2015 Volume 40 Issue 2 Pages 38-43
2015 Volume 40 Issue 2 Pages 38-43
Pyflubumide is a novel acaricide with a unique chemical structure that contains a methoxy-substituted hexafluoroisopropyl group on the anilino moiety. The compound has shown remarkable activity against spider mites including strains resistant to existing acaricides collected from the fields of Japan. Since carboxin was developed in 1966, various carboxamides with the same mode of action, i.e., succinate dehydrogenase inhibitors, have been created and developed. Although succinate dehydrogenase plays an important role in energy metabolism in aerobic organisms, the practical usage of the carboxamides has been primarily limited to disease control and an acaricidal activity has never been reported. The study to create a new carboxamide molecule revealed that introducing a fluoroalkyl group at the 4′-position of the aniline molecule remarkably enhanced the acaricidal activity. This finding prompted extensive research to ultimately identify pyflubumide. In this report, details of the structure–activity relationships from the lead compound to pyflubumide are described.
Phytophagous mite species, especially Tetranychus and Panonychus, are known as serious pests of various crops cultivated worldwide because damage from their feeding causes a significant loss of crop yield and quality. Some mite species are also notorious for their rapid development of resistance to agrochemicals, which makes it more difficult to control them. Although many acaricides with a different mode of action have been developed as a result of those properties of spider mites, a number of cross-resistances have been reported not only in the same chemical class of acaricides but also between compounds possessing different modes of action.1,2) Therefore, it is desired to constantly discover and develop novel acaricides that will be effective against the population that is resistant to existing agrochemicals.
The discovery of pyflubumide originated from our interest in the research of various carboxamides in the late 1990s’. Carboxin-related carboxamides had been considered relatively narrow spectrum fungicides, however, it was reported that carboxamide 1 with an ortho-branched alkyl substituent on the anilino moiety exhibited a broader spectrum of activity.3,4) Meanwhile, a novel insecticide 2 with a unique heptafluoroisopropyl substituent was reported by our colleague.5,6)
We initially designed and synthesized a hybrid analogue 3 with substituents characteristic in such fungicides and insecticides together, but it showed only low fungicidal activity. It seemed that this low fungicidal activity was attributed to the high lipophilicity of derivative 3. To improve the fungicidal activity, the less lipophilic derivative 4 was synthesized. Although the fungicidal activity of derivative 4 was not improved, it showed some larvicidal activity against Tetranychus urticae.7) This acaricidal activity drew our attention because of the importance of developing a novel acaricide with a new chemical structure.
Carboxamide fungicides such as flutolanil8) are known as succinate dehydrogenase inhibitors (SDHIs).9) Considering the structural similarity between 4 and SDHI fungicides, we assumed that the acaricidal activity of 4 could be derived from the inhibition of mitochondrial complex II. Therefore, the acid moiety of 4 was initially modified by referring to SDHI carboxamides9): mepronil, boscaild, penthiopyrad and furametpyr.10)
As a result, we have found that only derivative 5 shows highly potent larvicidal and adulticidal activity against T. urticae.11,12)
The chemical structure of compound 5 is evidently new as an acaricide and is surely promising as a novel acaricide. The optimization of the acaricidal performance of the compounds was started, which led to the identification of pyflubumide (6, NNI-0711).
We here describe the discovery, chemistry, structure-activity relationships and some biological features of pyflubumide together with its derivatives (Fig. 1).

Chemical structures were confirmed by 1H NMR spectroscopy using a Bruker ARX-400 NMR spectrometer with tetramethylsilane as an internal standard. Melting points were measured with a Mettler FP80 melting point apparatus and are uncorrected.
1.1. Synthesis1,3,5-Trimethylpyrazole-4-carboxylic acid (7) and aniline 8 were prepared by the methods reported in the literature.13,14) Anilide 9 was obtained by reacting 1,3,5-trimethylpyrazole carboxylic chloride with aniline 8. Pyflubumide was obtained from the acylation of anilide 9. The procedure used to synthesize pyflubumide is described below.15)
1.2. 3′-Isobutyl-1,3,5-trimethyl-4′-[2,2,2-trifluoro-1-methoxy-1-(trifluoromethyl)ethyl]pyrazole-4-carboxanilide (9)To a suspension of 1,3,5-trimetylpyrazole-4-carboxylic acid (7) (18.48 g, 120 mmol) and dimethylformamide (DMF, 600 mg) in toluene (60 mL) was added thionyl chloride (SOCl2, 17.48 g, 144 mmol). The mixture was refluxed for 2 hr and evaporated to give 1,3,5-trimetylpyrazole-4-carboxylic acid chloride. This compound was used for the next reaction without further purification.
