2015 Volume 40 Issue 3 Pages 130-137
2015 Volume 40 Issue 3 Pages 130-137
The bioconcentration and metabolism of 14C-labeled trans-isomers of tetramethrin (I) [3,4,5,6-tetrahydrophthalimidomethyl (1RS)-trans-chrysanthemate] in bluegill sunfish (Lepomis macrochirus) were examined under flow-through conditions. Ester cleavage followed by rapid liberation of a hydroxymethyl group from the alcohol moiety resulted in the formation of trans-chrysanthemic acid (VI) and 3,4,5,6-tetrahydrophthalimide (III) as primary metabolites in fish. III was either hydrolyzed to the diacid (V) or reduced at the 1,2-double bond (IV). VI and its metabolites formed through successive oxidation at the isobutenyl moiety were further conjugated with glucuronic acid or taurine. The rapid and extensive metabolism of trans-I with a short depuration half-life of 0.54–0.72 days resulted in a much lower bioconcentration factor (BCF), 180–310, than expected from its hydrophobicity (log Kow=4.6). The BCF value of its cis-isomer was conveniently estimated to be 230–400 by taking into account the different degradation rates between both isomers evaluated through an in vitro metabolism study.
Pyrethroids are one of the most important chemical classes of insecticide for both agricultural and public hygiene uses, because they exhibit not only excellent efficacy but also low environmental impact due to rapid dissipation.1–4) Pyrethroids are generally considered hydrophobic from their high octanol-water partition coefficient (log Kow) and low water solubility, implying a possible high bioconcentration in biota. However, many pyrethroids exhibit the lower BCF values in fish than estimated by the empirical equation proposed by Veith,5) log BCF=0.85×log Kow−0.70. The measured BCF values of eight pyrethroids (log Kow, 4.53–7.00) range from 359 to 6090,6,7) which are much lower than the estimated values (1400–180,000). These differences can be explained by large pyrethroid molecules’ low permeability through gill membranes and their rapid metabolism in fish.8–10) With regard to the metabolic profiles of chrysanthemic pyrethroids in fish, not only the chemical structures of the acid and alcohol moieties but also the cis/trans geometrical isomerism in the acid moiety influences the relative contribution of hydrolytic and oxidative routes.2,11,12) Ester cleavage predominates for trans isomers, while oxidative transformation is mainly observed for cis isomers.
Tetramethrin (I) [Neo-Pynamin®, 3,4,5,6-tetrahydrophthalimidomethyl (1RS)-cis,trans-chrysanthemate] is a synthetic pyrethroid insecticide characterized by rapid knockdown activity. Tetramethrin consists of four isomers in a ratio of approximately 4/4/1/1(1R-trans/1S-trans/1R-cis/1S-cis); the 1R-trans isomer is most biologically potent.13–15) The typical use patterns of I are indoor, residential outdoor, and limited outdoor for public hygiene,14,15) which indicates insignificant contamination of surface water bodies. Furthermore, even if contaminated, tetramethrin is most unlikely to persist in the aquatic environment due to its rapid hydrolysis.16) However, ecotoxicologcal profiles of I in aquatic bodies, especially for pyrethroid-sensitive taxa such as fish,17) should be further clarified to conduct robust hazard characterization and risk assessment.
Incidentally, several metabolism studies of I in mammals have shown that the main metabolic pathways are cleavage of the ester and imido linkages, oxidation in the chrysanthemic acid moiety, and reduction and/or hydroxylation of the alcohol moiety followed by conjugation with glucuronic or sulfonic acids.18–26) These circumstances suggest that for its bioconcentration in fish as well as its metabolism behavior, tetramethrin is one of the most suitable pyrethroids to have been investigated in detail. In order to obtain the relevant information of I on metabolism and bioconcentration in fish, we have conducted an in vivo fish study using 1RS-trans isomers of I (trans-I). For 1RS-cis isomers (cis-I) as minor components (ca. 20%), we conveniently estimated their BCF values by combining the in vivo kinetic parameters of trans-I and the in vitro values newly obtained for trans- and cis-I using fish homogenates.
