2021 Volume 46 Issue 2 Pages 152-159
2021 Volume 46 Issue 2 Pages 152-159
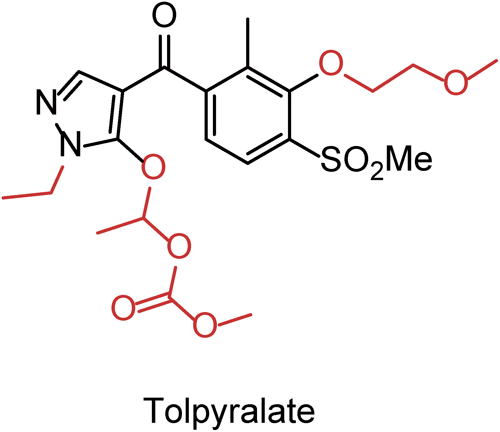
Tolpyralate, a new selective postemergence herbicide developed for the weed control in corn, possesses a unique chemical structure with a 1-alkoxyethyl methyl carbonate group on the N-ethyl pyrazole moiety. This compound shows high herbicidal activity against many weed species, including glyphosate-resistant Amaranthus tuberculatus. Tolpyralate targets 4-hydroxyphenylpyruvate dioxygenase (4-HPPD), which is involved in the tyrosine degradation pathway. Inhibition of the enzyme destroys the chlorophyll, thereby killing the susceptible weeds. Details of tolpyralate discovery, structure optimization, and biological activities are described.
The resistance to known commercial herbicides targeting most modes of action including the inhibition of 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) is a great threat for the future food production, and a challenge for modern agriculture science.1) The resistance mechanism of herbicides can be classified as target-site resistance (TSR) caused by specific resistance-conferring mutations or non-target-site resistance (NTSR) derived from enhanced herbicide metabolism. According to the Herbicide Resistance Action Committee (HRAC), bleaching herbicides associated with carotenoid biosynthesis are classified into several different mechanisms of action.2) Among them, 4-hydroxyphenylpyruvate dioxygenase (4-HPPD) inhibitor is one of the most actively researched target for herbicide discovery because of low application rate, broad weed spectrum and also usefulness for controlling glyphosate-resistant weeds.3–5) To date, no major herbicide with a new mode of action has been introduced to the corn market for more than two decades. However, the resistance to 4-HPPD inhibiting herbicide has been detected in only two weed species worldwide, mainly due to enhanced metabolism.6) Therefore, the new generation 4-HPPD inhibitor meets the demands of growers from the perspective of resistant weed management.7)
The herbicidal mechanism is the inhibition of 4-HPPD that catalyzes the transformation of 4-hydroxyphenylpyruvate (4-HPPA) to homogentisate (HGA) in the biosynthesis pathway of plastoquinone (PQ), which gives rise to bleaching symptoms on target weeds.8,9) These symptoms result from an indirect inhibition of carotenoid biosynthesis due to the involvement of PQ as a cofactor of phytoene desaturase (PDS).
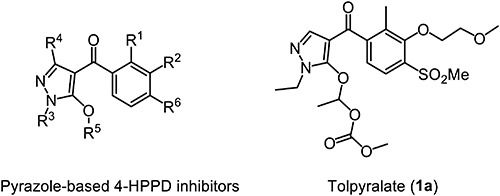
Among several herbicide families of 4-HPPD inhibitors, the pyrazole structure was found at the earliest stage of discovery, but the diversity of structural changes continues to attract researchers’ attention. In the 1980s, several pyrazolones such as pyrazolynate, pyrazoxyfen and benzofenap were commercialized as bleaching herbicides10) for paddy weed control at a rate of 3–4 kg a.i./ha by Japanese companies. These pioneering works have been successfully imparted practical herbicidal activity and crop selectivity by giving an appropriate structure design. Recently, N-methylpyrazole-based herbicide topramezone11) has been introduced to the corn market.
Based on the knowledge of such previous findings, we started a new HPPD inhibitor discovery research program toward the goal of creating a new herbicide with excellent herbicidal activity and selectivity for all types of corn. Here, we present the details of the discovery, synthesis, structure–activity relationships and biological properties of tolpyralate (1a; Fig. 1)12–14) and related derivatives.
The melting points of the products were determined by a Büchi melting point apparatus M-565 and were uncorrected. 1H NMR and 13C NMR were recorded at room temperature using a JEOL GSX-400 MHz spectrometer, a Bruker Avance III HD 300 MHz spectrometer, and a Varian Mercury Plus 300 MHz spectrometer with the solvent residual peak as the internal standard unless otherwise noted.15) High-resolution mass spectra (HRMS) were obtained on a Thermo Fisher LTQ Orbitrap XL mass spectrometer using positive electrospray ionization (ESI). All reagents were commercially available and used as received.
2. Preparation of compoundsAs shown in Fig. 2, benzoic acids 2 were converted to the corresponding acid chlorides 3 by using a chlorinating reagent such as thionyl chloride and then condensed with 1-alkyl-5-hydroxypyrazole in the presence of base to afford benzoylpyrazoles 4 through Fries rearrangement. Subsequent substitution of the hydroxy group on the pyrazole ring of compounds 4 afforded tolpyralate 1a and related analogs 1.16) As an alternative synthetic route, intermediates 8 were condensed with 1-alkyl-5-hydroxypyrazole in the presence of palladium catalyst under CO atmosphere to give compounds 4.

