2015 Volume 40 Issue 3 Pages 106-110
2015 Volume 40 Issue 3 Pages 106-110
Imazethapyr is widely used in soybean, and leguminous crops for control of wide variety of broad leaf weed species. A study was conducted to evaluate residues of imazethapyr in the soil and soybean grain at five different locations in India. Imazethapyr was applied at 100 g/ha, as a post emergence herbicide to control weeds in soybean fields. Residues of imazethapyr were found in the range from 0.006 to 0.018 µg/g in the soybean grains samples in all five locations. However in the soil, residues were found to be below 0.0010 µg/g in four locations, and 0.0015 µg/g in one location. Less residues were found in soils as compared to plant samples. Based on this study a pre-harvest interval of 90–102 days is suggested for soybean crops after imazethapyr application. This indicates the judicious use of imazethapyr by farmers in soybean fields.
Herbicides play an important role in higher crop yields by controlling a variety of weeds; however, at the same time its longer persistence in the soil surface due to the adsorption process may potentially affect the quality and yield of the next crop cultivated on the same field and may cause numerous environmental problems. Longer persistence also increases the chance of contaminating surface and groundwater through leaching and runoff.1,2) Imazethapyr, 5-ethyl-2-[(RS)-4-isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl]nicotinic acid belongs to the imidazoline group that has a toxicity class of III and is used as a selective herbicide for the control of a broad spectrum of weed species.3–6) Extremely effective weed control and good crop tolerance in pulses and other leguminous crops have contributed to an increase in the popularity of this herbicide.7,8) Imazethapyr acts by reducing the levels of three branched-chain aliphatic amino acids, isoleucine, leucine and valine by inhibiting acetohydroxy acid synthase (AHAS), a common enzyme of the biosynthetic pathway for these amino acids. This inhibition causes a disruption in protein synthesis which in turn, leads to interference in DNA synthesis and cell growth. Under field conditions, imazethapyr dissipates in the soil by microbial degradation and photolysis.9)
Imidazolinone herbicides are generally weakly adsorbed by soil.10,11) Organic matter and pH significantly affect imazethapyr behavior in soil.8,12) However, imazethapyr does not readily leach under field conditions,13) even though some authors have reported leaching of imazethapyr below 25 cm in soil during four months.8,14) As a result of leaching and runoff, imazethapyr residues were reported in streams and rivers in the Midwestern USA at concentrations above the maximum residue limits in 71% of samples.15) Due to leaching and persistence that may damage subsequent rotation crops, some researchers have recommended re-cropping periods of 6–34 months for imazethapyr and have reported that imazethapyr has a rapid initial phase of degradation, followed by a slower second phase leading to long-term persistence, especially in clay soil.16,17) A half-life of 18–21 day was reported of imazethapyr in silty clay soil.18) Stable herbicides may be taken up by plants, which results in unwanted terminal residues.14,19) Reports have shown that residues of ALS inhibitors or their metabolites can persist into the following growing seasons.20) Phytotoxicity to sensitive crops grown in rotation such as canola and lentils, mustard, or sugar beets18,21–25) is reported as a result of prolonged persistence in the soil.
The soybean (Glycene max) is one of the most important crops in the world and a major rainy-season crop in Madhya Pradesh, India. During the last two years soybean has been grown in an area of 40.4 million hectares producing approximately 3.9 million tones, with an average productivity of 796–885 kg/ha in this region. Today, the use of herbicides has become indispensable for managing weeds in cultivated lands, due to the scarcity labors in almost all parts of the globe. Hence, heavy reliance on chemical weed control enhances the risk of residue in the soil and in crop plants. Therefore the presence of terminal residues in plants or/crop produced at harvest is of great concern. Most of the residue studies of the imidazoline group of herbicides have been conducted in water sources of the southern and central United States.
Currently, research on the terminal residues of imidazoline herbicides in the soil and soybean plants in large cultivated areas in sub-tropical climate conditions has been exceedingly rare. The wide range of crops that are treated with the imidazoline group of herbicides can result in repeated applications of imazethapyr on the same land. The soybean farmers studied have been applying imazethapyr continuously over the last 6–7 years. In some parts of the Madhya Pradesh province, phytotoxicity to soybean plants has also been reported by farmers. Thus, this study was undertaken in farmers’ fields to determine terminal residues of imazethapyr in soil, soybean grain, and straw following its application to soybean.