14C-trans-I separately labeled at the 1- and 2-positions of the 3,4,5,6-tetrahydrophthalimido moiety ([Alc-14C]) or the 1-position of the cyclopropyl ring ([Acid-14C]) was prepared in our laboratory16,25,26) (Fig. 1): specific radioactivity, 1.83[Alc-14C] and 1.62 GBq/mmol [Acid-14C]; radiochemical purity,>98% by silica gel thin-layer chromatography (TLC). Non-radiolabeled isomers of I and potential metabolites, except IX (Fig. 1), were prepared in accordance with the reported methods.19) IX was synthesized from VI,21,27) with its chemical structure confirmed by LC-APCI-MS (Hitachi M-1000 spectrometer) and 1H NMR (Varian Unity 300 spectrometer at 300 MHz): MS m/z 201 ([M−H]−); 1H NMR δH (CDCl3): 1.22 (3H, s, CH3), 1.31 (3H, s, CH3), 1.33 (3H, s, CH3), 1.34 (3H, s, CH3), 3.01 (1H, m, CHC=O), 5.81 (2H, d, J=3 Hz, CH=CH). The chemical purity of each standard was determined by HPLC to be greater than 90% except for IV (85.6%). Eschericia coli β-glucuronidase (type VII-A), Helix pomatia sulfatase (type H-1) and D-saccharic acid-1,4-lactone were purchased from Sigma Chemical Co. Other chemicals were of a reagent grade and purchased from commercial suppliers unless otherwise noted.

The standard species recommended in OECD 305,28) bluegill sunfish (Lepomis macrochirus) and common carp (Cyprinus carpio), were used. Juvenile bluegill sunfish supplied from Nango Fisheries Center (Shiga, Japan) were used to examine bioconcentration. The fish were acclimated in tap water dechlorinated with activated charcoal for 8 months under a 16-hr daylight photoperiod and were fed a standard commercial fish food (Nisshin Flour Milling Co.) daily in an amount equivalent to ca. 1% of their body weight. The fish culture at the test initiation had a body weight of 1.9±0.2 g, a standard body length of 4.1±0.1 cm, and fat content29) of 3.7%.
An in vitro metabolism study was conducted using juvenile common carp obtained from Sumika Technoservice Co., Ltd. (Hyogo, Japan). The fish were acclimated for more than 1 month under conditions similar to those of the bluegill sunfish and fed daily with a standard commercial fish food in an amount equivalent to ca. 2% of their body weight (ca. 1 g). The ranges of body weight and total length of the subject fish (n=5) were 1.1–1.4 g and 4.4–4.5 cm, respectively.
3. RadioassayRadioactivity in water, organic extracts of water and fish, and scraped gel from TLC plates were individually measured by liquid scintillation counting (LSC) with a Packard Model 4640 or 460 Tri-Carb liquid scintillation counter using the liquid scintillation cocktail Emulsifier-Scintllator 299™ (Packard). The unextractable 14C residues in fish were quantified by LSC after combustion using a Packard Tri-Carb Model 306 Sample Oxidizer and Carbosorb®-CO2 absorber with a Permafluor®-V oxidizer scintillator (14C recovery, ≥95%).