The obtained acid chloride was added to a stirred suspension of aniline 8 (32.9 g, 0.1 mol) and sodium bicarbonate (NaHCO3, 13.1 g, 0.15 mol) in ethyl acetate (180 mL). The mixture was stirred for 3 hr at 50°C and extracted with tetrahydrofuran (THF, 400 mL), washed with water (100 mL) and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure and the residue was washed with a mixed solution of ethyl acetate (20 mL) and hexane (40 mL) to give anilide 9. Yield: 37.8 g (81%). 1H NMR δH(DMSO-d6): 9.76 (s, 1H), 7.87 (d, 1H), 7.68 (dd, 1H), 7.38 (d,1H), 3.69 (s, 3H), 3.42 (s, 3H), 2.85 (d, 2H), 2.50 (m, 1H), 2.36 (s, 3H), 2.26 (s, 3H), 0.91 (d, 6H).
1.3. 3′-Isobutyl-N-isobutyryl-1,3,5-trimethyl-4′-[2,2,2-trifluoro-1-methoxy-1-(trifluoromethyl)ethyl]pyrazole-4-carboxanilide: pyflubumideTo a stirred solution of anilide 9 (33.5 g, 72 mmol) in THF (350 mL), 60% sodium hydride (NaH, 3.6 g, 90 mmol) was added and stirred for 15 min at room temperature. To the mixture, isobutyryl chloride (9.8 g, 92 mmol) was added and stirred for 3 hr at room temperature. The mixture was dissolved in ethyl acetate (400 mL) and washed with water (200 mL). The organic phase was dried over anhydrous magnesium sulfate and evaporated. The resulting residue was purified by silica gel column to give pyflubumide. Yield: 26.0 g (68%). 1H NMR δH(DMSO-d6): 7.45 (d, 1H), 7.25 (dd, 1H), 7.15 (d, 1H), 3.61 (s, 3H), 3.41 (s, 3H), 2.94 (m, 1H), 2.83 (d, 1H), 2.30 (s, 3H), 2.11 (s, 3H), 1.95 (m, 1H), 1.16 (d, 6H), 0.68 (d, 6H). Water Solubility: 0.27×10−3 g/L (20°C). Partition coefficient log Po/w 5.34 (25°C).
1.4. Other derivativesThe other derivatives were synthesized in a similar way by the reaction of the corresponding carboxylic acids16) and anilines,7,14) and commercially available reagents and solvents were used unless otherwise noted (Fig. 2).

For the biological assay of pyflubumide derivatives, T. urticae Koch and Panonychus citri (McGregor) were used. Both spider mites had been continuously reared for more than 30 years at the Research Center, Nihon Nohyaku Co., Ltd., using leaves of the kidney bean Phaseolus vulgaris L., or citrus fruits (Rutaceae), respectively, as feed under the condition of 25±1°C, 60–70% R.H. and a 16L : 8D photoperiod without any acaricidal treatment.
2.2. Leaf disk assayTo investigate the acaricidal activity of candidate compounds against T. urticae and P. citri, ten adult female spider mites on leaf disks (2 cm diameter) of kidney bean or citrus Citrus unshiu Marcovitch were prepared on wet filter paper. The compound was prepared as 100 g a.i./L of EC formulation and diluted to various concentrations (300, 100, 30, 10, 3, 1 mg a.i./L) with water containing a wetting agent (Mai-Rinoh®, 0.1 mL/L). The leaf disk and spider mites were sprayed with an equivalent of 500 L per hectare of test solution. Control plots were treated with water containing the wetting agent. Each treatment consisted of two replicates. The spider mites on the leaf disk were kept at 25±1°C, 60–70% R.H. and a 16L : 8D photoperiod. Two days after treatment, the surviving mites were counted. Biological activity was expressed using the corrected mortality (%), which was calculated according to the formula shown below.
