2021 Volume 46 Issue 1 Pages 60-67
2021 Volume 46 Issue 1 Pages 60-67
Insect juvenile hormone (JH) mimics (JHMs) are known to have ovicidal effects if applied to adult females or eggs. Here, we examined the effects of exogenous JHMs on embryonic development of the bean bug, Riptortus pedestris. The expression profiles of JH early response genes and JH biosynthetic enzymes indicated that JH titer was low for the first 3 days of the egg stage and increased thereafter. Application of JH III skipped bisepoxide (JHSB3) or JHM on Day 0 eggs when JH titer was low caused reduced hatchability, and the embryos mainly arrested in mid- or late embryonic stage. Application of JHMs on Day 5 eggs also resulted in an arrest, but this was less effective compared with Day 0 treatment. Interestingly, ovicidal activity of synthetic JHMs was much lower than that of JHSB3. This study will contribute to developing novel insecticides that are selective among insect species.

Insect juvenile hormone (JH) is a sesquiterpenoid hormone synthesized and secreted from the endocrine organ, the corpora allata. JH has been known as an anti-metamorphic hormone.1,2) In the larval stage, insects repeat larva–larva molts in response to high JH titer. Once they reach the proper body size, JH biosynthesis ceases, and this decline in JH titer triggers metamorphosis into a pupa and then into an adult. Besides its role in regulating molting and metamorphosis, JH plays pleiotropic roles in regulating reproduction, diapause, caste differentiation, etc.3)
Insecticides known as JH mimics (JHMs) have been developed and utilized as insect growth regulators (IGRs). In the mode of action classification by Insecticide Resistance Action Committee (IRAC), these are classified as “Group 7, Juvenile hormone mimics,” which includes chemicals such as hydroprene, kinoprene, methoprene, fenoxycarb, and pyriproxyfen.4) When applied to insects in their pre-final larval instar, JHMs disrupt and prevent metamorphosis into pupa or adult stages, which finally results in lethality. Recent studies to dissect the molecular mode of action of JHMs revealed that the target of JHMs is the JH receptor, i.e., the heterodimer of Methoprene-tolerant (Met)5–7) and Taiman (Tai)8,9): JHMs work as agonists of JH by binding to the receptor protein Met-Tai competitively.10) The JHM-bound Met-Tai then upregulates the transcription of an early JH-response gene Krüppel homolog 1 (Kr-h1).2,11)
Besides their inhibitory activity on insect metamorphosis, JHMs have ovicidal effects if applied to adult females or eggs.12) Morphogenesis of the corpora allata starts in the embryonic stage; in the fruit fly Drosophila melanogaster, formation of the corpora allata seems to be completed by stage 17 in the late embryonic stage.13) In the red flour beetle Tribolium castaneum, the morphogenesis of the corpora allata is completed by mid-embryogenic stage (at 54–60 hr after egg laying in 90-hr embryonic stage).14) Studies have been done to elucidate the role of JH in the embryonic stage. For example, in D. melanogaster, suppression of JH biosynthesis or signaling by genetic techniques did not result in lethality in embryonic or larval stages, but in arrest at the larva–pupa transition.15,16) In the silkworm Bombyx mori, overexpression of a JH-degrading enzyme resulted in precocious larva–pupa metamorphosis in the third larval instar without any detectable physiological change in embryonic development.17) In B. mori, JH-deficient mod mutants that lack the function of the JH-biosynthetic enzyme CYP15C1 survived through the embryonic stage and died in the larval stage18); knockout of JH acid O-methyltransferase (JHAMT) encoding the key enzyme in JH biosynthesis19) resulted in reduced hatchability, but these unhatched embryos could be rescued via removing eggshells manually.20) In addition, knockout of Met1 in B. mori resulted in a slightly reduced hatch rate and arrest in the larval stage.20) Thus, suppression of JH biosynthesis or JH signaling during embryonic stages of B. mori caused minor defects but was not lethal for embryonic development. By contrast, in T. castaneum, RNA interference (RNAi)-mediated knockdown of Met caused lethality in the embryonic stage and in the first larval instar.21) In the cockroach Blattella germanica, parental RNAi targeting JH biosynthesis and signaling resulted in an arrest in mid embryogenesis,22) suggesting that JH signaling is essential for embryonic development in this species. Taken together, the roles of JH in the embryonic stage seem to be diverse among insect species.