A study was meticulously planned to determine terminal residues of imazethapyr in the soil, soybean grain, and straw at farmers’ fields in the soybean growing area. Field experiments were conducted at five different locations in the Majholi Tehsil near Jabalpur, Madhya Pradesh, in 2012 (Table 1). Farmers’ field were located at 23.130N latitude, 79.580E longitude at an altitude of approximetly 390 m above mean sea level. Jabalpur is located in the centre of India and fall in the agroclimatic region of Kymore plateau and Satpura Hills agroclimatic region. The climate of the region is subtropical, with an average rainfall of 1500 mm. The soils in the study area are mostly black, known as vertisols, and belongs to kheri-series of fine montmorillonite, Hyperthermic family of Typic Haplusterts. Rice and soybean are the major crops grown in the rainy season.
| Soil characteristics | Field location | ||||
|---|---|---|---|---|---|
| Location 1 | Location 2 | Location 3 | Location 4 | Location 5 | |
| pH | 8.04 | 8.06 | 8.11 | 8.09 | 8.14 |
| EC (dS/m) | 0.12 | 0.110 | 0.16 | 0.15 | 0.12 |
| N (kg/ha) | 156.80 | 146.30 | 219.5 | 167.25 | 203.80 |
| K (kg/ha) | 11.59 | 16.762 | 19.72 | 24.86 | 25.09 |
| Organic carbon (%) | 0.377 | 0.405 | 0.418 | 0.423 | 0.29 |
Soils collected from farmers’ field in different locations have different physicochemical characteristic. Soybean variety JS 9560 was sown, and imazethapyr (10% SL) was sprayed as post emergence, i.e. 20 days after sowing of soybean seeds at rates of 100 g/ha in each location as per the recommended guidelines. The crop was grown under irrigated conditions with the recommended package of practices of Madhya Pradesh State. During 2012, the soybean fields under study received approximately 2200 mm of rainfall. For the determination of terminal residues, soil and plant samples were collected at harvest (110 days, which is equivalent to 90 days after spraying the herbicide on soybean crop) randomly from ten to fifteen different places, representing an area of 500 m2 area in each location, so that the sample areas covered a wide range of soybean-growing areas under various farming practices. Soil cores of each approximately 3 kg of soil were randomly taken from untreated and treated plots using a soil auger to a depth of 15 cm from the surface in each location. Pebbles and other unwanted materials were removed manually. The bulk soil samples from each location were air dried under shade, powdered, and passed through a 3-mm sieve. Approximately 3 kg of representative soybeans plant sample was collected randomly from the imazethapyr-treated and control plots. Grains were separated from the plants samples. The straw samples were cut in small pieces and air-dried. Soybean grains and straw samples were then ground in a mechanical grinder.
2. Determination of imazethapyr residues in soil and plant samplesImazethapyr residues were determined as described by Sondhia.8) Representative soil (20 g) and plant samples (grain and straw) were palced in Erlenmeyer flasks (250 mL) and extracted with 0.5 N NaOH (50 and 100 mL, respectively) in a horizontal shaker (repeated twice). Additional methanol (50 mL) was added to the flask, which was then shaken vigorously, filtered, and adjusted to a pH to 2 with 6 N hydrochloric acid. The content was transferred to a separatory funnel (500 mL) and partitioned with dichloromethane (50 mL, twice). The lower dichloromethane layer was collected, combined, dried on anhydrous Na2SO4, and passed through activated charcoal. The solvent was evaporated completely to dryness using a rotary vacuum evaporator. Finally residues were dissolved in 5 mL of methanol and subjected to cleanup.
Soybean grains and straw samples were cleaned on a glass column (10 cm×2 cm i.d.) packed with Celite (1 g) and activated charcoal (0.25 g) between anhydrous sodium sulfate (2 g) at each end. The column was conditioned with methanol. The concentrated extract was added at the surface of the column and eluted with methanol and water (60 : 40 v/v). Elutes were collected, and the solvent was evaporated completely using a rotary vacuum evaporator. Residues were dissolved in 5 mL of methanol and filtered through Pall Nylon 0.45-µm filter paper prior to HPLC analysis.
Quality control (QC) and quality assurance (QA) procedures were used to determine and maintain the quality of the analytical data. QC and QA procedures were adopted at the sampling, extraction, and analytical stages of the studies. During the sampling stage, control samples were collected from untreated plots. The spiked samples (soyebean grains, straw, and soil) were fortified with a mix of standard solutions to obtain 0.01 and 0.5 µg/g concentrations, and the extraction and cleanup processes were described above as the percentage of the recovery of imazethapyr. Known concentrations of imazethapyr (0.01, 0.05, 0.5, 1.0, and 5.0 µg/mL) were prepared in methanol by diluting a stock solution (1000 µg/mL) to obtain the calibration curve (Table 2). Certified reference imazethapyr (AccuStandard, USA) was used for calibration (Figs. 1 and 2). The concentration of imazethapyr was determined by comparing the peak area of the samples and calibration curves of five levels of standards. A reporting limit of 0.01 µg/g was used for the calculation. The limit of determination [(LOD), estimated to be three times the background noise] and the limit of quantification [(LOQ) estimated to be 10 times the background noise] were found to be 0.001 and 0.01 µg/mL, respectively.26)
| Injected concentration of imazethapyr (µg/mL) | Av. area (mabs)a) | Std. deviation |
|---|---|---|
| 5 | 897940 | ±50942.80 |
| 1 | 208830 | ±9689.39 |
| 0.5 | 159884 | ±4536.66 |
| 0.1 | 48077 | ±784.14 |
| 0.01 | 15624 | ±642.05 |
a) Average of three replications; mabs: milli absorbance.

