2014 Volume 39 Issue 3 Pages 152-158
2014 Volume 39 Issue 3 Pages 152-158
The target site of tolprocarb, a novel systemic fungicide used for controlling rice blast, was investigated. Tolprocarb decolorized the mycelia of Magnaporthe grisea; the decolorization was reversed by adding scytalone or 1,3,6,8-tetrahydroxynaphthalene (1,3,6,8-THN). This result suggested that the target site of tolprocarb was polyketide synthase (PKS), which regulated polyketide synthesis and pentaketide cyclization in melanin biosynthesis. Further, we produced a transgenic Aspergillus oryzae, which possessed the PKS gene of M. grisea, and performed in vitro assays of PKS using membrane fractions from the transgenic A. oryzae. Compared with some conventional melanin biosynthesis inhibitors (cMBIs), tolprocarb only inhibited PKS activity in vitro. These results indicated that tolprocarb’s target protein in M. grisea was PKS, which differentiates this fungicide from other cMBIs.
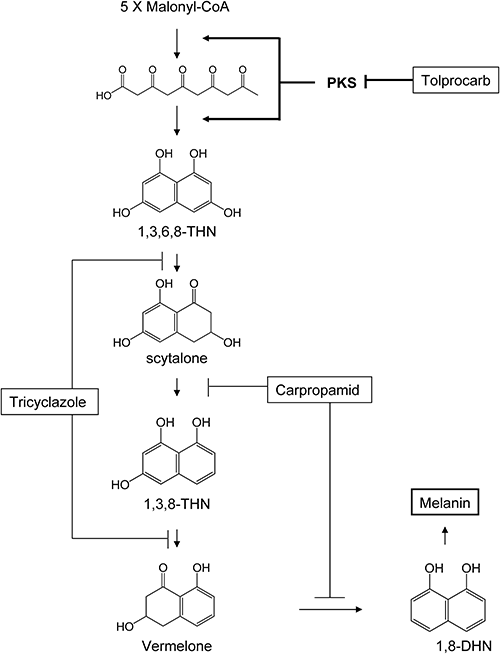
Rice blast caused by Magnaporthe grisea is a major fungal disease affecting rice production worldwide. In its infection process, M. grisea produces unicellular infection structures, called appressoria, which adhere tightly to the host surface and produce slender infection pegs that pierce the underlying cell wall of the host. The cell wall of appressoria contains a dense layer of 1,8-dihydroxynaphthalene (1,8-DHN)-melanin synthesized from 1,8-DHN.1) The accumulation of the dark-colored 1,8-DHN-melanin between the plasma membrane and the cell wall is an essential step before the appressoria of Magnaporthe and other fungal species can penetrate host plants.2–9) It is also known that 1,8-DHN-melanin-deficient mutants of M. grisea, which cannot produce the dark gray pigment typical of wild-type mycelia failed to infect intact host plants.10) In various fungal species (e.g., M. grisea, Verticillium dahliae, and Colletotrichum lagenarium), biochemical analysis of enzymes involved in the melanin biosynthetic pathway has been reported.1,8,10–12) The biosynthetic pathway for melanin in fungi starts from pentaketide synthesis and cyclization to form 1,3,6,8-tetrahydroxynaphthalene (1,3,6,8-THN) by polyketide synthase (PKS). The subsequent steps entail reduction of 1,3,6,8-THN to scytalone, dehydration of scytalone to 1,3,8-trihydroxynaphthalene (1,3,8-THN), reduction of 1,3,8-THN to vermelone, and dehydration of vermelone to 1,8-dihydroxynaphthalene (1,8-DHN). 1,8-DHN is then polymerized and oxidized to yield melanin.