Effects of exogenous JHM treatment of the embryos have been examined in several insect species. In the migratory locust Locusta migratoria, it has been inferred that JH titer is low until mid-embryogenic stage and increases through mid- and late embryonic stages.23,24) In the orthopteran cricket Acheta domesticus, application of the JHM pyriproxyfen in early embryonic stage resulted in precocious formation of nymphal cuticle as well as failure in katatrepsis and dorsal closure.23) This suggested that these embryos were sensitive to an exogenous JHM in the early embryonic stage in which JH titer was still low. In the greenhouse whitefly Trialeurodes vaporariorum, treatment with pyriproxyfen was effective against Day 0–3 eggs, whereas Day 3–5 eggs were not sensitive.12) In addition, it has been reported that JHMs have ovicidal effects in several insect species such as fleas,25,26) psyllids,27) and mosquitoes28); in the psyllids and mosquitoes, eggs in the early embryonic stage were more sensitive to exogenous JHM than those in late embryonic stage.27,28) The detailed molecular mechanism underlying embryonic lethality caused by JHM remains largely unknown.
In this study, we examined the ovicidal activity of several synthetic JHMs, and observed effects in embryonic development of the bean bug, Riptortus pedestris. JHM treatment of the eggs was conducted on Day 0 (early embryonic stage) and Day 5 (mid- or late embryonic stage) eggs: through qRT-PCR of JH biosynthetic and signaling genes, we presumed that JH biosynthesis has not started on Day 0, whereas JH was abundant on Day 5. Application of JHMs on Day 0 caused arrest in mid- or late embryonic stage, but they were much less toxic compared with JH III skipped bisepoxide (JHSB3), the major endogenous JH in R. pedestris.29) Application of JHM on Day 5 eggs also resulted in arrest, but this was less effective compared with Day 0 treatment.
The bean bugs were kindly provided by Professor Numata and Dr. Suzaki (Kyoto University). They were reared in long-day conditions (16L/8D) at 25°C and were fed with soybean grain (Hasebe Corporation, Hokkaido, Japan), red clover seeds (Takii Seed, Kyoto, Japan), and water containing 0.05% sodium ascorbate. Under these conditions, the nymphs hatched approximately 8 days after egg laying. To collect staged eggs for qRT-PCR and chemical treatment, a small piece of cotton (5 cm×5 cm) was put in a rearing container with approximately 20 adults, and the eggs laid on the cotton were collected every 24 hr.
2. ChemicalsJHSB3 and JH III were synthesized in accordance with the reported synthetic method.30) Pyriproxyfen and fenoxycarb were purchased from Fujifilm Wako Pure Chemical Corporation (Osaka, Japan), (S)-(+)-methoprene from Funakoshi Corporation (Tokyo, Japan), and (S)-hydroprene from Sigma-Aldrich Japan (Tokyo, Japan). JHSB3 and JH III were dissolved in n-hexane, whereas pyriproxyfen was dissolved in acetone, and fenoxycarb, (S)-(+)-methoprene, and (S)-hydroprene were dissolved in methanol.
3. cDNA cloning and quantitative RT-PCRThe cDNA sequences of Kr-h1 and JHAMT were obtained from the transcriptomic database (accession number, DRA004114).31) The nucleotide sequence of Kr-h1 was confirmed by RT-PCR as follows. Total RNA was extracted from pooled nymphs in the third and fourth nymphal instars using TRIzol reagent (Thermo Fisher Scientific, MA, USA), and was reverse transcribed using a PrimeScriptII 1st strand cDNA Synthesis Kit using Oligo-dT primer (TaKaRa Bio Inc., Shiga, Japan). Primers used in RT-PCR are listed in Table S1. PCR products were purified, subcloned into pGEM-T Easy Vector (Promega Corporation, WI, USA) and sequenced. The obtained nucleotide sequence of Kr-h1 was deposited in the DDBJ/EMBL-Bank/GenBank International Nucleotide Sequence Database with the following accession number, LC587232. Nucleotide and amino acid sequences of JHAMT are shown in Figure S1A. Alignment of protein sequences of JHAMT and Kr-h1 from several insect species were conducted using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) as shown in Figures S1B and S2. Nucleotide sequence of ribosomal protein L32 (rpL32) was obtained from the DDBJ/EMBL-Bank/GenBank International Nucleotide Sequence Database (accession number, AK417074.1). Primers for qRT-PCR were designed based on the obtained sequences (listed in Table S1).
