2015 Volume 40 Issue 3 Pages 146-151
2015 Volume 40 Issue 3 Pages 146-151
Ecdysteroids and brassinosteroids are steroid hormones that regulate insect molting and plant growth, respectively. In this study, novel ecdysteroids and brassinosteroids were designed and synthesized, and their biological activity was evaluated. Structure-activity relationship details between the structure of the steroid skeleton of ecdysteroids and their molting hormone receptor binding activity, as well as between the side chain structure of brassinosteroids and their plant hormonal activity were investigated. The results of these investigations suggested that in order to exhibit hormonal activity in insects or plants, the structure of the side chain in these hormones should be more important than that of the steroidal core skeleton.
Ecdysteroids (ESs) are steroids that exhibit molting hormonal activity in insects.1,2) Ecdysone, the first isolated ES (Fig. 1), was obtained from Bombyx mori,3) and during the following decade, ecdysone and its biologically active form 20-hydroxyecdysone (20E) were also isolated from a few other insects and a member of Crustacea.4) However, ESs are not only contained in insects, but also in plants. For example, ponasterone A (PoA) was isolated from Podocarpus nakaii Hay in 1966,5) and both ecdysone and 20E were obtained from Pteridium aquilinum (L.) Kuhn in 1967.4) Encouraged by these findings, other steroidal hormones were extracted from plants,6) and in 1979, brassinolide (BL), a steroid exhibiting plant growth promoting activity, was isolated from Brassica napus L. pollen.7) Three years later, Yokota and co-workers found castasterone (CS), a steroid with a similar structure and biological activity to BL, in Castanea crenata.8) Initially, BL and CS were considered to represent a new group of plant hormones, and an examination of their biosynthesis and perception mechanism revealed that BL and CS are required for normal plant growth. Nowadays, it is widely accepted that BL and CS are plant steroidal hormones. So far, more than 70 BL and CS analogs have been obtained from natural sources,9) and they are categorized as brassinosteroids (BRs).10,11)

As shown in Fig. 1, ESs and BRs share some structural features, such as e.g. hydroxy groups at position C-2, C-3, and C-22, as well as a carbonyl group at C-6. Although the structure-activity relationship between ESs and the molting hormone receptor binding activity,12) as well as between BRs and the plant hormonal activity,13–15) have been extensively investigated, it still remains unclear how insects and plants are able to distinguish those structurally closely related steroidal compounds and use them as hormones. In order to address this issue, we designed and synthesized a series of novel ESs and BRs derivatives, and evaluated their insect molting and plant hormonal activity.
Initially, we designed and synthesized a CS/PoA hybrid that contained the core steroidal skeleton of CS and the side chain structure of PoA. This compound was obtained as a stereoisomer mixture at C-22 (R/S-1, Fig. 2).16)

The ES receptor binding activity of R/S-1 was evaluated using Kc (Drosophila melanogaster) and Sf-9 (Spodoptera frugiperda) cells, and the inhibition of [3H]PoA binding to the ES receptor was measured as an index of activity.17,18) As shown in Fig. 3A and 3B, R/S-1 inhibited the binding of [3H]PoA to the ES receptor in a dose-dependent manner, and was found to be approximately 3 and 10 times more potent than ecdysone in Sf-9 and Kc cells, respectively (Table 1). Similar structure-activity relationships for ESs have been reported in Kc and Sf-9 cells.17) R/S-1 can accordingly be classified as a normal ES (Fig. 3C), even though it exhibits the steroidal skeleton of the plant hormone CS (Fig. 2). In these assays, the binding activity of nonsteroidal ES agonists such as RH-5849, tebufenozide, and methoxyfenozide usually depend on the cell lines, and Sf-9 cells are more sensitive to these nonsteroidal ES agonists than Kc cells (Fig. 3C). In contrast, CS inhibited only 45% of the [3H]PoA binding to the ES receptor, even at concentrations as high as 50 µM (Fig. 3A, B).