4. Chromatography and spectroscopyTwo-dimensional thin-layer chromatography (2D-TLC) was conducted for analysis of extracts using a precoated silica gel 60F254 chromatoplate (20×20 cm, 0.25 mm thickness, E. Merck) with the following solvent systems: n-hexane/toluene/acetic acid (3/15/2, v/v/v, development twice in the same direction) and toluene/ethyl formate/formic acid (5/7/1, v/v/v) for [Alc-14C]-trans-I, and n-hexane/ethyl acetate/acetic acid (10/4/1, v/v/v) and toluene/ethyl formate/formic acid (5/7/1, v/v/v) for [Acid-14C]-trans-I. In order to isolate and analyze polar metabolites (conjugates) in fish exposed to [Acid-14C]-trans-I, one-dimensional TLC with 1-butanol/acetic acid/water (6/1/1, v/v/v) was additionally conducted. Radioactive spots on the TLC plates after development were visualized by autoradiography, following exposure to the X-ray SB film (Eastman Kodak Company) for one week, and then scraped off for 14C quantification. The non-radiolabeled standards were detected by exposing the chromatoplate to ultraviolet light. Reversed-phase HPLC was carried out in addition to TLC, using a Hitachi L-6200 pump linked in series with an L-4000 UV monitor (230 or 215 nm) and a Radiomatic FLO-ONE/Beta A-120 radio detector (500 µL liquid cell, Packard PICO-AQUA™ scintillator, flow rate 1 mL/min). A Sumipax ODS A-212 (5 µm, 6 mm i.d.×15 cm) column was typically operated at a flow rate of 1 mL/min with the following linear gradient program: 0 min, %A (acetonitrile): %B (0.05% trifluoroacetic acid in water), 10 : 90; 30 min, 50 : 50; 60 min, 100 : 0; 75 min, 100 : 0. Typical HPLC retention times (tR) and TLC Rf values of I–IX are summarized in Table 1. Chiral HPLC analysis of trans-I was conducted for representative water and fish samples, using two SUMICHIRAL OA-2000 (5 µm, 4 mm i.d.×25 cm) columns linked in series (n-hexane/ethanol=400/1 (v/v) at 1 mL/min). The typical retention times of 1R-trans and 1S-trans isomers were 45.0 and 47.0 min, respectively. LC-APCI-MS (negative ion mode, drift voltage −35 or −25 V) was performed using a Hitachi M-1000 spectrometer equipped with a Hitachi L-6200 series liquid chromatograph for mass spectrometric analysis of conjugated metabolites.
| TLC Rf | HPLC tR (min) | ||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| trans-I | 0.48 | 0.61 | 0.60 | — | 60.2 |
| cis-I | 0.47 | 0.60 | 0.60 | — | 59.6 |
| II | — | 0.43 | 0.18 | — | 22.7 |
| III | — | 0.50 | 0.31 | — | 23.7 |
| IV | — | 0.46 | 0.26 | — | 16.7 |
| V | — | 0.38 | 0.14 | — | 17.2 |
| VI-free | 0.56 | 0.46 | — | — | 43.4 |
| -taurine conjugate | — | — | — | 0.44 | 28.1 |
| VII-free | 0.23 | 0.46 | — | — | 22.9 |
| -glucronide conjugate | — | — | — | 0.34 | 35.3 |
| VIII-free | 0.31 | 0.48 | — | — | 26.9 |
| -taurine conjugate | — | — | — | 0.39 | 11.4 |
| VIII-conjugatea) | — | — | — | 0.37 | — |
| IX | 0.08 | 0.27 | — | — | 10.1 |
Solvent systems; A) n-hexane/ethyl acetate/acetic acid (10/4/1, v/v/v), B) toluene/ethyl formate/formic acid (5/7/1, v/v/v), C) n-hexane/toluene/acetic acid (3/15/2, v/v/v, twice), D) 1-butanol/acetic acid/water (6/1/1, v/v/v).HPLC conditions: See Sect. 4.a) Chemical identity of VIII was conducted through hydrolysis of the conjugate. Amino acid conjugate was proposed.
In the in vitro study, a Waters LC-MS system, a Micromass ZQ spectrometer equipped with a Waters Separation Module 2695 as a liquid chromatograph with an ODS column (Symmetry, 3.5 µm, 3.0 mm×50 mm; Waters), was operated at a flow rate of 0.2 mL/min under an isocratic condition (acetonitrile/0.05% formic acid in water=8/2, v/v, tR=ca. 3.0 min). The quantification of each isomer of I was carried out by monitoring m/z 354 ([M+Na]+) in an electrospray ionization (ESI) positive mode with a cone voltage of 30 V.
5. Bioassay5.1. Bioconcentration of trans-I in bluegill sunfishIn accordance with OECD 305,28) the flow-through condition of the fifteen volume replacement per day was kept at 25±1°C with 16-hr daylight photoperiod. Good water quality was maintained during the study (DO: 6.6–7.7 mg/L, pH: 6.5–7.9). Throughout the experiments, fish were fed 1.5% of their body weight daily, and their health was monitored. Each 14C label dissolved in N,N-dimethylformamide (DMF) at 10 mg/L and dechlorinated tap water was continuously mixed in a glass flask to obtain a nominal concentration of 1 µg/L (ppb), and the test solution was introduced into each exposure aquarium by maintaining a 40 L water volume. The nominal exposure concentration was selected in consideration of the toxicity (bluegill, 96 hr-LC50=15.9–19 µg/L)30) with an appropriate margin. A solvent control group was similarly prepared using only DMF. Exposure was initiated by introducing 85 acclimated fish into each aquarium and was continued for 28 days. At the end of the exposure, all of the remaining fish were individually transferred to clean water in a 20-L aquarium to initiate 14-day depuration phase.