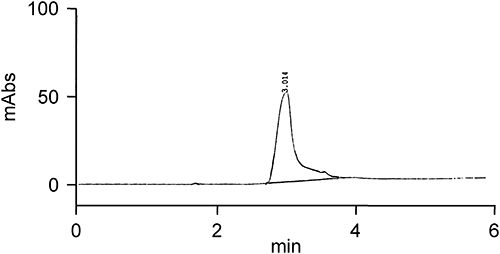
Imazethapyr residues were analyzed with a Shimadzu HPLC coupled with a diode array detector (DAD) with λmax of 250 nm for detection purposes. A Phenomenex C-18 (ODS) column (250×4.6 mm) and methanol: water (70 : 30 v/v) as a mobile phase at a flow rate of 0.9 mL/min were used to separate imazethapyr residues. A 20-µL aliquot of the samples and standard were injected into the column with a microsyringe.
The limit of quantification of imazethapyr in soil, soybean grain and straw was found to be 0.01 µg/g (Table 2). At this detection level the signal to noise ratio was 3 : 1. Due to low organic matter content soil blanks did not exhibit any peak interfering with the retention time of imazethapyr; therefore, soil samples were not subjected to a cleanup step. Equations of analytical calibration graphs, obtained by plotting peak areas on the y axis against concentrations of imazethapyr on the x axis within a range of 5 to 0.01 µg/mL was, y=172627x+37859, showed good linearity. At this concentration range, the correlation coefficient was found 0.99.
The retention time of imazethapyr was found to be approximately 3.345 min. Imazethapyr recoveries varied from 80–78%, 86–74%, and 90–76% for soil, soybean grains, and soybean straw fortified with 0.01 and 0.5 µg/g of imazethapyr, respectively (Figs. 3–5). The recovery for soybean grain and straw was acceptable up to fortification level of 0.01 µg/g (Table 3). Recoveries of imazethapyr from various matrixes at different concentration levels were satisfactory.
| Matrix | Fortified concentration (µg/g) | Concentration found (µg/g)a) | Recovery (%) | Average recovery |
|---|---|---|---|---|
| Soil | 0.50 | 0.400 | 80.0 | 79.0 |
| 0.01 | 0.007 | 78.0 | ||
| Soybean grain | 0.50 | 0.430 | 86.0 | 80.0 |
| 0.01 | 0.007 | 74.0 | ||
| Soybean straw | 0.50 | 0.450 | 90.0 | 83.0 |
| 0.01 | 0.007 | 76.0 |
a) Average of three replications.
Mature plant samples collected at harvest were analyzed for terminal residues of imazethapyr in the fields of various farmers. In the mature soybean grain samples, the highest residual concentration of 0.018 µg/g was found in location 1. In the third and fourth locations, the imazethapyr residues were found to be 0.013 and 0.015 µg/g, respectively. However, the lowest residues concentration of imazethapyr residues was detected from location 2. Residual concentrations of 0.010 and 0.011 µg/g were detected in straw from locations 1 and 2, respectively, whereas, in the other three locations, imazethapyr residues were found to be <0.01 µg/g (Table 4). This shows the fast degradation of imazethapyr residues in the soil and plants under reported agroclimatic conditions, although imazethapyr has a soil photolysis half-life of 33 months, and, in some field dissipation studies, the consistently persistence of imazethapyr was reported regardless of the soil type, agriculture practice and climatic effects.27)
| Location | Farmers name | Terminal residues of imazethapyr (µg/g) | ||
|---|---|---|---|---|
| Soil | Grain | Straw | ||
| 1 | Rajesh | <LOQa) | 0.018 | 0.010 |
| 2 | Ramkumar | <LOQ | <LOQ | <LOQ |
| 3 | Kummaz | <LOQ | 0.013 | <LOQ |
| 4 | Bhagwant | <LOQ | 0.015 | 0.011 |
| 5 | Shyam | <LOQ | <LOQ | <LOQ |
a) Limit of quantification (0.01 µg/g).