We further assessed the molting hormonal activity of R/S-1, using a cultured integument of Chilo suppressalis,19) and the uptake of N-acetyl [14C] glucosamine during chitin biosynthesis induced by ESs was measured as an index of the molting hormonal activity. Hybrid compound R/S-1 exhibited a typical bell-shaped hormonal activity (Fig. 3D), and its potency is identical to that of ecdysone (Table 1). A clear relationship between the hormonal activity and the ES receptor binding activity in Sf-9 cells could be observed (Fig. 3E), and this relationship is applicable not only to ESs, but also to nonsteroidal ES agonists.18) The behavior of R/S-1 shown in Fig. 3E suggested that the molting hormonal activity of R/S-1 was induced via the same molecular mechanism as for ESs and nonsteroidal ES agonists.
Subsequently, we stereoselectively synthesized the 22R- and 22S-isomers of hybrid 1 (Fig. 2) in order to examine the effect of the stereochemistry of the hydroxy group at position C-22 on the biological activity.20) In the ES receptor binding assay, the potency of R-1 was by ca. two orders of magnitude higher than that of S-1, and the binding activity of R-1 in Sf-9 cells was almost the same as for 20E, which is the biologically active molting hormone in insects (Table 1). Furthermore, R-1 exhibited clear activity as a molting hormone in the cultured integument of C. suppressalis, whereas S-1 was inactive under the same conditions.20) On the basis of these results, it is feasible to conclude that the stereochemistry of the hydroxy group at C-22 is of pivotal importance for the molting hormonal activity. Even though Bathori et al. isolated the 22S-isomer of 20E (22-epi-20E) from Serratula tinctoria L. (Compositae),21) Hedtmann et al. reported that their synthetic 22-epi-20E showed neither ES receptor binding nor molting hormonal activity.22)
The plant hormonal activity of R-1 and S-1 was evaluated by using the rice lamina inclination assay,23) which is a well-established and highly sensitive method for the detection of BR-like activity. In this assay, rice lamina bends from the leaf sheath when test compounds exhibit BR-like activity, and the co-application of indole-3-acetice acid (IAA) enhances the response. However, as shown in Fig. 3F, both R-1 and S-1 were inactive in this assay. This result indicated that hydroxylation at C-20 is detrimental for the plant hormonal activity of BRs. Cleavage of 20,22-diols after C-20 hydroxylation of BRs has been observed in cell suspension cultures of Ornithopus sativus, and is considered to be one of the metabolizing pathways for BRs.24) Seto and co-workers synthesized BL derivatives containing additional hydroxy groups, and reported that the plant hormonal activity of 20-hydroxy-BL decreased to approximately 1/250 (with IAA) or 1/1000 (without IAA) of that of BL.25)
The previously discussed results suggested that the side chain structures of both ESs and BRs are more important for the hormonal activity than the structure of the steroidal core skeleton. Voigt et al. synthesized another type of ES/BR hybrid that comprised ES-like steroid skeletons and BR-type side chains.26) The molting hormonal activity of their hybrid compounds was at least three orders of magnitude lower than that of 20E. In insects, the hydroxylation of C-20 in ecdysone represents the last step in the biosynthesis of 20E, which is the active component of the molting hormone. Conversely, hydroxylation of C-20 is considered to be one of the metabolizing pathway of BRs. Therefore, hydroxylation of C-20 may act as a biological switch for insect and plant steroidal hormones, whereby the function is diametrically opposed: “on” for insects means “off” for plants.
