major concern, and one that is not adequately addressed [4]. Another anticariogenic agent, casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) has been proved to be successful for management of early caries lesions [15]. The CPP-ACP nanocomplex incorporates into dental plaque, bonds to bacteria, acting as a reservoir for calcium and phosphate [16]. That way, Ca and P in plaque help maintain a state of supersaturation with respect to enamel, thereby depressing enamel demineralization and enhancing remineralization [17,18]. Under acidic conditions, CPP-ACP buffers plaque pH by increasing the concentration of Ca and P ions, balancing any fall in pH, and preventing enamel demineralization [15]. In addition, CPP-ACP reduces WSL number and appearance when used as supplement to regular oral hygiene with fluoridated toothpaste during orthodontic treatment [19].
Given the potential remineralization effect of CPP-ACP on enamel lesions [20] without aggressively influencing the pH of saliva, like topical fluoride, it was hypothesized that intraoral exposure of orthodontic NiTi wires to CPP-ACP would not provoke corrosion, or a change in Ni content and Ni/Ti ratio on the wire surface. The aim of the present study was to investigate the surface topography, elemental composition and Ni content of NiTi orthodontic wires exposed to a prophylactic regimen with CPP-ACP during orthodontic treatment.
This was a randomized in vivo study, performed at the University Clinic of Orthodontics and Faculty of Technical Sciences. As-received and retrieved NiTi archwires from orthodontic patients following two different oral hygiene protocols were analyzed. Adolescents requiring orthodontic treatment with fixed appliances were randomly enlisted for participation in the study at the University Clinic of Orthodontics through a process of consecutive sampling. The inclusion criteria were as follows: orthodontic patients 13 to 16 years of age with permanent dentition, no extractions, no active caries or periodontal disease, no known milk allergy or allergy to oral hygiene products used in the study, no systemic conditions, no medication taken regularly, and absence of orofacial syndromes. The exclusion criteria included any pathological change in the oral cavity, known milk allergy or allergy to oral hygiene products used in the study, the use of any remineralizing or fluoridated products (gels, varnishes, or mouthrinses) within 30 days prior to the study.
All patients and parents were willing to follow the oral hygiene protocol and participate in the study, after signing a consent form. Materials used in the study are presented in Table 1. The metal brackets (Equilibrium 2, Dentaurum, Ispringen, Germany) and non-convertible buccal tubes (slot size 0.018" × 0.025") (Ortho cast, Dentaurum) were bonded to the maxillary teeth with light-cured composite (Enlight, Ormco, Brea, CA, USA). Archwires were ligated with elastic ligatures (Dentalastics, Dentaurum) into the slot of the brackets. Nickel-titanium archwires (0.014") (Rematitan Lite, Dentaurum) were used in the first phase of orthodontic treatment and investigated in the present study. Twenty new NiTi Rematitan 0.014" archwires from the same batch (lot#83962) served as a baseline, and were assigned to Group A. Intraorally used archwires were collected from patients randomly assigned to either Group B or Group C. To ensure blinding, dental assistants scheduled patients for bonding appointments based on their availability, without consulting the doctors. Block randomization, with a block size of 4, was used to allocate patients to one of the following groups:
Group B – to maintain regular oral hygiene (ROH) by means of brushing in the morning and evening with a soft regular toothbrush (TePe, Malmo, Sweden) and dentifrice with 1,450 ppm (Colgate Total, Colgate-Palmolive, New York, NY, USA);
Group C – to maintain ROH and use a chemoprophylactic agent containing 10% CPP-ACP (Tooth Mousse, GC Corp., Tokyo, Japan). After regular brushing twice daily, patients were instructed how to use CPP-ACP (apply a pea sized amount of chemoprophylactic agent on the dry toothbrush for each arch, brush for 1 min, and leave the paste for a minimum of 3 min. After that, expectorate; avoid rinsing, eating and drinking for 30 min).