When R2 was an effective leaving group such as halogen, a regioselective aromatic nucleophilic substitution reaction (SNAr) proceeded by using a nucleophile such as an alkoxy anion to give compounds 5, which was subsequently converted to compounds 6.
2.1. 1,3-Dichloro-2-methyl-4-(methylsulfonyl)benzene (7a)A mixture of 2,6-dichlorotoluene (100 g, 621 mmol) and methanesulfonyl chloride (85.3 g, 745 mmol) was heated to 80°C and then iron (III) chloride (101 g, 623 mmol) was added portionwise. The resulting mixture was further heated to 120°C and stirred for 6 hr. After completion of the reaction, the reaction mixture was cooled to 90°C. Next, 10% of hydrochloric acid (230 mL) was added slowly while maintaining the temperature above 80°C, and 2-propanol (230 mL) was added slowly at 80°C. The mixture was stirred vigorously and allowed to cool to room temperature. The resulting precipitate was collected by filtration and washed with a mixed solvent of water (150 mL) and 2-propanol (150 mL). The obtained solid was dissolved in ethyl acetate (600 mL) under heating, followed by filtration to remove insoluble material. The filtrate was concentrated under reduced pressure to give the titled compound 7a (105 g, 439 mmol, 71% yield) as a pale yellow solid.
mp: 124–125°C; 1H NMR (400 MHz, CDCl3): δ=2.57 (3H, s), 3.28 (3H, s), 7.50 (1H, d, J=8.4 Hz), 7.97 (1H, d, J=8.4 Hz); 13C NMR (75 MHz, CDCl3): δ=18.0, 42.8, 128.2, 128.7, 133.6, 137.2, 137.7, 141.4; HRMS calcd. for C8H9Cl2O2S+ [M+H]+ 238.9695, found 238.9693.
2.2. 1-Chloro-3-(2-methoxyethoxy)-2-methyl-4-(methylsulfonyl)benzene (8a)To a suspension of compound 7a (13.1 g, 54.8 mmol) in toluene (40 mL), 2-methoxyethanol (4.49 g, 59.0 mmol) and sodium hydroxide (4.45 g, 111 mmol) were added, followed by refluxing for 3 hr. After completion of the reaction, the mixture was cooled to room temperature, and the solvent was distilled off under reduced pressure. To the obtained residue, a mixed solvent of methanol (12 mL) and water (48 mL) was added and the resulting mixture was stirred for a while. The resulting solid was collected by filtration, washed with water and dried to afford the titled compound 8a (12.4 g, 44.5 mmol, 81% yield) as a pale yellow solid.
mp: 83.5–85°C; 1H NMR (300 MHz, CDCl3): δ=2.41 (3H, s), 3.25 (3H, s), 3.48 (3H, s), 3.78–3.81 (2H, m), 4.20–4.22 (2H, m), 7.33 (1H, d, J=8.4 Hz), 7.76 (1H, d, J=8.7 Hz) ; 13C NMR (75 MHz, CDCl3): δ=13.7, 43.6, 59.2, 71.6, 74.3, 125.5, 127.2, 132.8, 133.3, 142.2, 155.8; HRMS calcd. for C11H16ClO4S+ [M+H]+ 279.0452, found 279.0451.
2.3. 3-(2-Methoxyethoxy)-2-methyl-4-(methylsulfonyl)benzoic acid (2a)To a mixed solvent of water (12.5 mL) and 2-methyl-2-propanol (238 mL), nitrogen gas was blown for 5 min to remove dissolved oxygen thereby to prepare a reaction solvent. Into a 500 mL autoclave, compound 8a (50.0 g, 179 mmol), sodium carbonate (28.5 g, 269 mmol), 1,4-bis(diphenylphosphino)butane (1.50 g, 3.52 mmol), 5% Pd/C (wet, 1.50 g), and the above reaction solvent were introduced. Nitrogen flushing (5 MPa) was carried out twice under vigorous stirring, followed by flushing carbon monoxide (5 MPa) twice. Finally, carbon monoxide (2.5 MPa) was filled. The autoclave was heated at 160°C for 7 hr under stirring (300 rpm). The resulting mixture was cooled to room temperature and poured into a mixed solvent of water