Imazethapyr residues in mature plants were also reported by other researchers. Mallipoodi et al.28) demonstrated that though imazethapyr metabolizes rapidly in corn plants through oxidative hydroxylation at the α-carbon atom of the ethyl substituent on the pyridine ring to yield α-(hydroxylethyl)-imazethapyr, it left 0.08 and 0.02 ppm of imazethapyr residues in mature stalk, and seed of corn.
3. Terminal residues of imazethapyr in soilThe persistence or dissipation of a chemical is mainly controlled by its physicochemical properties, by crop management practices, and by environmental conditions that include climate, soil physicochemistry and microbial activity in the soil. In contrast to the plant samples, at four locations in farmers’ fields, imazethapyr residues were found to be below 0.001 µg/g in the top 15 cm of soil, however 0.0015 µg/g imazethapyr residues in soil at 15 cm of soil depth was detected at 100 g/ha of the applied dose of imazethapyr (Table 4). In the literature, some studies demonstrated longer imazethapyr in soil. Marsh and Lloyd29) reported that imazethapyr persisted for a longer period in Romanian soil and showed residual effects on succeeding barley and winter wheat even after 2–3 years.
In the samples collected from various locations, less imazethapyr residue was detected in the soil. Imazethapyr had low Koc values (19.8–83.9), which suggests that little adsorption would be expected for any of the soils; it also indicated that imazethapyr has very high mobility, and consequently a high potential to leach.6,8) During the experimental period in 2012, the soybean field received approximately 2200 mm of rainfall, which was greater than the average rainfall in this region. Due to the higher solubility of imazethapyr in water (1.4–3.7 g/L), the higher rainfall (2200 mm) might have enhanced herbicide leaching in the soil after application of imazethapyr8); this may have resulted in reduced availability of imazethapyr in the soil, causing there to be no residues found in the soil at harvest at four locations.8,19,30–32) Less adsorption and a slightly alkaline pH 8.04–8.14, also favored the fast dissipation of imazethapyr residue in the surface soil (0–15 cm); thus, imazethapyr residue was consequently, not detected at harvest in the soil at four locations.
The soil pH affects imazethapyr sorption–desorption, which in turn can affect persistence and bioavailability. Long-term imazethapyr carryover has been observed in soil that is below a pH of 6.5, resulting in significant sugar beet damage.16) Selectivity in soybeans is attributed to a rapid detoxification via hydroxylation and glycosylation in the course of days1–6,31) and the persistence of the imazethapyr herbicide in the soil is very long in soils with low pH.30) In another study conducted in soils with a high pH (alkaline, cracking clay, pH 8.6) no residue was found in the top 10 cm of soil; however, 0.6–1.6 ng/g of imazethapyr was reported at 20–40 cm soil depths after four months of imazethapyr application.21) O’Sullivan et al.21) demonstrated the marked effect of rainfall on imazethapyr persistence and concluded that the herbicide was lost from the profile either by leaching to below 40 cm or by microbial breakdown due to the wetter years’ being more conducive to microbial activity in the topsoil. The bioassay results showed a marked effect of rainfall on residue persistence.
Soil pH plays an important role in the degradation and bioavailability of the imidazoline group of herbicides.6,8,21,26–32) Dissipation of imazethapyr is faster in soils with a high pH and low adsorption since the amount available in the soil solution for microbial transformation is greater. A low concentration of imazethapyr in soil is also compensated for high microbial activity, which increases the rate of degradation.6,8,30,32–36) O’Sullivan et al.21) reported imazethapyr residues mainly in the top 0–10 cm soil fraction; however, some quantity of imazethapyr residue was found at 10–20 and 20–40 cm depths, also. Besides the organic matter, clay can also play an important role in the degradation of pesticides. However Hollaway et al.34) reported the persistence of imazethapyr residues for 24 and 5 months in clay and sandy soils, respectively. Though the soils in all five locations were low in organic carbon contents, residues were detected below the maximum residue limits in plant samples.
These results suggest that there is less risk of imazethapyr carryover to succeeding crops planted the year following post emergence application to soybeans in subtropical conditions. Similar conclusions were reached by Johnson and Talbert.37) However, imazethapyr carryover has been noted infrequently in soybean fields in some parts of Madhya Pradesh during low rainfall conditions (author’s personal observations).
It can be concluded that soil with an alkaline pH, less soil adsorption capacity with high rainfalls total might leads to less terminal imazethapyr residues. The terminal imazethapyr residue in plant parts was found to be below the maximum residue limit in soybean plants set by the U.S.A. and some European countries (0.1 mg/kg).38,39) Based on this study, a pre-harvest interval of 90–102 days after herbicide application is suggested for soybean crops.