Four weeks after the bonding appointment and the beginning of the orthodontic treatment, the archwires were retrieved, washed with distilled water to detach loose precipitates, marked and put in labeled airtight bags (marked with the ID of the patient, the hygiene regimen, and the archwire insertion and retrieval dates).
A total of 60 as-received and retrieved archwires (20 wires from each group) with marked areas at the incisor, canine and molar regions were subjected to further analysis using scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) at the Faculty of Technical Sciences by one investigator. A tabletop SEM (TM3030Jeol JSM-6460LV, Jeol Industries Ltd., Hitachi, Tokyo, Japan) with a 15-kV accelerating voltage, at 10 mm distance, accompanied by EDX, were used for analysis at various magnifications. The EDX spectra were analyzed, and the X-ray peak intensities for each element were converted to atomic and weight fractions. Percentage weight concentrations of nickel (Ni wt%) and titanium (Ti wt%,) were calculated and compared to reveal any changes among the groups. In addition, for standardization purposes, the Ni content was presented as the Ni/Ti ratio, as proposed by Eliades et al. (2004) [21].
| Material | Name | Manufacturer | Composition |
|---|---|---|---|
| Brackets | Equilibrium 2 | Dentaurum, Ispringen, Germany | stainless steel wt%: C ≤ 0.06; Si ≤ 1.0; Mn ≤ 2.0; Cr 17.0-19.0; Ni 11.0-13.0; P ≤ 0.045; S ≤ 0.03; N ≤ 0.11; Fe Residue |
| Buccal tubes | Ortho cast | Dentaurum | stainless steel wt%: C ≤0.06; Si ≤1.0; Mn ≤ 2.0; Cr 17.0-19.0; Ni 11.0-13.0; P ≤ 0.045; S ≤ 0.03; N ≤ 0.11; Fe Residue |
| NiTi Archwire | Rematitan Lite | Dentaurum | wt%: Ni 54.5-57; Fe < 0.005; Cr < 0.005; Ti Residue C < 0.0029; Co 0.005; O < 0.05; H <0.005 N < 0.002; Cu < 0.005; Nb < 0.005 |
| Elastic ligatures | Dentalastics | Dentaurum | natural rubber |
| Orthodontic adhesive | Enlight | Ormco, Brea, CA, USA | resin-poly(oxy-1,2-ethanediyl), α,α'-[(1-methylethylidene)di-4,1-phenylene]bis [ω-[(2-methyl-1-oxo-2-propen-1-yl)oxy]-trimethoxysilylpropyl methacrylate |
| Dentifrice | Colgate Total | Colgate-Palmolive, New York, NY, USA | glycerin, aqua, hydrated silica, calcium pyrophosphate, sodium methyl cocoyl taurate arginine, aroma, cellulose gum, zinc oxide, benzyl alcohol, poloxamer 407, zinc citrate, tetrasodium pyrophosphate, cocamidopropyl betaine, xanthan gum, sucralose; sodium fluoride, sodium saccharin, phosphoric acid |
| CPP-ACP | Tooth Mousse | GC Corp., Tokyo, Japan | pure water, glycerol, Recaldent (CPP-ACP), D-sorbitol, CMC-Na, propylene glycol, silicon dioxide, titanium dioxide, xylitol, phosphoric acid, flavoring, zinc oxide, guar gum, propyl p-hydroxybenzoate, butyl p-hydroxybenzoate, soybean |
| Toothbrush | Select Soft | TePe, Malmö, Sweden |
The sample size was calculated using G*Power software version 3.1.9.2 3.1.9.4 (Heinrich-Heine-Universität, Düsseldorf, Germany) after performing a power analysis, based on data from the literature [22], for the difference between mean values of new and retrieved archwires. The sample size was determined to be 58 to test the difference in the arithmetic means for a significance level of 0.05 and a statistical strength of 80%. The final sample size was rounded up to 60 (20 wires in each group). Post hoc analysis was performed in the same program based on the results for Ni and Ti, and powers for 20 samples in each group were calculated for the difference between means, α = 0.05 (Wilcoxon-Mann Whitney test).