and ethyl acetate. The resulting mixture was filtered through a short pat of Celite to remove insoluble solid and the filtrate was partitioned between ethyl acetate and water to remove impurities. The aqueous layer was acidified by the addition of 10% hydrochloric acid to pH 1 and then extracted with ethyl acetate twice. The combined organic layer was washed with saturated brine twice, dried over anhydrous sodium sulfate and then the solvent was removed under reduced pressure. Hexane (150 mL) was added and the obtained solid was collected by filtration to afford the titled compound 2a (48.1 g, 167 mmol, 93% yield) as a white solid.
mp: 102–104°C; 1H NMR (300 MHz, CDCl3): δ=2.63 (3H, s), 3.31 (3H, s), 3.50 (3H, s), 3.82–3.85 (2H, m), 4.22–4.25 (2H, m), 7.92 (2H, s); 13C NMR (75 MHz, CDCl3): δ=13.9, 43.5, 59.2, 71.7, 74.1, 126.6, 127.1, 136.1, 136.7, 138.3, 155.8, 171.3; HRMS calcd. for C12H17O6S+ [M+H]+ 289.0740, found 289.0736.
2.4. 3-(2-Methoxyethoxy)-2-methyl-4-(methylsulfonyl)benzoyl chloride (3a)To a stirred suspension of compound 2a (100 g, 347 mmol) in toluene (300 mL) were added thionyl chloride (47.5 g, 399 mmol) and N,N-dimethylformamide (DMF) (2.50 g, 34.2 mmol) at room temperature. The resulting mixture was heated at refluxed temperature for 2 hr and then 180 mL of toluene was distilled off under reduced pressure to obtain a toluene solution of the titled compound 3a for the use in the next reaction without further purification.
2.5. (1-Ethyl-5-hydroxy-1H-pyrazol-4-yl)(3-(2-methoxyethoxy)-2-methyl-4-(methylsulfonyl)phenyl)methanone (4a)To a stirred suspension of 1-ethyl-1H-pyrazol-5-ol (42.8 g, 382 mmol) in toluene (150 mL) was added trimethylamine (38.6 g, 382 mmol) at room temperature to obtain a clear solution. The toluene solution of acid chloride 3a prepared in the above-mentioned method was added dropwise, keeping the temperature below 30°C. After stirring for 1 hr at room temperature, the mixture was heated to 80°C and the stirring was continued for 30 min. The resulting solution was cooled to room temperature and poured into water (150 mL). The water phase was extracted with toluene (200 mL) twice and the combined organic layer was dried over anhydrous magnesium sulfate. The resulting mixture was filtered to remove insoluble solid and the residue was washed with a small portion of toluene to give a solution of 1-ethyl-1H-pyrazol-5-yl 3-(2-methoxyethoxy)-2-methyl-4-(methylsulfonyl)benzoate as an intermediate for the next reaction.
The toluene solution of 1-ethyl-1H-pyrazol-5-yl 3-(2-methoxyethoxy)-2-methyl-4-(methylsulfonyl)benzoate prepared above was transferred to a three-necked round bottom flask equipped with a Dean-Stark trap, and a part of toluene (about 100 mL) was removed with water by azeotropic distillation. The solution was cooled to 80°C, and then DMF (40 mL) and powdered potassium carbonate (33.6 g, 243 mmol) were added. The resulting mixture was heated at refluxed temperature and the solvent was removed (about 100 mL) again during 3 hr to give a toluene solution containing a potassium salt of the titled compound 4a.