Eggs were collected every 24 hr after egg laying, and ten pooled eggs were homogenized. Total RNA was extracted using TRIzol reagent. First-strand cDNA was synthesized using a PrimeScript RT reagent with gDNA Eraser (TaKaRa Bio). To quantify mRNA levels, qRT-PCR reactions were performed using SYBR Premix Ex Taq (TaKaRa Bio) and a Thermal Cycler Dice Real-Time System TP800 (TaKaRa Bio) as described previously.21) The PCR conditions were 95°C for 30 sec followed by 45 cycles at 95°C for 5 sec and 60°C for 30 sec. The homogeneity of the amplicon was confirmed by melting curve analysis. The threshold cycle number for each mRNA in the sample was determined based on the second derivative of its primary amplification curve by relative quantification. The amount of target mRNA in each sample was normalized using R. pedestris rpL32 as the reference gene.
4. Application of JHMs to Riptortus pedestris eggsEggs either 0–24 hr (Day 0) or 120–144 hr (Day 5) after egg laying were aligned on double-sided sticky tape, and 1 µL of chemical solution was applied per egg. Ten eggs were included in one replicate, and experiments were done in 3–4 biological replicates except for 10 mM JH III and 10 mM JHSB3 (two biological replicates). Hatch rate was examined on Day 12. Based on the hatch rates of the solvent-treated group and test chemical-treated group, corrected mortality was calculated using Abbott’s formula.32)
The median lethal concentration (LC50) was calculated from corrected mortality at different concentrations by non-linear regression analysis with GraphPad Prism 8 (GraphPad Software, CA, USA).
5. Microscopic observation of embryosEggs that had not hatched by Day 12 after chemical treatment were observed using a microscope. The eggshells were removed with forceps, and the embryos were observed using a microscope (Olympus, model SZX12). Normal eggs were collected every 24 hr, and the embryos were observed after removing eggshells, too. Based on the external morphology of the unhatched embryos, they were classified into the following categories (Figs. 3 and 4).
Multiple alignment of protein sequences of JHAMT (Fig. S1B) and Kr-h1 (Fig. S2) revealed that R. pedestris JHAMT and Kr-h1 homolog sequences are similar to their sequences reported from other insects.
The expression profiles of JHAMT and Kr-h1 were analyzed using qRT-PCR. The JHAMT mRNA levels remained low from Day 0 to Day 2, increased on Day 3 and Day 4, and remained high until Day 7 (Fig. 1A). The expression profile of the JH inducible gene Kr-h1 was similar to that of JHAMT, although Kr-h1 mRNA was also detected on Day 0 (Fig. 1B).

Firstly, we observed the morphology of normal embryos of R. pedestris after removing eggshells manually. It was difficult to take out embryos from Day 0 to Day 3 from the eggshell because they were too small and fragile. Observation of the embryos indicated that the morphogenesis of appendages proceeded from Day 4 to Day 7 (Fig. 2). Embryos looked flat and thin dorsoventrally on Day 4, and became thicker afterwards. Morphogenesis of the nymph was almost complete on Day 7 prior to hatching (Fig. 2).

As described above, we estimated through JHAMT gene expression analysis that JH biosynthesis started on Day 3 in the embryonic stage, and that transient peak in Kr-h1 on Day 0 might be due to maternal JHs or maternal Kr-h1 mRNA. Therefore, application of JHs and JHMs was conducted on Day 0 eggs in which JH biosynthesis had not yet started presumably. Application of JHSB3 caused a reduction in hatch rate, and the LC50 of JHSB3 was calculated as 2.8 µM (Table 1). By contrast, the ovicidal activity of JH III was relatively low, and the corrected mortality was only 16% at 10 mM. Synthetic JHMs were also applied to Day 0 eggs, and mortality was examined. The LC50 of fenoxycarb, (S)-hydroprene, (S)-(+)-methoprene, and pyriproxyfen were 1.6 mM, 15 mM, 3.9 mM, and 92 mM, respectively (Table 1).
