2016 Volume 41 Issue 3 Pages 83-86
2016 Volume 41 Issue 3 Pages 83-86
Trypsin-modulating oostatic factor (TMOF) is an effective mosquito larvicide, but information on its potential toxicity to non-target organisms is limited. To investigate this, triplicate groups of 10 Macrobrachium rosenbergii were exposed to 0, 10, 50 or 100 mg/L nominal TMOF concentrations for 12 days. Tail moisture, crude protein, and hepatopancreatic glycogen/histopathology were unaffected, but increasing TMOF linearly decreased survival and growth. TMOF at the lowest concentration employed significantly decreased trypsin and chymotrypsin activities.
Mosquitos are well-known disease vectors and while pesticides are often used to control their populations, these can pose dangers to various non-target organisms, including humans. This can be compounded by increased pesticide resistance among insects, thus requiring higher concentrations to be effective. Subsequently, there is increasing interest in more environmentally friendly chemicals, and among these, trypsin-modulating oostatic factor (TMOF) has been receiving increasing attention1–3) and has reached the stage of commercial production.
TMOF is a decapeptide hormone found in high concentrations within mosquito ovaries and the mode of toxicity is the inhibition of the proteolytic enzyme, trypsin, during the early larval stage of mosquitos, causing mortality by starvation.1,4,5) A similar action of disrupting trypsin and/or chymotrypsin biosynthesis was shown in the cat flea Ctenocephalides felis, stable fly Stomoxys calcitrans, housefly Musca domestica, midge Culicoides variipennis,6,7) fall webworm moth Hyphantria cunea, and lesser mulberry pyralid Glyphodes pyloalis.8) On the other hand, TMOF had no effect on the survival, growth, or reproduction of the freshwater crustacean Daphnia magna2) and no acute/sub-chronic toxicity to tilapia (Oreochromis sp.).3) After a series of toxicity tests with terrestrial vertebrate animals in which TMOF was non-toxic, Thompson et al.2) suggested that the enzyme leucine aminopeptidase degraded TMOF into smaller components.
Although it has been reported that the freshwater prawn Macrobrachium rosenbergii also possesses leucine aminopeptidase,9) it is unclear whether TMOF is potentially toxic to this species. In addition to the high commercial importance of M. rosenbergii around the world, with production exceeding 200,000 tons/year since 2007,10) they can be particularly susceptible to insecticide exposure due to runoff into their natural habitats or when being farmed in close proximity to agricultural areas.11,12) The aim of the experiment was to subject post-larval M. rosenbergii to a commercial TMOF product at nominal concentrations of 0 (control), 10, 50 and 100 mg/L and after 12 days their survival, growth, hepatopancreatic trypsin and chymotrypsin activity, tail crude protein, and histopathology and glycogen reserves of the hepatopancreas were measured.
A total of 200 prawns were slowly acclimated in a 1000-L fiberglass tank and then 120 apparently healthy prawns were equally distributed among 12 glass aquaria filled with dechlorinated tap water. Each aquarium contained 10 prawns. The prawns (initial mean±standard deviation length and weight of 3.03±0.85 cm and 0.45±0.25 g, respectively) were further acclimated over three days and fed commercial prawn pellets (Gold Coin Ltd., Malaysia) to satiation. Any uneaten food and waste from the preceding days were siphoned out. Each aquarium received gentle aeration and an equal number of PVC pipes, totaling 15, were provided to act as shelters. After 3 days, there was a total water exchange (50 L), the treatments were randomly assigned in triplicate, and the TMOF product was directly added at 10, 50, or 100 mg/L, while in the control treatment, no TMOF product was added. The TMOF product was purchased from EntoGenex and was produced by extracting TMOF from the ovaries of mosquitoes and expressed in yeast cells along with Bacillus thuringiensis israelensis serotype H-14 (7,000 ITU/mg) to act as an entomopathogenic bacterium.