2.6. 1-((1-Ethyl-4-(3-(2-methoxyethoxy)-2-methyl-4-(methylsulfonyl)benzoyl)-1H-pyrazol-5-yl)oxy)ethyl methyl carbonate (1a, tolpyralate)To the stirred toluene solution of potassium salt of 4a prepared in the procedure 2.5. was added dropwise 1-chloroethyl methyl carbonate (62.5 g, 451 mmol) in the presence of tetra-n-butylammonium bromide (5.60 g, 17.4 mmol) at 90°C. After completion of the addition, the resulting mixture was heated at 100°C for 3 hr and then cooled to 50°C. Hexane (150 mL) was added thereto, and the stirring was continued for 30 min. Water (300 mL) and 3 N hydrochloric acid (300 mL) were sequentially added and the resulting mixture was allowed to cool to room temperature. Precipitated crystals were collected by filtration, washed with water and a mixed solvent of hexane and toluene (1 : 2, 300 mL) to give tolpyralate 1a (120.9 g, 249.5 mmol, 72% yield over 3 steps) as a slightly brown solid.
mp: 124–126°C; 1H NMR (300 MHz, CDCl3): δ=1.42 (3H, t, J=7.2 Hz), 1.79 (3H, d, J=5.1 Hz), 2.37 (3H, s), 3.32 (3H, s), 3.48 (3H, s), 3.73 (3H, s), 3.81–3.83 (2H, m), 4.01–4.13 (2H, m), 4.24–4.27 (2H, m), 6.80 (1H, q, J=5.1 Hz), 7.28 (1H, d, J=8.1 Hz), 7.31 (1H, s), 7.90 (1H, d, J=8.1 Hz); 13C NMR (75 MHz, CDCl3): δ=13.0, 14.4, 20.3, 42.6, 43.6, 55.2, 59.1, 71.6, 73.8, 100.9, 108.7, 123.1, 126.8, 131.8, 135.7, 142.3, 147.3, 152.1, 154.2, 155.7, 189.1; HRMS calcd. for C21H29N2O9S+ [M+H]+ 485.1588, found 485.1581.
The other intermediates and derivatives were synthesized by the similar method mentioned above or the method described in the literature.17) 1H NMR spectrum data of the other derivatives are described in the Supplementary materials (Table S1).
3. Biological evaluation 3.1. Herbicidal activity test under greenhouse conditionsBiological experiments were conducted in the greenhouse at Central Research Institute of Ishihara Sangyo Kaisha, Ltd. in Shiga, Japan for evaluating herbicidal efficacy and corn selectivity.
The synthesized compounds were formulated as either 10% WP (wettable powders) or 10% EC (emulsifiable concentrates), which were diluted with water to the required application dose rate and applied to the pot-grown plants using hand sprayer with 500 L/ha water volume containing spray adjuvant (nonionic adjuvant, 0.05% (v/v)) in greenhouse. Treated weeds species were Echinochloa crus-galli (ECHCG), Setaria viridis (SETVI), Amaranthus retroflexus (AMARE) and Abutilon theophrasti (ABUTH), and Zea mays (ZEAMX) was used as a crop. A plastic pot (13.5 cm×24.0 cm) was filled with sandy loam soil (2000 mL) and kept wet by watering. A certain amount of each seeds were sown to the soil surface and covered with the soil about 1–3 mm, and grown until the weeds and crop reached 2–4 leaf stage at 20–25°C. When they reached such growth stage, each compounds were applied and the herbicidal activity and crop selectivity were observed visually 21 days later, using growth inhibition rate 0 (no effect/injury) to 100 (completely control or death).
3.2. Herbicidal activity test under field conditionsField trials of tolpyralate were conducted to evaluate the herbicidal efficacy at Central Research Institute of Ishihara Sangyo Kaisha, Ltd. in Shiga, Japan from 2011 to 2017. Weed seeds of ECHCG, Setaria faberi (SETFA), Eleusine indica (ELEIN), Solanum nigrum (SOLNI), Chenopodium album (CHEAL), Polygonum lapathifolium (POLLN), Ambrosia artemisiifolia (AMBEL), AMARE, Ipomoea hederacea (IPOHE), and field corn were sown in experimental plot and immediately mixed with soil. The trial site had a natural population of Digitaria sanguinalis (DIGSA), Portulaca