| LC50 | 95% CL | |
|---|---|---|
| JHSB3 | 2.8 µM | 1.3–5.8 µM |
| JH IIIa) | >10 mM | |
| Fenoxycarb | 1.6 mM | 0.67–3.5 mM |
| (S)-Hydroprene | 15 mM | 7.8–33 mM |
| (S)-(+)-Methoprene | 3.9 mM | 1.4–11 mM |
| Pyriproxyfen | 92 mM | 50–190 mM |
a) The corrected mortality at 10 mM was 16%.
To identify the cause of lethality of chemical-treated eggs, unhatched eggs after chemical treatment on Day 0 were observed after removing eggshells. In this experiment, a few unhatched eggs after solvent treatment on Day 0 were pooled and observed as the control. As shown in Table 2, unhatched embryos after n-hexane or methanol treatment were arrested mainly in early embryonic stages (57% for n-hexane, and 85% for methanol). By contrast, unhatched embryos after 10 µM JHSB3 treatment on Day 0 were arrested in early, mid-, and late embryonic stages (33%, 22%, and 44%, respectively). Unhatched embryos after application of fenoxycarb and (S)-hydroprene at 3 mM also showed similar phenotype: they were arrested in early, mid-, and late embryonic stages in similar proportions (Table 2). The majority (64%) of unhatched embryos after application of (S)-(+)-methoprene on Day 0 were arrested in the late embryonic stage (categorized as Phenotype 3 and 4 in Table 2; see Fig. 3 for details of each phenotype). Thus, the cause of lethality of solvent-treated eggs could be attributed to an arrest in the early embryonic stage. In contrast, the cause of lethality of JHSB3- or JHM-treated eggs could be attributed to arrest in early, mid-, and late embryonic stages.
| Number of eggs observed | Unfertilized or arrested in early embryonic stage | Arrested in mid- and late embryonic stages | ||||
|---|---|---|---|---|---|---|
| Phenotype 1: embryos cannot be removed from eggshells | Phenotype 2: arrest in mid-embryonic stage (around Day 4–5) | Phenotype 3: arrest in late embryonic stage (around Day 6–7), with morphological defects | Phenotype 4: arrest in late embryonic stage (around Day 6–7), with normal external morphology | |||
| n-Hexane (control) | 7 | 4 (57%) | 1 (14%) | 0 | 2 (29%) | |
| JHSB3 | 10 µM | 9 | 3 (33%) | 2 (22%) | 3 (33%) | 1 (11%) |
| Methanol (control) | 20 | 17 (85%) | 2 (10%) | 0 | 1 (5%) | |
| Fenoxycarb | 3 mM | 14 | 3 (21%) | 5 (36%) | 1 (7%) | 5 (36%) |
| (S)-Hydroprene | 3 mM | 10 | 4 (40%) | 4 (40%) | 2 (20%) | 0 |
| (S)-(+)-Methoprene | 3 mM | 14 | 4 (29%) | 1 (7%) | 1 (7%) | 8 (57%) |
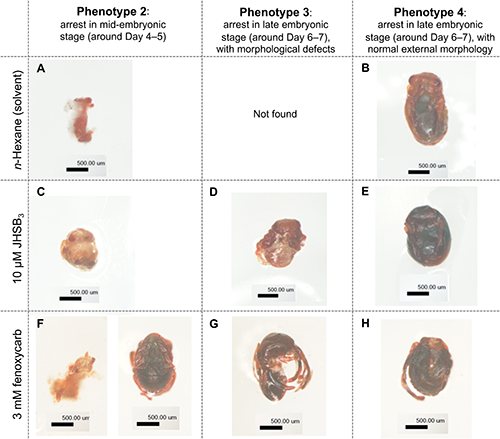
As shown in Fig. 3, some individuals were arrested in the mid-embryonic stage, and their morphogenesis had not proceeded (panels A, C, and F; categorized as Phenotype 2). In addition, morphological defects were found in embryos that were arrested after treatment with JHSB3 or JHM; the abdomen seemed to be small (panels D and G in Fig. 3; categorized as Phenotype 3). Some embryos were arrested in the late embryonic stage without obvious morphological defects (panels B, E, and H in Fig. 3; categorized as Phenotype 4).