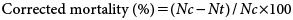 |
We collected field populations of T. urticae and P. citri from major crop-producing fields in Japan in 2008–2009 to investigate the susceptibility of mite populations to pyflubumide and commercial acaricides. Collected populations were maintained in the same conditions used for continuous rearing. Offspring to the 3rd generation from the field population was used for biological assay. All experiments were treated with leaf disk assay (see 2.2) and were replicated two times. The dose–response data were subjected to probit analysis17) and the activities were calculated as LC50 values.
The structure of lead compound 5 consisted of three major parts: a pyrazole ring, a phenyl ring and an amide bridge. Optimization and structure–activity relationship were attempted as described as below.
1.1. Pyrazole ringTable 1 summarizes the effect of the substituents of the pyrazole moiety. A methyl group was the best substituent for every position (11). However, no or larger substituents decreased the acaricidal activity. This tendency was remarkable for the R2 position (12, 13).
 | ||||
|---|---|---|---|---|
| No. | R1 | R2 | R3 | LC50 (mg a.i./L) |
| 5 | CH3 | CH3 | Cl | 30 |
| 10 | CH3 | CH3 | H | 100–300 |
| 11 | CH3 | CH3 | CH3 | 3–10 |
| 12 | CH3 | H | CH3 | >300 |
| 13 | CH3 | Cl | CH3 | 300 |
| 14 | H | CH3 | CH3 | 30–100 |
| 15 | n-Pr | CH3 | CH3 | 10–30 |
The most favorable 1,3,5-trimethylpyrazole moiety is synthetically advantageous because this acid moiety is easily prepared from commercialized materials. In view of manufacturing as well as the acaricidal activity, the acid moiety was fixed as 1,3,5-trimethylpyrazole and the effect of the other substructure was examined.
1.2. Phenyl ringTable 2 summarizes the effect of the substituents on the 2′- and 3′-position. First, the effect of the substituents on the 2′-position was examined. 1,3-Dimethylbutyl derivative 11 showed good acaricidal activity. Further investigation revealed that the 1,3-dimethylbutyl group was the most favorable substituent for the 2′-position (16–20). It was noteworthy that the most favorable substituent was the same as that of penthiopyrad and penflufen.18)
 | |||
|---|---|---|---|
| No. | Y | LC50 (mg a.i./L) | |
| 2′ | 3′ | ||
| 16 | H | H | >300 |
| 17 |  | H | >300 |
| 18 |  | H | 100–300 |
| 19 |  | H | 10–30 |
| 11 |  | H | 3–10 |
| 20 |  | H | 10–30 |
| 21 | H |  | 30–100 |
| 22 | H |  | 300 |
| 23 | H |  | 30–100 |
| 24 | H |  | 3–10 |
| 25 | H |  | 10–30 |
Next, the effect of the substituents on the 3′-position was examined. Based on the 2′-substituted compound’s substructural similarity with the SDHI carboxamides, we first chose the isopropyloxy group as the substituent that is employed for 3′-substituted SDHI carboxamides, such as mepronil and flutolanil. Isopropyloxy derivative 21 showed acaricidal activity, but the activity level was moderate.
The lower activity of derivative 21 seemed to be attributed to the oxygen of the isopropyloxy group and substituting carbon for oxygen was thought to be effective. Taking this assumption into consideration, isobutyl derivative 24 was synthesized. As expected, it showed greater acaricidal activity than did isopropyloxy derivative 21 and the activity level was comparable to that of 2′-(1,3-dimethylbutyl) derivative 11. A further investigation of the other 3′-alkyl derivatives revealed that the isobutyl group was the most favorable substituent for the 3′-position (22–25).
In short, activity was strongly influenced by the number of carbon atoms and type of chain branching. An alkyl chain at the 2′- or 3′-position was required for activity, and the favorable substituent was different on each position. The 1,3-dimethylbutyl group was the best substituent for the 2′-position, and the isobutyl group was best for the 3′-position.