In this study, we synthesized further PoA analogs with varying steroidal skeletons in order to investigate structural requirements for ES receptor binding in detail.27) As summarized in Table 2, an indispensible structural requirement for ES receptor binding activity is the presence of a hydroxy group at C-3 (see 2 and 3 vs. 4–12). Conversely, the introduction of a hydroxy group at C-6, regardless of its stereochemistry, lead to a significant decrease of the ES receptor binding activity (7 and 8). With respect to the stereochemistry of the hydroxy group at C-3, it was observed that an α-hydroxy group with A/B trans configuration (9) exhibited high ES receptor binding activity. However, a β-hydroxy group with A/B cis configuration, which is a commonly encountered structural motif in natural ESs (e.g. ecdysone, 20E and PoA) was even more potent (12). Hybrid compound R-1 with a pEC50 value of 6.49 in Kc cells also contains a 3α-hydroxy group with A/B trans configuration (Fig. 2). In contrast, α-hydroxy groups with A/B cis configuration (10) or a β-hydroxy group with A/B trans configuration (6 and 11) showed lower binding affinity to the ES receptor. This observation should most likely be attributed to the spatial orientation of the hydroxy group at C-3, as a 3α-hydroxy group with A/B trans configuration and a 3β-hydroxy group with A/B cis configuration occupy the same space (Fig. 4). Therefore, the presence of a functional group that is able to form hydrogen bonds in this space should be highly important for an effective binding of ESs to their specific receptors. In case of BRs, the presence of 6α- or 6β-hydroxy groups did not decrease the plant hormonal activity substantially, and a hydroxy group at C-2 rather than at C-3 is important in order to exhibit BR-like activity.14,15)
| Compound | pIC50 (M)a) | Compound | pIC50 (M)a) | Compound | pIC50 (M)a) | |||
|---|---|---|---|---|---|---|---|---|
| No. | Structure | No. | Structure | No. | Structure | |||
| 2 |  | < 3.61 (8%) | 6 |  | 5.02 | 10 |  | 4.05 |
| 3 |  | < 3.61 (23%) | 7 |  | < 3.61 (19%) | 11 |  | 4.84 |
| 4 |  | 4.38 | 8 |  | < 3.61 (42%) | 12 |  | 7.23 |
| 5 |  | 4.38 | 9 |  | 6.10 | Side chain structure: |  | |
a) Values in parentheses represent the inhibition % at the corresponding concentration.

The critical importance of the side chain structures of the CS/PoA hybrid compounds for the plant hormonal activity described above prompted us to investigate the structure-activity relationship of the side chain in BRs in more detail. Therefore, we synthesized BR derivatives containing various side chains (Table 3).28,29) The steroid core skeleton of these derivatives was based on the structures of BL (A), CS (B), or 2,3-diepi-CS (C). The potential plant hormonal activity of the synthetic compounds thus obtained was evaluated by the rice lamina inclination assay,23) and the reciprocal logarithmic value of the 50% effective dose (pED50), calculated from a dose-response curve like that shown in Fig. 3F was used as an activity index.
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | St | R | pED50 (mol)a) | No. | St | R | pED50 (mol)a) | No. | St | R | pED50 (mol)a) |
| BL | A |  | 13.6 | 21 | A |  | 10.1 | 29 | B |  | 9.3 |
| 13 | A |  | < 8.0 (13%) | 22 | A |  | 8.3 | 30 | C |  | < 8.0 (4%) |
| 14 | A |  | < 8.0 (41%) | 23 | A |  | < 8.7 (16%) | 31 | C |  | < 8.0 (5%) |
| 15 | A |  | < 8.0 (48%) | 24 | A |  | 9.1 | 32 | C |  | < 8.0 (13%) |
| 16 | A |  | < 8.0 (44%) | 25 | A |  | 8.2 | 33 | C |  | < 8.0 (11%) |
| 17 | A |  | 8.6 | CS | B |  | 12.3 | 34 | B |  | 10.7 |
| 18 | A |  | 9.6 | 26 | B |  | < 8.0 (46%) | 35 | B |  | 8.5 |
| 19 | A | 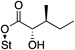 | 9.3 | 27 | B |  | 8.5 | 36 | B |  | 8.7 |
| 20 | A |  | 10.5 | 28 | B |  | 9.0 | ||||
a) Values in parentheses represent the inclination % at the corresponding dose: 100% for the BL treatment and 0% for the control. IAA (25 nmol/plant) was used as a synergist. Data for 13–24 as well as for BL and CS were obtained from Ref. 28. Data for 34–36 were obtained from Ref. 29. Others are unpublished results.