4. Effects of JHM treatment of Day 5 eggsWe then conducted an application of a JHM fenoxycarb on Day 5 eggs in which JH biosynthesis had already started presumably. The corrected mortality of fenoxycarb was 17% at 3 mM, and 65% at 10 mM.
Unhatched eggs after fenoxycarb treatment on Day 5 were observed after removing the eggshells. As shown in Table 3, unhatched embryos after methanol treatment were arrested mainly in early or mid-embryonic stages (60% and 28%, respectively). Unhatched embryos after 3 mM fenoxycarb treatment were arrested mainly in early or mid-embryonic stages (21% and 64%, respectively); treatment with 10 mM fenoxycarb also caused arrest mainly in early or mid-embryonic stages (58% and 17%, respectively). The number of unhatched embryos arrested in late embryonic stage, categorized as Phenotypes 3 and 4 in Table 3, was comparable between the methanol-treated group (12%) and the 3 mM fenoxycarb-treated group (14%), whereas it increased slightly after 10 mM fenoxycarb treatment (25%).
| Number of eggs observed | Unfertilized or arrested in early embryonic stage | Arrested in mid- and late embryonic stages | ||||
|---|---|---|---|---|---|---|
| Phenotype 1: embryos cannot be removed from eggshells | Phenotype 2: arrest in mid-embryonic stage (around Day 4–5) | Phenotype 3: arrest in late embryonic stage (around Day 6–7), with morphological defects | Phenotype 4: arrest in late embryonic stage (around Day 6–7), with normal external morphology | |||
| Methanol (control) | 25 | 15 (60%) | 7 (28%) | 0 | 3 (12%) | |
| Fenoxycarb | 3 mM | 14 | 3 (21%) | 9 (64%) | 1 (7%) | 1 (7%) |
| 10 mM | 24 | 14 (58%) | 4 (17%) | 2 (8%) | 4 (17%) | |
After fenoxycarb treatment, some individuals were arrested in mid- and late embryonic stages, and the abdomen seemed to be smaller in some individuals (panels D and G in Fig. 4; categorized as Phenotype 3), whereas others were arrested in late embryonic stage without obvious morphological defects (panels E and H in Fig. 4; categorized as Phenotype 4). Overall, the morphology of arrested embryos that were treated with fenoxycarb on Day 5 was similar to that after chemical treatment on Day 0.

In this study, we examined the effects of JHM treatment of R. pedestris eggs. We first estimated JH titer from the expression profiles of a JH biosynthetic enzyme, JHAMT, and an early JH-response gene, Kr-h1. The transcript levels of JHAMT and Kr-h1 started to increase in the mid-embryonic stage (Day 3), suggesting that JH biosynthesis in the embryo starts in this stage. This observation was consistent with that in T. castaneum: the corpora allata are formed and start to synthesize JH in mid-embryogenic stage (at 54–60 hr after egg laying in 90-hr embryonic stage).14) We found that there was transient expression of Kr-h1 mRNA on Day 0, whereas JHAMT mRNA was undetectable on Day 0 (Fig. 1). We assumed that the transient peak of Kr-h1 on Day 0 could be due to maternal Kr-h1 mRNA. Alternatively, it is possible that a small amount of maternal JH induced the transcription of Kr-h1.
Thus, we decided to apply JHs and JHMs in the beginning of the embryonic stage (Day 0), in which JH biosynthesis was presumed not to have started. The ovicidal activity of JHSB3, the major endogenous JH in R. pedestris,29,33) was much more active than JH III: the LC50 of JHSB3 was 2.8 µM, whereas the mortality with JH III was only 16% at 10 mM (Table 1). Because we were not able to prepare JH III solution at higher concentrations, we could not determine its LC50. Unexpectedly, the ovicidal activities of synthetic JHMs were much lower compared with JHSB3. The LC50 values of fenoxycarb, (S)-hydroprene, (S)-(+)-methoprene, and pyriproxyfen were 1.6 mM, 15 mM, 3.9 mM, and 92 mM, respectively (Table 1). In our preliminary experiments, the metamorphosis-inhibiting activity of these JHMs against the pre-final instar nymphs was lower than that of JHSB3 (Nakagawa et al., unpublished). The molecular mechanism of how JHMs showed low activity against R. pedestris remains unknown. There could be several possibilities: JHMs could be easily degraded by metabolic detoxifying enzymes in R. pedestris or the binding affinity of JHMs to the JH receptor (Met-Tai) of R. pedestris might be lower compared with that of JHSB3.