The effect of Z on the fluoroalkyl groups at the 4′-position is shown in Table 3. First, the effect of Z of the 2′-(1,3-dimethylbutyl) derivatives (type A) was examined. Fluorine derivative 26 showed less activity, however, hydrogen derivative 11 showed high acaricidal activity. It seemed that less lipophilic substituents were good for activity. To examine the importance of lipophilicity, hydroxy and alkoxy derivatives were also synthesized. Although hydroxy derivative 27 did not show activity, alkoxy derivatives (28–30) showed acaricidal activity, especially methoxy derivative 28, whose activity was as good as that of compound 11.
 | |||
|---|---|---|---|
| No. | type | Z | LC50(mg a.i./L) |
| 26 | A | F | 100–300 |
| 11 | A | H | 3–10 |
| 27 | A | OH | >300 |
| 28 | A | OCH3 | 3–10 |
| 29 | A | OEt | 10–30 |
| 30 | A | O-n-Pr | 10–30 |
| 31 | B | F | >300 |
| 24 | B | H | 3–10 |
| 32 | B | OH | 30 |
| 9 | B | OCH3 | 10–30 |
| 33 | B | OEt | 30–100 |
| 34 | B | O-n-Pr | >300 |
The effect of Z on the 3′-isobutyl derivative (type B) was also examined. Fluorine derivative 31 did not show activity, but hydrogen derivative 24 showed good activity. Methoxy derivative 9 showed slightly lower activity than did hydrogen derivative 24, but its activity was highest among the hydroxy- and alkoxy derivatives (9, 32–34). Except when Z was hydroxy group, the effect on Z on type B was similar to that on type A.
These structure–activity relationships would be explained by the physicochemical profile of these fluoroalkyl groups. Introducing fluorine to the hexafluoroisopropyl derivatives (11, 24) sharply decreased activity. The low activity was attributed to its high lipophilicity. Acaricidal activity was strongly influenced by the introduction of alkoxy groups. A methoxy group maintained high activity, however slightly more lipophilic groups (ethoxy or propyloxy) were not acceptable.
The 3′-isobutyl aniline moiety was easily prepared from inexpensive commercialized materials.19) Moreover, the isobutyl group has no chiral center. From a manufacturing point of view, the alkyl chain branch of the aniline moiety was fixed to a 3′-isobutyl group and the effect of the amide moiety was examined.
1.3. Amide bridgeThe effect of the substituent R4 on the amide moiety is shown in Table 4. N-Acyl-substituted derivatives showed good to excellent results.
 | |||
|---|---|---|---|
| No. | Z | R4 | LC50 (mg a.i./L) |
| 35 | H | COCH3 | 1–3 |
| 36 | H | COEt | 1–3 |
| 37 | H | CO-i-Pr | 1–3 |
| 38 | H | COCH2C(CH3)3 | 3–10 |
| 39 | H | COPh | 3–10 |
| 40 | OCH3 | COCH3 | 1–3 |
| 41 | OCH3 | COEt | 1–3 |
| Pyflubumide | OCH3 | CO-i-Pr | 1–3 |
| 42 | OCH3 | COCH2C(CH3)3 | 3–10 |
| 43 | OCH3 | COPh | 3–10 |
The less-hindered acyl-substituted derivatives (35–37, 40–41 and pyflubumide) showed excellent activity. The activity of acyl-substituted derivatives (35–37) was three times higher than that of the corresponding non-substituted derivative 24, whereas the activity of acyl-substituted derivatives (40–41 and pyflubumide) with the methoxy group was ten times higher than that of 9. Hindered acyl- or benzoyl-substituted compounds (38–39, 42–43) also showed high activity, but their activity level was not comparable to those of less-hindered acyl-substituted derivatives.
These results could be explained by considering acyl-substituted derivatives as propesticides. Compared to non-substituted derivatives, acyl-substituted derivatives could easily penetrate into the mite body and then be metabolized to the deacylated derivatives (9 and 24), which are the active forms of these derivatives. It seemed that improved penetration and metabolic activation might deliver the excellent acaricidal activity of the substituted derivatives (35–43 and pyflubumide).
Considering that the moderate activity of 9 was attributed to its poor penetration, the remarkable activity improvement by acylation of methoxy derivatives (40–43 and pyflubumide) could be explained by the improved penetration. Offsetting the drawback by acylation of 9, the activity of R4-substituted methoxy derivatives (40–43 and pyflubumide) was comparable to that of substituted hydrogen derivatives (35–39).