A group of compounds that specifically block melanin biosynthesis in the pathogen, here referred to as conventional melanin biosynthesis inhibitors (cMBIs), has long been used for practical control of plant disease. These cMBIs inhibit the penetration of the pathogen into intact host plants by preventing the accumulation of 1,8-DHN-melanin in the appresoria although they barely inhibit mycelial growth, spore germination, and/or appressorial formation of fungi. cMBIs include tricyclazole,2–5) pyroquilon,13) phthalide,5,14,15) carpropamid,16,17) diclocymet,18) and fenoxanil.19) Tricyclazole, pyroquilon, and phthalide inhibit the two reduction steps between 1,3,6,8-THN and scytalone and between 1,3,8-THN and vermelone.3,5,8,14) Carpropamid, diclocymet, and fenoxanil inhibit the two dehydration steps between scytalone and 1,3,8-THN and between vermelone and 1,8-DHN.16–19) At present, the reduction and dehydration steps are common target sites of cMBIs. However, developing resistance to some cMBIs that inhibit dehydration steps has been a problem in controlling rice blast and other plant diseases.20) For effective disease control, fungicides having different modes of action are required.
Tolprocarb, 2,2,2-trifluoroethyl N-[(1S)-2-methyl-1-[[(4-methylbenzoyl)amino]methyl]propyl carbamate, which is being developed by Mitsui Chemicals Agro, Inc., has proven highly effective in controlling rice blast. However, tolprocarb’s target site has not been revealed. Considering the possibility of cross-resistance with conventional fungicides, it is important to pinpoint this target site. In this report, through recovery tests, tolprocarb’s target site was suggested as the polyketide synthesis and/or the pentaketide cyclization steps catalyzed by PKS. Further, we produced transgenic Aspergillus oryzae, which possessed the PKS gene of M. grisea, and using the membrane fraction from the transgenic A. oryzae, we verified that tolprocarb clearly inhibited pentaketide synthesis and/or pentaketide cyclization in melanin biosynthesis.
Three fungicides were used in this study: tolprocarb (Fig. 1, purity 99.0%), tricyclazole (purity 98.4%), and carpropamid (purity 98.5%). These fungicides and two melanin biosynthesis-related substances, 1,3,6,8-THN and flaviolin, were synthesized at Agrochemicals Research Center, Mitsui Chemicals Agro, Inc. (Chiba, Japan). Malonyl-CoA was purchased from Sigma-Aldrich (St. Louis, MO, USA). Potato dextrose agar (PDA) and potato dextrose broth (PDB) were purchased from Kyokuto Seiyaku (Tokyo, Japan) and Difco Laboratories Inc. (Detroit, MI, USA), respectively. Czapek Dox broth and yeast extract were also purchased from Difco. Soluble starch was purchased from Wako (Osaka, Japan). Scytalone was purchased from Indofine Chemical Company, Inc. (Hillsborough, NJ, USA). Restriction enzymes and plasmid vector pBSSK+ were purchased from TAKARA (Osaka, Japan). Fungal expression plasmid pTAex3 was obtained from Professor Katsuya Gomi (Tohoku University, Miyagi, Japan). M. grisea 40901 maintained at Agrochemicals Research Center, Mitsui Chemicals Agro, Inc., was used as a wild-type strain of M. grisea.

M. grisea 40901 was pre-incubated on a PDA plate to form a mycelial colony. The resultant mycelial colony disc (5 mm in diameter) was removed and inoculated onto fresh PDA plates containing tolprocarb or tricyclazole at 10 ppm and scytalone or 1,3,6,8-THN at 100 ppm. The plates were subsequently incubated at 25°C for 2 weeks and visually observed for any mycelial color change.
3. Construction of expression plasmidFungal expression plasmid pTAex3 possessing the α-amylase (amyB) promoter, terminator of A. oryzae, and auxotrophic marker argB of A. nidulans was used.21) A PKS gene of M. grisea expression plasmid pTA-pks was constructed on the basis of pUC-pks (Fig. 2). The PKS gene of M. grisea (National Center for Biotechnology Information (NCBI) accession number XP003715434), with an amino acid sequence similar to that of the PKS1 protein of C. lagenarium, was retrieved from NCBI’s genetic data base.22) The four fragments of DNA that covered the whole PKS ORF region were amplified by a polymerase chain reaction (PCR) using DNA of M. grisea 40901 maintained at Mitsui Chemicals Agro, Inc., as a template. The primers are mentioned in Supplemental Table S1. These four DNA fragments, designated as pks 5-8, 21-10, 13-12, and 15-14, were digested by appropriate restriction enzymes and then ligated into cloning vector pUC118 DNA Hinc II/BAP (TAKARA, Osaka, Japan), yielding pUC5-8, pUC21-10, pUC13-12, and pUC15-14, respectively. The pUC 5-8 and 21-10 plasmids digested by NcoI/PstI were ligated into the pUC 5-8 plasmid digested by NcoI/PstI to produce the plasmid pUC-5′-pks. The pUC15-14 digested by XbaI/PstI was ligated into pBSSK+ digested by XbaI/PstI to produce the plasmid pBS-3′-pks. The pUC-5′-pks digested by PstI/HindIII, the pUC13-12 digested by PstI/SphI, and pBS-3′-pks digested by SphI/HindIII were all ligated to yield pUC-pks. The pUC-pks digested by EcoRI was ligated into pTAex3 digested by EcoRI to produce pTA-pks. Finally, A. oryzae M-2-3 was transformed with pTA-pks through the protoplast-polyethylene glycol method as described previously.23) As a result, the PKS gene from M. grisea comprised 6522 base pairs with an estimated molecular mass of 236 kDa.