Each day, the prawns were fed twice to apparent satiation; the following day, any uneaten food was removed, and the dissolved oxygen (DO), pH and temperature were recorded. Throughout the experiment, the DO, pH, and temperature were >5.5 mg/L, 7.7±2, and 28±2°C, respectively. On days 5 and 10, each aquarium received a complete water exchange; on day 12, each prawn was measured for total weight and length. The heads of all prawns were then separated from the tails using dissecting scissors. From these, the heads of two prawns in each aquarium (6 prawns/treatment) were immediately immersion-fixed in an FAAC C (4% formaldehyde, 5% acetic acid, and 1.3% calcium chloride) formalin solution, and the tails were stored at −20°C. The hepatopancreas was dissected from each remaining prawn and immediately stored at −80°C, and the tails from these prawns were stored at −20°C.
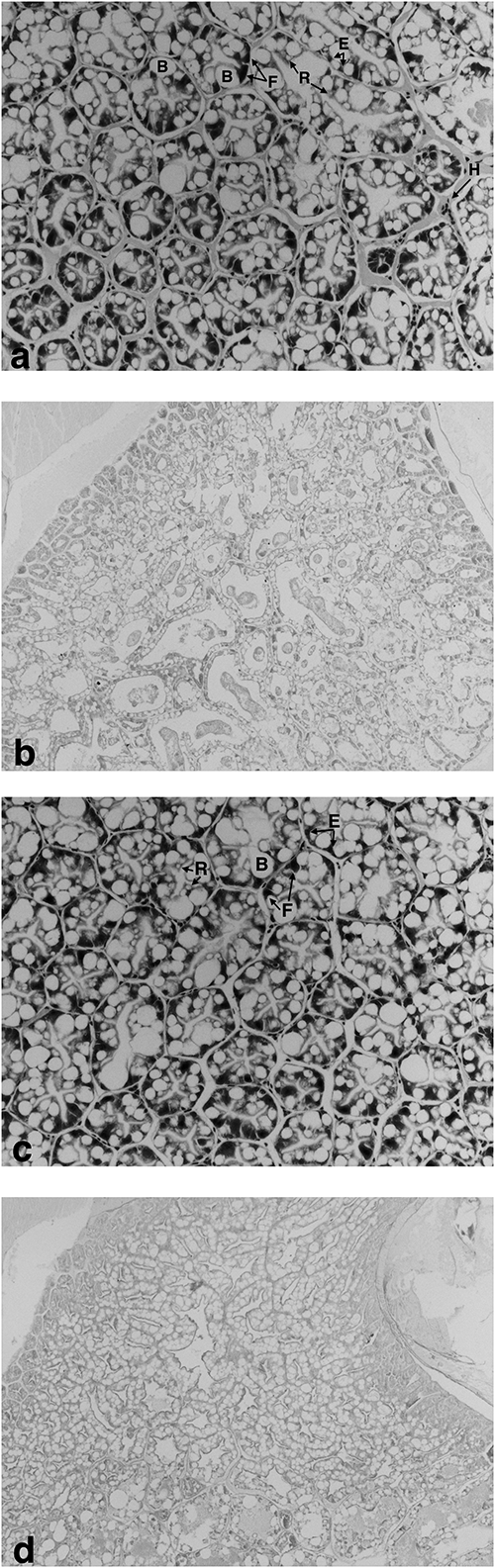
All prawn tails were pooled from all replicates (n=3) and their moisture and crude protein content were measured according to standard Association of Official Analytical Chemists (AOAC) methods.13) After less than a week at −80°C, hepatopancreatic samples were thawed on ice for trypsin and chymotrypsin analysis according to Rick,14,15) respectively. Soluble protein in the samples was measured using a Bio-Rad protein test kit according to Bradford16) and the specific enzyme activities were expressed as U/mg of protein. For histology, a total of 24 prawn heads (six/treatment) were left in the FAAC C formalin for three days to allow the shell to soften, and then they were transferred into 70% (v/v) ethanol until processing at increasing ethanol concentrations. Sections were stained for hematoxylin and eosin using standard procedures while separate sections were then stained with Periodic-acid Schiff according to Karami et al.17) The cell prevalence was quantified by counting the number of cells in 10 tubules from six replicate prawns; the PAS staining intensity was quantified from 12 pictures for each treatment according to Karami et al.17) In addition, the B-cell sizes were quantified from a total of 60 cells equally obtained from six replicate prawns, using ImageJ software (v 1.46d). All data were subjected to linear regression to determine any relationship with increasing TMOF levels. After prior confirmation of data homogeneity and normality, a one-way ANOVA was used to determine any significant differences, and if any were found, Tukey’s post-hoc test was used to identify the differences between treatments.