Less-hindered acyl groups seemed to be more easily metabolized than were hindered (or bulky) acyl groups. Taking into consideration the possible decomposability of these functional groups, the excellent activity of less-hindered acyl-substituted derivatives (35–37, 40–41 and pyflubumide) could be explained by the readiness of metabolic activation to non-N-substituted derivative 9 or 24.
In fact, when pyflubumide was incubated with spider mite (T. urticae) homogenate, its deacylated derivative 9 was observed.20) This result strongly supports our hypothesis that the deacylated derivatives would be the active forms and excellent acaricidal activity is derived by improving penetration and metabolic activation.
The compounds that showed excellent activity against T. urticae were further investigated to determine whether they had acaricidal activity against P. citri, another important phytophagous species of mite.
These compounds also showed acaricidal activity against P. citri, and particularly 35, 40, 41, and pyflubumide showed excellent activity (Table 5). In view of its residual efficacy in pot testing (data not shown), pyflubumide was finally selected as the developed compound.
 | |||
|---|---|---|---|
| No. | Z | R4 | LC50 (mg a.i./L) |
| 35 | H | COCH3 | 1–3 |
| 36 | H | COEt | 3–10 |
| 37 | H | CO-i-Pr | 3–10 |
| 40 | OCH3 | COCH3 | 1–3 |
| 41 | OCH3 | COEt | 1–3 |
| Pyflubumide | OCH3 | CO-i-Pr | 1–3 |
The activity of pyflubumide was examined against field populations of spider mites collected from various crops in Japan. Pyflubumide showed excellent activity against field populations of P. citri and T. urticae that had developed resistance to conventional acaricides (Table 6).
| Species | Collected site | LC50 (mg a.i./L) | |||||
|---|---|---|---|---|---|---|---|
| Pyflubumide | Fenpyroximate | Acequinocyl | Bifenazate | Etoxazol | Milbemectin | ||
| T. urticae | Itayanagi, Aomori pref. | 0.86 | > 50 | > 150 | 40–200 | > 50 | > 10 |
| Suzaka, Nagano pref. | 2.3 | > 50 | > 150 | > 200 | > 50 | 2–10 | |
| Kumamoto, Kumamoto pref. | 0.52 | > 50 | > 150 | 40–200 | > 50 | > 10 | |
| Susceptible strain | 1.2 | 0.32 | 3.3 | 2.4 | 0.036 | 0.44 | |
| P. citri | Arida, Wakayama pref. | 5.0 | > 50 | 150 | > 200 | — | > 10 |
| Iyo, Ehime pref. | 1.7 | > 50 | > 150 | > 200 | — | 2–10 | |
| Karatsu, Saga pref. | 1.5 | > 50 | > 150 | > 200 | — | 2–10 | |
| Susceptible strain | 1.3 | 8.6 | 8.9 | 15 | — | 0.059 | |
These results suggest that pyflubumide could be a promising new acaricide that provides excellent control efficacy even against resistant populations that are hard to controll with conventional acaricides.
Pyflubumide was discovered as a novel carboxanilide acaricide with a methoxy-substituted hexafluoroisopropyl group on the anilino moiety. It showed remarkable acaricidal activity against important species of spider mites (Tetranychus and Panonychus species) for crop protection and also showed excellent activity against field populations of mites that had developed resistance to conventional acaricides. Furthermore, our study revealed that pyflubumide-deisobutylated metabolite 9 inhibits electron transport in mitochondrial complex II of spider mites, as with the cases of beta-ketonitrile acaricides.21,22) However, it was also suggested that their binding sites on mitochondrial complex II and/or the manners of binding are not identical.20,23,24) These results demonstrate that pyflubumide could be a promising new acaricide. Pyflubumide has been tested in official trials with the code number NNI-0711 in Japan since 2007, and a registration petition for this acaricide has already been made in 2012.
The authors are very thankful to the JPPA (Japan Plant Protection Association) and the national, prefectural and incorporated administrative agency research institutions for their technical support and biological evaluations of pyflubumide.