The transformants appearing on minimal plates after 1 week’s incubation were precultured on a PDA plate and inoculated into an induction medium (Czapek Dox broth containing 1% starch and 0.4% yeast extract) in a baffled Erlenmeyer flask at 1×104 spores/mL. The transformants were grown for 2 days at 25°C on a rotary shaker at 150 rpm. The mycelia and culture medium were separated by a Buchner funnel. The culture medium was acidified with 1N HCl and extracted with ethyl acetate for analysis of 1,3,6,8-THN produced by the transformants. The organic phase was desiccated by vacuum drying and dissolved in 50 µL of ethanol for HPLC analysis. The HPLC conditions are described later. Mycelia were washed with distilled water and then used for the following cell-free extraction.
5. Cell-free extract preparationCell-free extract preparation was performed by a method described previously.24) Freshly harvested mycelia were flash frozen in liquid nitrogen and pulverized in a mortar with a pestle. The mycelial powder was then suspended in a dilution buffer (50 mM potassium phosphate buffer, pH 7.2, containing 30% glycerol, 1 mM dithiothreitol, 1 mM EDTA, and 0.1 mM benzamidine). The mixture was occasionally stirred on ice for 20 min and subsequently centrifuged at 10,000×g for 20 min. The supernatant was then filtered through a four-layered gauze to remove residual cell debris (crude cell-free extract). The extract was then centrifuged at 38,900×g for 80 min. The supernatant was further fractionated by centrifugation at 38,900×g for 11 hr. The precipitate was suspended in a dilution buffer. The precipitate was subjected to SDS-PAGE using the NuPAGE Novex Tris-Acetate Gels system. NuPAGE 3–8% Tris Acetate Gel with NuPAGE Tris-Acetate SDS Running Buffer was used for the assay. The gel was stained by SimplyBlue Safestain (Life Technologies, Carlsbad, CA, USA), which indicated the expression of 236 kD of protein. The molecular mass of this protein corresponded well with the deduced molecular mass of PKS. The suspension of precipitate was then used as an enzyme solution in the following experiments.
6. In vitro assay of PKSEnzymatic synthesis of 1,3,6,8-THN by PKS was performed by a method described previously.24) A reaction mixture containing 435 µL of 50 mM potassium phosphate buffer, pH 7.2, 15 µL of malonyl-CoA solution, 50 µL of enzyme solution, and 1 µL of tolprocarb or cMBIs was incubated for 1 hr at 25°C. Final protein concentration in the mixture was 0.91 mg/mL on average. The reaction mixture was then boiled at 100°C for 30 min for oxidation of 1,3,6,8-THN to flaviolin and termination of reaction. After acidification with 100 µL of 6 M HCl, the reaction mixture was extracted with 500 µL of ethyl acetate. The organic phase was desiccated by vacuum drying and dissolved in 50 µL of ethanol for HPLC analysis. The 1,3,6,8-THN and flaviolin formed by the enzymatic reaction were identified with LS-MS (date not shown).
7. HPLC analysisReverse-phase HPLC, used to analyze the 1,3,6,8-THN and flaviolin, was performed under the following conditions: column, ODS-80Ts column (4.6×150 mm; Tosoh Corp., Tokyo, Japan); column temperature, 25°C; mobile phase, linear gradient from 5% CH3CN in H2O to 50% CH3CN in H2O (each contained 2% acetic acid) over 30 min; flow rate, 1.0 mL/min; detection, UV detector at 254 nm.