Descriptive statistics were applied to summarize the data, including median and interquartile range (IQR) of Ni weight % (wt%), Ti wt%, and Ni/Ti ratios for groups of as-received and retrieved archwires (Jamovi software, version 2.3.24, R Core Team, 2021). For comparative intergroup statistics, Kruskal-Wallis test was utilized, with level of significance set at P < 0.001. Dwass-Steel-Critchlow-Fligner pairwise test was used to further examine the significance of differences between groups.
After EDX analysis of the wires from Group C, two distinctive variations in spectra were noticed (presence or absence of calcium and phosphorus on the surface). Subsequently, Group C was divided into two subgroups (C1 and C2). Additional post hoc analysis between subgroups was done to further explore noticed findings. Descriptive statistics were used to summarize the Ni wt%, Ti wt%, and Ni/Ti ratios of the subgroups (C1 and C2). The Shapiro-Wilk test was employed to assess the normality of the data, and the level of significance was set at P < 0.001. Dwass-Steel-Critchlow-Fligner pairwise test was used to examine the differences between subgroups.
Representative SEM images of new and retrieved archwires are presented respectively in Fig. 1. The SEM images (representative magnification ×1,000) of new NiTi archwires showed variations in surface topography, despite originating from the same batch. Representative images of three typical types of surface topographies of new wires are presented in Fig. 1, and were characterized as the following surfaces: (a) a grain-like morphology, (b) a uniform pattern of longitudinal striations with processing marks, and (c) a smooth surface with rare indentations. The surface of all as-received wires demonstrated the same patterns, which tended to alter or intensify after intraoral use (Fig. 1d-i).