Three fish were periodically taken, weighed, cut into small pieces and homogenized in methanol (10,000 rpm, on ice, 5 min) with an ACE homogenizer (Nihon Seiki Co.). After centrifugation of the homogenate at 2500 rpm for 5 min, the separated residues were similarly extracted three times. The exposure water (1 L) was also periodically taken. After radioassay, the water was extracted thrice with ethyl acetate (500, 300, 300 mL; 10-min shaking) in the presence of ammonium sulfate (500 g) and conc. HCl (0.8 mL). The combined extracts from the fish and the exposure water were individually subjected to chromatographic analyses.
5.2. In vitro metabolism of cis- and trans-I in carpIn vitro bioassay using the supernatant of common carp homogenate31) was conducted to estimate the metabolic dissipation rates (km) of cis- and trans-I. The fish homogenate in 0.1 M Tris–HCl buffer (fish/buffer=1/5, w/w) at pH 7.4 was centrifuged at 1000 g at 4°C for 20 min. A 2-mL aliquot of the supernatant was transferred into a 5-mL glass vial in duplicate, and 0.01 mL of 200 mg/L acetonitrile solution of non-radiolabeled cis- or trans-I was added. The protein concentration in each supernatant was determined to be 2.7–3.5 mg/mL by Protein Assay Rapid Kit (Wako Pure Chemical Industries, Ltd.). The samples were incubated for 5 hr at 25±1°C and 120 rpm with periodic sampling for LC-MS quantification of the remaining cis- or trans-I.
6. Identification of metabolitesIdentification of each metabolite formed in the bioconcentration study was first conducted by HPLC and 2D-TLC co-chromatographies with non-radiolabeled authentic standards (I–IX). Conjugated phase-II metabolites were isolated by preparative one-dimensional TLC and subjected to hydrolysis by β-glucuronidase (10 mg/mL) in 0.1 M phosphate buffer (pH 6.8) at 37°C overnight or in sulfatase (20 mg/mL) with D-saccharic acid-1,4-lactone in 0.1 M sodium acetate buffer (pH 5) at 37°C overnight and/or 3 M NaOH at 110°C for up to 18 hr. The released aglycons were extracted with ethyl acetate/methanol (5/1, v/v) and subjected to 2D-TLC and HPLC co-chromatographies. The chemical identity of some conjugates was further confirmed by LC-MS.
7. Kinetic analysis7.1. Bioconcentration in bluegill sunfishAccording to the OECD 305 guideline, the following differential equation can describe the concentration of trans-I in fish, Cf(t) (µg/kg), under conditions of negligible growth dilution.
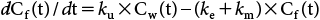 | (1) |
Depuration phase, Cf(t)=Cf(0)×exp{−(ke+km)×t}
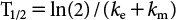 |
Uptake phase, Cf(t)=Cw(t)×(ku/(ke+km))×[1−exp{−(ke+km)×t}]
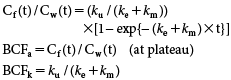 |
The degradation rate constant (kdeg) in the in vitro study was calculated from the following equation, assuming first-order kinetics.
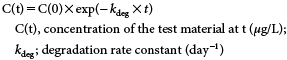 |
No adverse effects were confirmed for fish with a good biomass control (mean body weight of 2.4±0.4 g, ca. 120% of the initial weight) at the end of depuration phase. During the uptake period, the exposure concentration was kept almost constant for both 14C labels on the basis of total radioactive residue (TRR, 93–110% of the nominal concentration) and trans-I (80–120% of the mean measured concentration).