The M. grisea mycelia became black in a 2-week culture because of melanin formation on an ordinary PDA plate (Fig. 3A), whereas the mycelia became white and orange on a PDA plate with tolprocarb and tricyclazole, respectively (Fig. 3B and E) probably because of melanin biosynthesis inhibition at respective sites on the pathway. The mycelia treated with tolprocarb became black upon addition of scytalone or 1,3,6,8-THN (Fig. 3C and D). However, the mycelia treated with tricyclazole did not show any significant change in color upon addition of scytalone or 1,3,6,8-THN (Fig. 3F and G).

The accumulation of 1,3,6,8-THN in the culture of A. oryzae pTA-pks was examined. Although no accumulation of 1,3,6,8-THN was detected in the control culture of nontransformant A. oryzae and transformant A. oryzae pTAex3 (data not shown), 234 ppm of 1,3,6,8-THN was detected in the culture of A. oryzae pTA-pks (Fig. 4). In the culture of A. oryzae pTA-pks, accumulation of black pigment was also observed (data not shown). Furthermore, the accumulation of 1,3,6,8-THN and black pigment was reduced depending on the concentration of tolprocarb added to the culture media. The concentration of 1,3,6,8-THN in the culture was reduced to 13 ppm after adding 4.3 µM of tolprocarb (Fig. 4).

The enzymatically synthesized 1,3,6,8-THN was unstable in the reaction mixture, and a part of 1,3,6,8-THN was rapidly converted to flaviolin. The synthesized 1,3,6,8-THN was completely converted to flaviolin by heating at 100°C for 30 min.24) In this reaction mixture, the PKS product was formed depending on the protein amount and reaction time throughout 1 hr; the enzyme reaction then proceeded stably with pH 7.2 at 25°C, producing 43.8 µM of 1,3,6,8-THN in our assay system (data not shown). It was here that we first established the in vitro assay system of M. grisea PKS. The PKS inhibition rate increased depending on the increasing concentrations of tolprocarb. The IC50 and IC90 were 0.03 µM and 0.23 µM, respectively; when 0.6 µM of tolprocarb was added, the PKS inhibition rate reached 99% (Fig. 5). On the other hand, PKS was not inhibited when tricyclazole and carpropamid were added, even at 30 µM (Fig. 5).

M. grisea causes rice blast, one of the major diseases in rice. In its infection process, unicellular infection structures, called appressoria, and accumulation of 1,8-DHN-melanin in the appressoria are essential for the penetration of M. grisea into host plants.1–9) It was reported that 1,8-DHN-melanin-deficient mutants of M. grisea fail to produce the dark gray pigment in mycelia and fail to infect an intact host plant.10) Compounds that inhibit melanin biosynthesis are widely used as practical fungicides for M. grisea. Although cMBIs do not exhibit direct inhibitory activity on fungal growth, these compounds inhibit the accumulation of 1,8-DHN and melanin, which is key to the infection process. However, evolving resistance to some cMBIs that inhibit dehydration steps has become a problem in rice blast control as well as other plant diseases.20) Tolprocarb, which is being developed by Mitsui Chemicals Agro, Inc., showed a high controlling effect on rice blast by both nursery box application and broadcasting on paddy rice (data not shown), and its practical value has been verified. Therefore, in considering the possibility of cross-resistance, clarifying tolprocarb’s target site becomes of paramount importance.