Following a 4-week intraoral exposure period, wires from both Group B and Group C manifested changes in surface topography and displayed evident signs of corrosion. The increase in surface defects stemmed primarily from the pre-existing pits, producing a more irregular surface. Images of wires from Group B showed deeper and wider defects, and grooves on the surface. Longitudinal striations were more noticeable, deeper, and covered with corrosion debris. Perpendicular scratches along the length of the wire, signs of pitting corrosion, deep grooves, corrosion debris, and dark spots were present (Fig. 1d-f). Images of wires from Group C showed pitting corrosion, dark patches and pronounced longitudinal striations with microdefects (Fig. 1g, h). The smooth sites present on the surface showed no signs of destruction: only dark patches and deposits (Fig. 1i).

a-c: new archwires (Group A); d-f: retrieved archwires exposed to regular oral hygiene protocol (Group B); g-i: retrieved archwires exposed to hygiene protocol with CPP-ACP (Group C)
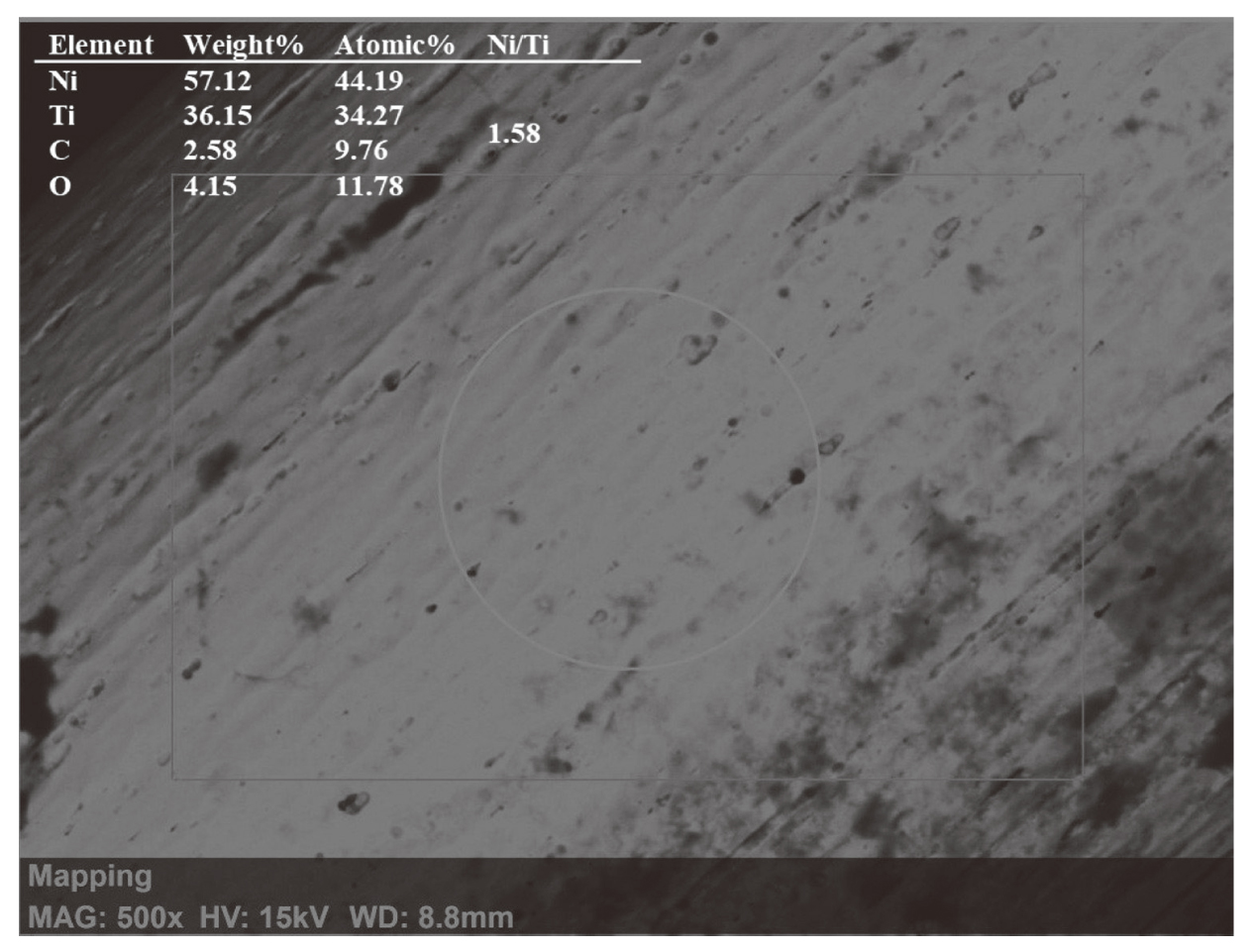
Representative EDX spectra of new and retrieved archwires are presented in Figs. 2,3,4. Quantitative EDX spectroscopy of NiTi wires demonstrated Ni and Ti as specific elements in all three groups (Table 2).
Elemental analysis of wires in Group A showed Ni and Ti as specific elements, with traces of carbon (C) (Fig. 2). The wires from Groups B and C showed spectra that were more complex (Fig. 3, 4). Wires from Group B, beside Ni and Ti, showed the presence of C and oxygen (O), as well as potassium (K), and nitrogen (N) (Fig. 3). Spots in the same group covered with corrosion debris, presenting as light deposits with uneven edges and height, showed higher contents of C, N, and K.
Pairwise comparisons (Dwass-Steel-Critchlow-Fligner test) showed statistically significant differences for Ni wt% and Ni/Ti ratio between groups A/B (P < 0.001), A/C (P <0.001) and B/C (P < 0.001) (Table 3). The highest Ni wt% was present on the surface of the wires in Group B, relative to Group A (P < 0.001) and Group C (P < 0.001) respectively. The Ni/Ti ratio was significantly higher in Group B in comparison to the baseline (P < 0.001) and Group C (P < 0.001). Group C showed a significantly lower Ni content and Ni/Ti ratio than Group B (P < 0.001) and Group A (P < 0.001).