oleracea (POROL), and Galinsoga quadriradiata (GASCI). When the field corn reached 4 leaf stage or more, tolpyralate 400SC (suspension concentrate formulation including 35.7% (w/w) of tolpyralate as an active ingredient) which is adjuvant non-built-in formulation was applied using a CO2-pressurized backpack sprayer calibrated to deliver 300 L/ha containing 0.5% (v/v) of methylated seed oil adjuvant (MSO) at a pressure of 275.8 kPa through TeeJet 8001VS flat fan nozzles (TeeJet® Spraying Systems Co., P.O. Box 7900, Wheaton, IL, USA). The growth state of respective weeds were visually observed to determine the herbicidal efficacy rate on 28–36 days after application [herbicidal efficacy; 0% (equivalent to the untreated plot) to 100% (completely killed)].
3.3. Corn selectivity test under field conditionsField trials of tolpyralate were conducted to evaluate the corn selectivity at Central Research Institute of Ishihara Sangyo Kaisha, Ltd. in Shiga, Japan from 2016 to 2017. The selectivity by corn variety was evaluated with 41 field corn and 16 sweet corn varieties. When the field corn reached approximately 5 leaf stage, tolpyralate 100OD (oil dispersion formulation including 10.4% (w/w) of tolpyralate as an active ingredient) which is adjuvant built-in formulation was applied using a CO2-pressurized backpack sprayer calibrated to deliver 1000 L/ha at a pressure of 275.8 kPa through TeeJet 8003VS flat fan nozzles (same as above). The chlorosis symptoms of aerial part were visually observed on 4–6 days after application, using a scale of 0 to 5 with 0 representing equal to untreated plot and 5 representing complete death. The culm length was measured on 47–59 days after application and the ratio with untreated plot each of variety was calculated.
3.4. Herbicidal activity test against glyphosate-resistant weeds under greenhouse conditionsGreenhouse experiments were conducted in the United States to evaluate the herbicidal efficacy of tolpyralate on 3 broadleaf weed species. Weed seeds of Amaranthus tuberculatus (AMATU), Ambrosia trifida (AMBTR), Erigeron canadensis (ERICA) were sown in the experimental pot and immediately mixed with soil. Tolpyralate 400SC was applied using a CO2-pressurized backpack sprayer calibrated to deliver 187 L/ha containing 0.5% (v/v) of MSO at a pressure of 241.3 kPa through TeeJet 80015EVS nozzles (same as above). The growth state of respective weeds was visually observed to determine the herbicidal efficacy rate on 29–30 days after application [herbicidal efficacy; 0% (equivalent to the untreated plot) to 100% (completely killed)].
Our previous results of intensive study on pyrazole-based 4-HPPD inhibitors (Supplemental Table S2) revealed that the introduction of an appropriate substituent R5 into the hydroxy group on the pyrazole ring resulted in improved herbicidal activity and safety in corn. Based on these findings, S-ethyl thiocarbonate18) or 1-alkoxyethyl alkyl carbonate12) was employed as a standard protecting group and various derivatives were synthesized for the structure optimization. Representative chemical structures and biological activities are shown in Table 1 and 2.
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound No. | R1 | R2 | R5 | Dose (g a.i./ha) | Growth inhibition rate (%) | ||||
| ECHCG | SETVI | AMARE | ABUTH | ZEAMX | |||||
| 9 | Cl |