Biochemical analysis of enzymes involved in the melanin biosynthetic pathway has been reported in various fungal species (Fig. 6). It is known that accumulation and polymerization of 1,8-DHN and its intermediates cause pigmentation of the mycelia of M. grisea.10) Tricyclazole inhibits the two reduction steps in the biosynthetic pathway of 1,8-DHN. In a recovery test, tolprocarb decolorized M. grisea mycelia, whereas tricyclazole turned them orange (Fig. 3B and E). The mechanism that induces these various color changes is not yet known; a possible explanation is that different sites of inhibition on the pathway entail different compositions of pigments. Addition of 1,3,6,8-THN and scytalone to the medium recovered the black mycelia only when the mycelia were treated with tolprocarb (Fig. 3C and D). These results suggested that tolprocarb’s target is at the upper site of scytalone and 1,3,6,8-THN synthesis, or, more precisely, where polyketide synthesis and/or pentaketide cyclization catalyzed by PKS occurs. Although there was another possibility of chemical suppression of PKS expression, here we examined the effect of tolprocarb and cMBIs on the activity of the PKS of M. grisea in the following tests.25)
We attempted to examine tolprocarb’s effect on PKS from M. grisea by establishing an in vitro evaluation system. Because the PKS gene has already been identified in C. lagenarium,22) the PKS gene of M. grisea was predicted on the basis of homology with the PKS gene of C. lagenarium. As deduced from the amino acid sequence, the PKS protein of M. grisea conserves active sites in the following order: β-ketoacyl synthase (KS), acyltransferase (AT), two acyl carrier proteins (ACP), and thioesterase (TE). This architecture is similar to those of other fungal type I polyketide synthases of Claisen-type cyclization, for example, C. lagenarium PKS1, A. nidulans WA, and STCA.26) Because M. grisea also produces 1–8-DHN-melanin much like C. lagenarium, it was expected that the PKS gene of M. grisea would similarly catalyze the synthesis of 1,3,6,8-THN from malonyl-CoA.24) The PKS gene of M. grisea was cloned and subsequently inserted into the pTAex3 plasmid for expression in A. oryzae (Fig. 2). Transformant A. oryzae with pTA-pks showed accumulation of 1,3,6,8-THN (Fig. 4) and black pigments in A. oryzae. This phenomenon suggested that functional PKS of M. grisea might be expressed in A. oryzae. Furthermore, as demonstrated by SDS-PAGE, a protein having the same size of PKS (236 kD) was expressed in A. oryzae pTA-pks (data not shown). These results indicate that expressed PKS produced 1,3,6,8-THN in A. oryzae. Because 1,3,6,8-THN is known to be unstable and easily polymerized to a black pigment or oxidized to flaviolin,24) it seems logical that a part of 1,3,6,8-THN would polymerize, causing the accumulation of black pigments. The accumulation of black pigments and THN was reduced by adding tolprocarb (Figs. 3 and 4), suggesting that tolprocarb inhibits PKS.
To confirm direct interaction between tolprocarb and PKS, we assayed PKS activity in a cell-free system. This process was reported by Fujii et al. with the PKS of C. lagenarium24); however, in this study, we achieved an in vitro enzymatic synthesis of 1,3,6,8-THN from malonyl-CoA by the PKS of M. grisea in the cell-free system. This reaction corresponded well with the reaction of C. lagenarium PKS.24) The inhibition study of this system revealed that tolprocarb inhibited the PKS of M. grisea at a 10–100 nM range. On the other hand, tricyclazole and carpropamid did not affect PKS activity (Fig. 5), nor did other cMBIs such as pyroquilon and diclocymet (data not shown). It is therefore suggested that tolprocarb is a novel PKS inhibitor. In other words, although this compound interferes with melanin biosynthesis in M. grisea, the site of action is completely different from that of cMBIs (Fig. 6). Tolprocarb is expected to show no or very low possibility of cross-resistance with cMBIs.
We conducted the same experiments on PKS from both C. lagenarium and M. grisea for a comparison because C. lagenarium is insensitive to tolprocarb in antifungal experiments (data not shown). The transgenic A. oryzae that possessed the PKS gene of C. lagenarium was created and used in an in vitro assay of PKS using a membrane fraction in the same manner as that for M. grisea. The inhibitory activity of tolprocarb on the PKS of C. lagenarium was found to be very low; the IC90 for C. lagenarium was more than 100 times higher than that for M. grisea (data not shown). These data support the assertion that PKS is the target protein of tolprocarb.
In conclusion, the target protein of tolprocarb, as opposed to that of cMBIs, was found to be the PKS of M. grisea. It is expected that tolprocarb will prove effective against resistant M. grisea because of the fungicide’s different target site compared with that of cMBIs. Tolprocarb, N-(benzoylamino-alkyl) carbamate, is a new class of fungicide that features a novel mode of action among agrochemicals.
We express our sincere gratitude to Professor Katsuya Gomi (Tohoku University) for providing the expression plasmid.