Two distinctive spectra on the surfaces of the wires retrieved from the group exposed to the chemoprophylactic protocol (Group C) were observed (Fig. 4). The surface of the majority of the wires, besides O, C, and K, demonstrated the presence of Ca and P. However, EDX spectra of the remaining wires, accounting for 30% of Group C, did not exhibit Ca and P. To further explore this phenomenon, based on the presence of Ca and P on the surfaces of the wires in Group C, retrieved archwires with Ca and P on the surface were subsequently allocated to Subgroup C1, while retrieved archwires without Ca and P on the surface were allocated to Subgroup C2 (Fig. 4a, b). Nickel content and Ni/Ti ratio were then compared between the subgroups. Post hoc statistical analysis revealed that wires in Subgroup C1, where Ca and P were present, exhibited a significantly lower Ni wt% and Ni/Ti ratio in comparison to Subgroup C2 (P < 0.001), as shown in Table 4.


| Group (n) | Element wt% | A (20) median ± IQR |
B (20) median ± IQR |
C (20) median ± IQR |
|---|---|---|---|---|
| Ni | 55.00 ± 0.80 | 57.30 ± 2.76 | 42.80 ± 4.04 | |
| Ti | 43.90 ± 1.26 | 39.80 ± 2.80 | 39.20 ± 5.06 | |
| Ni/Ti | 1.25 ± 0.04 | 1.44 ± 0.12 | 1.10 ± 0.22 |
n, number of archwires; Group A: new NiTi archwires; Group B: retrieved NiTi archwires exposed to the ROH protocol; Group C: retrieved NiTi archwires exposed to the CPP-ACP protocol
| Groups pairwise comparisons |
Element wt% | A/B P |
A/C P |
B/C P |
|---|---|---|---|---|
| Ni | <0.001 | <0.001 | <0.001 | |
| Ti | <0.001 | <0.001 | 0.830 | |
| Ni/Ti | <0.001 | <0.001 | <0.001 |
| wt% | Subgroups of Group C | P | |
|---|---|---|---|
| C1 median ± IQR |
C2 median ± IQR |
||
| Ni | 41.30 ± 3.86 | 46.40 ± 5.06 | <0.001 |
| Ti | 40.70 ± 3.03 | 35.40 ± 5.98 | <0.001 |
| Ni / Ti | 1.02 ± 0.01 | 1.32 ± 0.18 | <0.001 |
C1: retrieved archwires with Ca and P on the surface; C2: retrieved archwires without Ca and P on the surface
The acidic pH caused by the oral environment itself, as well as food, soft drinks, toothpaste, and bacterial byproducts, can provoke corrosion attacks and damage orthodontic NiTi alloys [23], as reflected in the SEM micrographs of retrieved wires in the present study. The retrieved archwires showed deep striations, grooves and gathered pits, with irregularly distributed deposits, but also sites with smooth surfaces. These observations align with previous findings reported for archwires retrieved after intraoral use [14,22]. The degradation of archwires, which are constantly immersed in saliva, results from simultaneous electrochemical, chemical and mechanical interaction. Stress, galvanic, fretting and pitting corrosion lead to degradation of the passive oxide layer and a change in surface topography. Finding a way to categorize and measure the factors influencing wire corrosion in the oral environment, such as friction, defects, and contact with brackets and ligatures, is a demanding task. In an effort to analyze the entire surface of wires exposed to different microenvironments, including areas subjected to friction forces, EDX analysis was conducted on both intra-bracket and inter-bracket wire surfaces in the present study. Archwires were retrieved after 4 weeks in the oral environment, where they had been exposed to similar conditions, excluding individual variations related to plaque accumulation, dietary preference, masticatory forces and bad habits. The composition of the sample was structured by selection of the participant age group, as well as by the adoption of inclusion and exclusion criteria. Although the intraoral conditions were authentic, many factors involved in the corrosion of orthodontic appliances, such as various forces, friction and other elements that can contribute to changes on the metal surface, could not be controlled for. However, no significant differences in either the appearance or elemental composition of the wire surfaces were noticed.