The concentration of trans-I in fish gradually increased, almost reaching a plateau at 120–210 ppb after 14 days of exposure (Fig. 2). The BCFa value was calculated to be 210–300 for [Alc-14C] and 180–310 for [Acid-14C]. During the depuration phase, more than 95% of the trans-I in fish dissipated within 7 days (Tables 2 and 3). The apparent dissipation rate constant (km+ke) was estimated with a high correlation (r2=0.99–1.0) to be 0.96–1.2 day−1, giving T1/2 of 0.54–0.72 days. By curve-fitting Cf(t)/Cw(t) with the km+ke value, the ku value was calculated to be 170–210 day−1 with low correlations (r2=0.014–0.033), which might be due to the experimental constraint of sampling intervals in the early phase of the study.
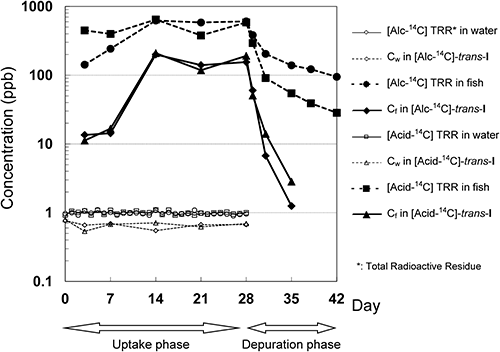
| Concentration, ppb (% in Total Radioactive Residue (TRR)) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Uptake phase | Depuration phase | ||||||||||
| Day 0 | Day 3 | Day 7 | Day 14 | Day 21 | Day 28 | Day 1 | Day 3 | Day 7 | Day 10 | Day 14 | |
| Water | |||||||||||
| TRR | 0.969 | 1.07 | 1.01 | 1.08 | 0.983 | 0.959 | — | — | — | — | — |
| trans-I | 0.766 | 0.659 | 0.693 | 0.549 | 0.669 | 0.670 | — | — | — | — | — |
| (79.0) | (61.6) | (68.6) | (50.8) | (68.1) | (69.9) | — | — | — | — | — | |
| Others | (21.0) | (38.4) | (31.4) | (49.2) | (31.9) | (30.1) | — | — | — | — | — |
| Whole fish | |||||||||||
| TRR | — | 142 | 242 | 624 | 584 | 606 | 383 | 204 | 139 | 122 | 94.6 |
| Unextractable | — | (19.2) | (17.9) | (24.5) | (26.8) | (32.0) | (36.1) | (53.7) | (67.9) | (69.5) | (69.2) |
| Extractable | — | (80.8) | (82.1) | (75.5) | (73.2) | (68.0) | (63.9) | (46.3) | (32.1) | (30.5) | (30.8) |
| trans-I | — | 13.5 | 14.3 | 200.3 | 139.6 | 154.5 | 60.1 | 6.7 | 1.3 | ND | — |
| — | (9.5) | (5.9) | (32.1) | (23.9) | (25.5) | (15.7) | (3.3) | (0.9) | ND | — | |
| III | — | (13.1) | (10.0) | (1.4) | (1.4) | (2.3) | (1.2) | (1.0) | (0.2) | (0.3) | — |
| IV | — | (13.0) | (8.5) | (2.3) | (2.0) | (2.4) | (1.7) | ND | (0.4) | ND | — |
| V | — | (2.6) | (2.1) | (0.5) | (0.9) | (1.2) | (0.8) | ND | ND | ND | — |
| Others | — | (42.6) | (55.6) | (39.2) | (45.0) | (36.6) | (44.5) | (42.0) | (30.6) | (30.2) | — |
—: Not applicable.ND: Not detected.