Microscopic observation of unhatched eggs revealed that treatment of JHSB3 or JHMs increased the number of embryos that were arrested in the mid- and late embryonic stage (Phenotypes 2–4 in Table 2), whereas unhatched eggs that had been treated with solvent were arrested mainly in the early embryonic stage (Phenotype 1 in Table 2). Thus, the cause of lethality of unhatched eggs after solvent treatment could be attributed to arrest in the early embryonic stage, but the cause of lethality of JHSB3- or JHM-treated eggs could be attributed to an arrest in early, mid-, and late embryonic stages. We concluded that treatment with JHSB3 and JHMs on Day 0 caused an arrest in mid- and late embryonic stages. Morphological defects such as undeveloped and smaller abdomens were found in some embryos that were arrested after JHSB3 or JHM treatment (Phenotype 3 in Table 2 and Fig. 3), whereas others were arrested in late embryonic stage without obvious defects in their external morphology (Phenotype 4 in Table 2 and Fig. 3). In the cricket A. domesticus, treatment of early embryos with JH III or JHM resulted in failure of katatrepsis or dorsal closure in mid-embryogenesis.23) In this study, R. pedestris embryos that received JHSB3 or JHM were arrested mainly in mid- and late embryogenesis, and some of them seemed to be arrested around the time of dorsal closure (Fig. 3F, left panel), suggesting that the effect of exogenous JHM on the embryos might be similar between A. domesticus and R. pedestris.
Our qRT-PCR analysis suggested that JH titer was low on Day 0–2, and it increased thereafter (Fig. 1). We performed JHM treatment on Day 5 eggs, in which JH biosynthesis was presumed to have already started, and compared the effects with those after Day 0 treatment. The corrected mortality with fenoxycarb after Day 5 treatment was 17% at 3 mM, and 65% at 10 mM, whereas that after Day 0 treatment was 88% at 3 mM, and 100% at 10 mM (LC50=1.6 mM). Thus, treatment on Day 5 was less effective than Day 0 treatment. This observation was somewhat consistent with a report in the whitefly T. vaporariorum, in which pyriproxyfen treatment was less effective in the late embryonic stage compared with treatment in the early embryonic stage.12) We assumed that Day 5 eggs of R. pedestris might be less sensitive to exogenous JHM than Day 0 eggs because of the presence of abundant endogenous JH. Moreover, metabolic detoxification might be more active in Day 5 embryos.
As stated above, the ovicidal activity of JHMs as well as their metamorphosis-inhibiting activity against R. pedestris was much lower than that of JHSB3. This was surprising because JHMs have been utilized as insecticides against a variety of pest insects such as whiteflies, mosquitoes, psyllids, flies, and fleas. Regarding the molecular mechanism how JHMs showed low activity against R. pedestris, several possibilities such as enhanced metabolic detoxification of JHMs and low binding affinity of JHM to the JH receptor (Met-Tai) have been suggested. Since heteropteran insects, including R. pedestris have a unique endogenous JH, i.e., JHSB3, there is a possibility that the structure of ligand-binding pocket of Met is different between R. pedestris and other species, which might cause different ligand-binding affinity of JHM. Detailed studies are ongoing to obtain more insights into the mechanism of selective toxicity of JHMs among insect species.
We thank Professor Numata and Dr. Suzaki (Kyoto University) for providing the bean bugs. This study was supported by a grant-in-aid for Pesticide Science from Pesticide Science Society of Japan.
The online version of this article contains supplementary materials (Supplemental Table S1, Figs. S1, S2), which are available at http://www.jstage.jst.go.jp/browse/jpestics/.