Although surface defects in the structure of new wires, such as those observed in the present study in the baseline group, cannot be considered the only factor involved in corrosion, they do provide predilection sites for material degradation [24]. Signs of corrosion were observed on all retrieved archwires, regardless of the oral hygiene protocol.
However, the results of EDX analysis revealed pronounced differences between the elemental compositions of the investigated wires. The ratio of Ni and Ti, and the presence of C on the surfaces of new wires indicated a nominal alloy composition. Considering the capabilities and limitations of the EDX technique for detection of elements with a low atomic number, the values presented in the figures for C and O provide qualitative information and quantitative estimates regarding the presence of these elements. It seems that new wires lack a protective oxide layer, likely due to mechanical polishing (as evidenced by characteristic marks on the surface) intended to improve resistance to localized corrosion, as reported previously [24,25].
Interestingly, the EDX spectroscopy in the present study demonstrated that, in comparison to the baseline, Ni and the Ni/Ti ratio were significantly elevated in the group with ROH and significantly lower in wires exposed to CPP-ACP. If a lower Ni content and Ni/Ti ratio are indicative of wire degradation, these results would suggest significant loss of Ni in the group exposed to CPP-ACP. In contrast, the findings in the group exposed to ROH, where the Ni content was even higher than in the baseline group, would suggest Ni accumulation on the wire. Nonetheless, the Ni content and Ni/Ti ratio on the wire surface does not directly correlate with the extent of observed corrosion, or Ni loss into the oral cavity [26], i.e., less Ni on the surface of the wire does not necessarily imply that Ni ions have been released into the mouth. Although Ti and Ni are highly reactive metals, the passive titanium oxide-based surface layer prevents leakage of corrosion products from NiTi alloy. The corrosion resistance of NiTi alloy is largely determined by the protective role of passive titanium oxide film and the composition and changes in the surrounding environment, such as saliva and plaque [4]. Any acidic change in pH could disrupt the titanium oxide layer, reducing its protective role and potentially leading to fatigue and corrosion of the NiTi wire.
The corrosion of NiTi alloy in a biological environment leads to the precipitation of dissolved Ni ions, thus increasing their content on the surface [27], and this corresponds to the findings in the group exposed to ROH. In addition, the deposition of corrosion products prevented significant release of Ni ions from the archwires by blocking the sites of corrosion, which further amplified the Ni content on the surface [28].
Contrary to the significantly higher content of Ni on the surfaces of wires from the group exposed to ROH relative to the baseline, EDX analysis revealed considerably less Ni on the wires in the CPP-ACP group. This may have been due to the lower Ni/Ti ratio on the wire surfaces where Ca and P were present, relative to those that lacked Ca and P. The decreased Ni content on the wire surfaces could have been due to accumulation of Ca and P, which formed a protective film. Biofilm may have protected the passive titanium oxide layer that covered the underlying layer containing nickel, as found in previous studies [22,29,30]. The protective surface layer of Ca and P ensured that the passive titanium oxide film remained undisturbed, thus exposing less Ni [31]. Given that Ni ions are insoluble in the titanium oxide layer, it could be assumed that Ni did not reach the surface, but was concentrated below the titanium oxide, similar to previous findings [26,32]. This phenomenon was not observed at either the baseline or in the group exposed to ROH, thus supporting this possibility.