| Concentration, ppb (% in Total Radioactive Residue (TRR)) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Uptake phase | Depuration phase | ||||||||||
| Day 0 | Day 3 | Day 7 | Day 14 | Day 21 | Day 28 | Day 1 | Day 3 | Day 7 | Day 10 | Day 14 | |
| Water | |||||||||||
| TRR | 0.925 | 1.06 | 1.08 | 1.10 | 0.984 | 1.00 | — | — | — | — | — |
| trans-I | 0.792 | 0.536 | 0.679 | 0.711 | 0.622 | 0.695 | — | — | — | — | — |
| (85.6) | (50.6) | (62.9) | (64.6) | (63.2) | (69.5) | — | — | — | — | — | |
| Others | (14.4) | (49.4) | (37.1) | (35.4) | (36.8) | (30.5) | — | — | — | — | — |
| Whole fish | |||||||||||
| TRR | — | 448 | 398 | 644 | 378 | 585 | 293 | 90.9 | 54.5 | 39.0 | 28.4 |
| Unextractable | — | (9.8) | (8.0) | (14.8) | (16.7) | (10.9) | (12.9) | (36.4) | (63.6) | (71.2) | (65.7) |
| Extractable | — | (90.2) | (92.0) | (85.2) | (83.3) | (89.1) | (87.1) | (63.6) | (36.4) | (28.8) | (34.3) |
| trans-I | — | 11.2 | 16.7 | 209.0 | 118.0 | 191.0 | 50.7 | 14.0 | 2.8 | — | — |
| — | (2.5) | (4.2) | (32.4) | (31.2) | (32.7) | (17.3) | (15.4) | (5.2) | — | — | |
| VI-free | — | (20.1) | (21.7) | (5.1) | (5.2) | (8.0) | (5.1) | ND | ND | — | — |
| -taurine conj. | — | (9.4) | (7.2) | (3.9) | (2.6) | (4.0) | (3.5) | (1.8) | ND | — | — |
| VII-free & unknowna) | — | (3.8) | (7.9) | (3.2) | (3.6) | (5.5) | (8.2) | (5.4) | (1.2) | — | — |
| -glucuronide | — | (1.7) | (4.5) | (4.5) | (1.9) | (3.6) | (3.3) | (1.8) | ND | — | — |
| VIII-free | — | (4.1) | (8.4) | (3.2) | (3.2) | (3.3) | (7.1) | (1.7) | (1.4) | — | — |
| -taurine conj. | — | (7.7) | (15.6) | (6.5) | (6.5) | (5.2) | (11.1) | (3.7) | ND | — | — |
| -conjugateb) | — | (6.0) | (10.8) | (5.9) | (3.9) | (4.9) | (7.5) | (2.5) | ND | — | — |
| IX | — | (0.5) | (1.1) | (6.2) | (7.7) | (5.6) | (8.2) | (5.4) | (1.2) | — | — |
| Others | — | (34.4) | (10.6) | (14.3) | (17.5) | (16.3) | (15.8) | (25.9) | (27.4) | — | — |
a) A single spot in 2D TLC exhibiting two HPLC peaks with an approximate ratio of 2 : 3.b) Amino acid conjugation was proposed.—: Not applicable.ND: Not detected.
Radioanalysis of the fish and water indicated no significant change in the isomeric ratio for the acid moiety.
Ester cleavage was a primary metabolic pathway in fish. The free forms of III, IV, and V were detected from [Alc-14C]-trans-I, amounting to 13.1, 13.0, and 2.6% TRR (Day 3), respectively, at maximum (Table 2). In the case of [Acid-14C]-trans-I, VI was predominantly detected as a primary metabolite and further transformed to VII and VIII via successive oxidation at the terminal methyl group of the isobutenyl moiety or to IX via oxidative ring-opening of the epoxide intermediate (Fig. 1). Free and taurine conjugates amounted to 21.7% TRR (Day 7) and 9.4% TRR (Day 3) for VI, and 8.4 and 15.6% TRR (Day 7) for VIII, respectively (Table 3). The LC-MS spectra in a negative ion mode confirmed the structure of taurine conjugates for VI and VIII with deprotonated molecular ion ([M−H]−) peaks at m/z 276 and 306, respectively. In addition to the free form (7.9% TRR, Day 7), VII was detected as a glucuronide conjugate (4.5% TRR, Day 7). VIII was released only by alkaline hydrolysis for another conjugate (10.8% TRR, Day 7), suggesting conjugation with an amino acid other than taurine. In contrast, IX existed only as a free form (7.7% TRR, Day 21).
The other fractions in fish and water consisted of minor metabolites as maximums of 36 and 27 for [Alc-14C] and 14 and 17 for [Acid-14C], respectively. Unextractable residues in fish reached 32.0% TRR for [Alc-14C] and 10.9% TRR for [Acid-14C] on Day 28, but two-thirds of them (66 and 71%, respectively) were steadily eliminated in the following 14-day depuration period.