In case of the synthetic BL derivatives with acyl-type side chains 13–24, the presence of a hydroxy group at the α position of the carboxy group was observed to be important for a BR-like activity (13–16 vs. 17–22). Conversely, methylation of the hydroxy group had a negligible effect on the plant hormonal activity (20 vs. 24), even though the introduction of a benzene ring at the end of the acyl side chain dramatically decreased the BR-like activity (23). With respect to the stereochemistry of the hydroxy groups at the α position, higher activity was observed for the R- relative to the S-configuration (17 vs. 18; 19 vs. 20; 26 vs. 27; and 28 vs. 29). In contrast, an additional introduction of a hydroxy group at the β position of the carboxy group decreased the potency by more than one order of magnitude (18 vs. 22).
Trends for the plant hormonal activity remained constant, when the structure of the steroidal core was changed from a BL- (A) to a CS-type (B) skeleton (17 vs. 26; 18 vs. 27; 20 vs. 29), and a tenfold increase in potency was observed for BL-type analogs relative to CS-type analogs. This result is in good agreement with the difference regarding the plant hormonal activity between BL and CS, which showed pED50 values of 13.6 and 12.3, respectively. In case of 2,3-diepi-CS-type analogs, such tendencies could not be observed, as their BR-like activity was extremely low (30–33). This is consistent with the results of Lee et al., who reported the isolation of 2,3-diepi-CS from Phaseolus vulgaris and described very low BR-like activity.30) Based on these observations, a similar recognition mechanism for the steroidal core skeleton of BRs, containing acyl-type side chains, to that BRs with alkyl-type side chains was proposed. The plant hormonal activity of a BR with an amide-type side chain was by approximately two orders of magnitude lower compared to that of the corresponding BR with an acyl-type side chain (25 vs. 20).
The BR-like activity of 20-epi-26,27-bisnor-CS (35) and 21,26,27-trisnor-CS (36) decreased by ca. two orders of magnitude relative to that of 26,27-bisnor-CS (34), and their activity was similar to that of the corresponding acyl-analog 27. This result suggested that both the presence and the stereochemistry of the methyl group at C-20 should be very important for the plant hormonal activity. The importance of a methyl group at C-20 was tentatively assigned to its ability to determine the conformation of the alkyl-type side chains in 34–36.29)
In this study, we revealed structure-activity relationships between ESs and BRs and their hormonal activities in insect cells and rice. The results suggested that the hormonal activity in insects or plants is governed by the structure of the side chain, rather than by the nature of the steroidal core skeleton. Especially, modifications at C-20 dramatically affected the hormonal activity of both ESs and BRs. The combination of the three-dimensional structures of ES and BR receptors, which were recently determined by co-crystallization with their respective ligands,31–36) with the results obtained in this study should allow the design and development of more effective agrochemicals with insect and/or plant growth-regulating activity.
This study was carried out at the Laboratory of Bioregulation Chemistry, Division of Applied Life Sciences, Graduate School of Agriculture, Kyoto University. The author would like to express his gratitude to Profs. Tamio Ueno, Hisashi Miyagawa, Yoshiaki Nakagawa, and Masahiro Miyashita for their kind guidance and warm encouragement during the course of this study. The author is greatly indebted to Profs. Miki Akamatsu (Kyoto University), Hiroshi Abe (Tokyo University of Agriculture and Technology), and Tadao Asami (The University of Tokyo) for their kind suggestions. The author would also like to thank Mitsui Chemicals Agro, Inc. for a generous gift of rice seeds. The author is moreover grateful to his co-workers: Mr. Shinya Uesusuki, Mr. Shuji Yamamoto, Ms. Junko Otsuki, Dr. Takehiko Ogura, Mr. Hirokazu Arai, and Dr. Kanako Sasaki.