It has been reported that CPP binds to the bacteria in oral biofilm found on teeth and surrounding areas, such as orthodontic wires [15,33]. One can argue that intraoral exposure to CPP-ACP in the present study facilitated accumulation of Ca and P on the wire surfaces. At low pH, the accumulated CPP-ACP bound to bacteria would form a reservoir of Ca and P, forming a protective layer, thereby acting as a barrier to keep the titanium oxide film intact [34]. More importantly, CPP-ACP buffers plaque and saliva from acid [35], thus protecting the surface from deterioration. The presence of Ca and P on the surface revealed a deficit in Ni content, keeping it mainly at an equiatomic ratio with Ti. In addition, wires retrieved from teenagers who followed the ROH regimen demonstrated a higher Ni content and Ni/Ti ratio on the surface, and no traces of Ca and P in comparison to the baseline group and the group exposed to CPP-ACP.
The composition of biofilm on a wire surface depends heavily on the individual intraoral environment, length of exposure, and diet [22]. The presence of Ca and P on the surface of the investigated wires might indicate precipitation of CPP-ACP in accordance with the use of prescribed chemoprophylactic paste. In the present study, all wires remained in the oral environment for 4 weeks. While the subjects were instructed in oral hygiene protocols, they were not asked to maintain a food diary. Thus, their dietary preferences and compliance were not controlled for.
The characteristic EDX spectra of six wires retrieved from the group exposed to CPP-ACP that had no surface Ca and P could have been due to either the patients’ non-compliance or their dietary choices that had destroyed the biofilm, thereby resembling the spectra in the group with ROH. These finding also reflected the limitations of the study. A specific age group (teenagers) was selected to represent patients who often struggle to maintain adequate oral hygiene and a low incidence of caries. They were also anticipated to be the age group least expected to follow rules and protocols, and their dietary habits and compliance could not be controlled for [7]. However, the EDX data for wires from six patients who were likely non-compliant with the instructions for CPP-ACP reinforced the importance of findings in the rest of the group exposed to CPP-ACP, in comparison to the group with ROH and those in the baseline group. Namely, the wires retrieved from potentially non-compliant patients showed the same characteristics as wires retrieved from patients who practiced solely ROH.
The results of this study should not be considered as conclusive evidence for the protective effect of CPP-ACP on NiTi wire. The strength of this study lay in its retrieval approach, which emphasized clinical relevance. However, the lack of control with respect to the individuality of complex environments such as that in the human mouth, and the highly multifaceted nature of each wire, did not permit the precise description and quantification of sequential alterations during intraoral ageing. For this reason, the influence of biofilm on the friction generated within the bracket-archwire-ligature system, corrosion rate, or the duration and characteristics of tooth movement during orthodontic treatment, were not investigated in the present study.
The potentially beneficial features of CPP-ACP should be further explored in a controlled environment via studies that are more extensive. Nevertheless, in accordance with the documented anticariogenic and remineralization potential of CPP-ACP [17,36,37], the present results might further encourage its use in orthodontic patients.
A biofilm, enriched with precipitated Ca and P as a result of exposure to CPP-ACP, might act as a protective layer and reduce the surface Ni content of orthodontic wire. The ongoing development of novel calcium phosphate coatings for NiTi alloys aimed at biomedical application [38,39,40] underscores the potentially protective and anticorrosive nature of CPP-ACP. The use of CPP-ACP holds promise for application as a safe anticariogenic alternative to fluoride in orthodontic patients, acting as a chemoprophylactic agent with anticorrosion properties. Further research should explore the potentially protective and anticorrosive qualities of CPP-ACP during orthodontic treatment with fixed appliances.
The study protocol was designed according to the ethical principles of the Declaration of Helsinki, and approved by the University of Belgrade Ethics Committee (document 36/9).
The authors have no conflicts of interest related to this article.
The authors would like to thank Dr Jovana Kuzmanovic Pficer from the Department of Medical Statistics and Informatics, School of Dental Medicine, University of Belgrade, and Lazar Milic from the Department of Power, Electronics and Telecommunications, Faculty of Technical Sciences, for their help and research support with this paper.