3. In vitro degradation rates of cis- and trans-ITrans-I was found to more rapidly degrade in the fish homogenates than did cis-I. The estimated degradation rate constants (kdeg) for cis- and trans-I were 1.8±0.51 (1.0–2.3) and 3.7±2.6 (2.4–8.3) day−1, respectively. The cis/trans ratio of the mean rate constants was calculated to be 0.48.
The metabolic pathways of trans-I in bluegill sunfish are proposed in Fig. 1. Cleavage of the ester linkage primarily proceeded for trans-I in fish, similarly but more prominently than in mammals.19,23–26) The resultant alcohol and acid moieties were further metabolized by oxidation, reduction and hydrolysis (phase-I reactions). All of the Phase-I metabolites in fish were common to mammals with the exception of IX, whose presence was unclear in mammalian metabolism studies. However, the administration of the epoxide derivative of I to rats resulted in the formation of a corresponding diol metabolite whose acid moiety was IX.21) Since epoxidation is one of the most familiar oxidative metabolic reactions in aquatic organisms,10) the higher hydrolytic ability in fish resulted in significant formation of IX. The phase-II metabolites of VI, VII, and VIII were formed via conjugation with glucuronic acid or amino acids such as taurine. Although no taurine conjugate was detected in mammalian metabolism of I, its existence has been reported for other pyrethroids32) in mice and various aquatic organisms.2,10,12,33)
The BCFa of trans-I, 180–310, was approximately one order of magnitude below the internationally recognized criteria for bioaccumulative chemicals such as 2000 for PBT (persistent, bioaccumulative and toxic substance) and 5000 for POP (persistent organic pollutant).34,35) Based on the lipophilicity of I (log Kow=4.6),15) the BCF was estimated to be 1600 by QSAR without consideration of metabolism,5) while the EPI Suite v.4.1136) program that well incorporated the metabolic contribution gave the lower value of 550–720, closer to our experimental BCFa. Similarly to other pyrethroids,6,7) trans-I’s excellent metabolic potential in fish has greatly reduced the BCF value.
In general, a trans isomer is more susceptible to ester cleavage than is a cis isomer, not only in mammals administrated with I13,19,23) but also in aquatic organisms exposed to permethrin, cypermethrin and phenothrin.2,11,12) The observed rapid degradation of trans-I, as compared to cis-I in the in vitro experiment, is considered to be the difference in susceptibility to esterases as reported in mammalian carboxylesterases with trans- and cis-permethrin.32) Incidentally, the BCF of the cis isomer was conservatively/conveniently estimated from the in vivo BCF of the trans isomer (180–310), in accordance with the assumption that ku is independent of the geometric isomerism and the cis/trans ratio of (ke+km) is identical to that of in vitro degradation rates (cis/trans=0.48). Then, the estimated BCF of the cis-I isomer was calculated to be 380–650, which was 2.1 times higher than the original BCF of trans-I but clearly below the international PBT and POP criteria of 2000–5000.
In order to estimate the BCF of cis-I more precisely, additional kinetic analysis was conducted to estimate the km value of trans-I, assuming a first-order kinetic compartment model not only for the parent compound but also for the primary metabolite, as shown in Fig. 3, by using the following equation:
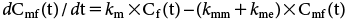 | (2) |
 |
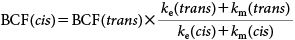 |

Since cis-I should have been more degraded by oxidation as observed with the other pyrethroids in fish,2,11,12,37) the present estimation is considered very conservative. We believe that such an estimation using a combination of in vivo and in vitro data possibly provides a more reasonable BCF value than that from sophisticated BCF modeling using in vitro data such as mass-balanced or physiologically based toxicokinetic models.38–40)
In conclusion, we have clarified the metabolic routes and kinetics of trans-I in bluegill sunfish. In addition, we have proposed a convenient method for estimating the BCF value of a certain isomer/analog compound by combining core data of in vivo bioconcentration and metabolism for a major active component of a pesticide with in vitro metabolism data for its minor component.
The authors are thankful for the help of Ms. Yoko Tagawa in in vivo experiments including metabolite identification works.