Results showed that prawn survival significantly decreased with increasing TMOF (r2=0.416; p=0.024) from a final survival of 96.6 to 86.6% in the control and 100 mg/L TMOF, respectively. Meanwhile, the specific growth rate (SGR) for length (r2=0.494; p=0.011) and weight (r2=0.498; p=0.010) significantly decreased with increasing TMOF concentrations (Fig. 1). No significant difference or relationship was detected between tail moisture (r2=0.19; p=0.668) or crude protein (r2=0.060; p=0.444) with increasing TMOF concentrations (Supplemental Table S1). The activities of trypsin and chymotrypsin from the hepatopancreas of the prawns are shown in Fig. 2, and at the lowest TMOF concentration, these significantly decreased (p<0.05). No significant relationship was detected for trypsin (r2=0.221; p=0.123) or chymotrypsin activity (r2=0.000; p=0.956) with increasing TMOF concentrations. The cell prevalence was not significantly different among treatments or B-cell sizes (p>0.05) (Supplemental Table S2). Similarly, the hepatopancreatic tubule structure and PAS staining intensity from the prawns were similar between the control and TMOF treatments (Fig. 3a–d).


While TMOF was safe for aquatic animals including Daphnia2) and tilapia3) as non-target organisms, TMOF disrupted the trypsin biosynthesis and/or chymotrypsin activity in various terrestrial insects6–8) indicating some degree of non-specific activity. Similarly, in the current study, the lowest tested TMOF concentration of 10 mg/L, which was similar to the recommended concentration of 6 mg/L to control mosquito populations in the field, significantly decreased both trypsin and chymotrypsin activities within the hepatopancreas of post-larval M. rosenbergii. This likely contributed to the significantly lower survival and growth of the prawns over the 12-day exposure period at increasing TMOF concentrations. Since the prawns were sometimes observed to consume the newly shed molts, and due to the possibility of these molts being hidden among the pipes, it was unclear whether reduced growth was the result of lower molting frequencies, reduced size increases at each molt or both. In fact, it should be pointed out that growth was not initially intended to be an endpoint in this experiment but was measured only after observing clear size differences on the final day, particularly at 100 mg/L. On the other hand, the tail crude protein and hepatopancreatic glycogen reserves were unaffected, possibly due to the relatively short experimental duration; however, this warrants further investigation, with a longer time frame, to include any potential lipid changes to the hepatopancreas.
Considering that both M. rosenbergii and Daphnia are freshwater crustaceans, the discrepancy between TMOF toxicities could be related to species-specific differences; however, the TMOF product used in the current experiment also contained Bacillus thuringiensis israelensis as a means of increasing TMOF efficacy. Nevertheless, it should be noted that the current study utilized between 10- and 100-fold lower concentrations than those of Thompson et al.2) On the other hand, histopathological examinations showed a similar hepatopancreatic structure and epithelial cell prevalence of M. rosenbergii among all treatments, indicating that a disruption of trypsin and chymotrypsin activities was not related to hepatopancreatic damage. Based on this information, hepatopancreatic histopathology may not be an accurate biomarker of TMOF exposure in the natural environment for this species.
In conclusion, TMOF showed non-specific action in M. rosenbergii, since this decreased their survival, growth and digestive activities. A contributor to this finding may also include the use of an entomopathogenic bacterium with TMOF. The results of this study indicate that further investigation of this species and other aquatic crustaceans is warranted before the widespread application of TMOF in natural environments to control mosquito populations.
This study was funded by a grant from Universiti Putra Malaysia (UPM); project no. GP-IPB/2014/9440403.