|
R5-a | 7 | 90 | 50 | 70 | 40 | 0 |
| 10 | Me |

|
R5-a | 7 | 95 | 95 | 90 | 75 | 0 |
| 11 | Me |
|
R5-a | 7 | 80 | 70 | 60 | 60 | 0 |
| 12 | Me |

|
R5-a | 7 | 95 | 90 | 80 | 90 | 0 |
| 13 | Me |
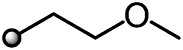
|
R5-a | 7 | 90 | 80 | 100 | 80 | 0 |
| 14 | Me |

|
R5-a | 7 | 75 | 30 | 70 | 70 | 0 |
| 15 | Me |

|
R5-a | 7 | 95 | 95 | 80 | 65 | 0 |
| 16 | Me |
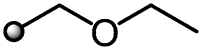
|
R5-a | 7 | 75 | 80 | 98 | 80 | 0 |
| 17 | Me |

|
R5-a | 7 | 90 | 70 | 60 | 60 | 0 |
| 18 | Me |

|
R5-a | 7 | 70 | 70 | 98 | 80 | 0 |
| 19 | Me |

|
R5-a | 31 | 30 | 0 | 10 | 0 | 10 |
| 20 | Me |

|
R5-a | 7 | 20 | 20 | 60 | 30 | 0 |
| 21 | Me |

|
R5-a | 7 | 60 | 95 | 95 | 98 | 0 |
| 22 | Me |
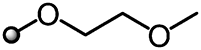
|
R5-b | 7 | 98 | 100 | 100 | 95 | 0 |



|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound No. | R3 | R4 | R5 | Dose (g a.i./ha) | Growth inhibition rate (%) | ||||
| ECHCG | SETVI | AMARE | ABUTH | ZEAMX | |||||
| 23 | Et | H | R5-b | 7 | 95 | 100 | 90 | 90 | 0 |
| 24 | i-Pr | H | R5-b | 7 | 95 | 90 | 95 | 95 | 0 |
| 25 | n-Pr | H | R5-b | 7 | 70 | 30 | 80 | 60 | 0 |
| 26 | Me | Me | R5-b | 7 | 90 | 90 | 80 | 60 | 0 |
| 27 | Me | c-Pr | R5-b | 7 | 60 | 30 | 80 | 20 | 0 |
| 28 | Et | H | Me | 31 | 50 | 0 | 80 | 80 | 0 |
| 29 | Me | H | benzyl | 7 | 95 | 90 | 60 | 100 | 0 |
| 30 | Me | H | acetyl | 7 | 98 | 100 | 100 | 100 | 10 |
| 31 | Me | H | R5-c | 7 | 100 | 100 | 100 | 100 | 10 |
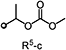
| 1a | Et | H | R5-c | 7 | 95 | 100 | 90 | 100 | 0 |


First, we examined the introduction of various linear ether substituents R2 at the 3-position of the benzene ring to evaluate the steric effect and the role of heteroatoms. Insertion of a CH2 or CH2CH2 linker between the benzene ring and the methoxy group of compound 11 improved herbicidal activities (12 and 13), whereas insertion of a CH2CH2CH2 linker depressed the activities (14). The position of oxygen also affected the biological activity of compound 16 which contains an oxygen atom at the beta position from the benzene ring. The herbicidal activity of 16 was good against AMARE and ABUTH but inferior to 15 against grass weeds such as ECHCG and SETVI. To our delight, an additional oxygen insertion (10) led to the enhanced overall activities, while one atom longer derivative 18 tended to be weaker on grass weeds. As a result, the side chain length was optimal at 3 to 5 chemical bonds at the longest, with any increase in chain length associated with a reduction of herbicidal activity. As mentioned above, the introduction of the heteroatom such as oxygen into the side chain resulted in a certain activity enhancement, whereas the introduction of sulfur caused the reduction of the activity (19). Furthermore, the activity was also reduced by introducing a methyl group into the side chain to form a branched structure (20 and 21) or a trifluoroethyl ether substituent (17).
The substituent effect of R1 on the benzene ring was also explored. The methyl derivative 10 clearly showed higher herbicidal activity against each weed species, compared with the chloro derivative 9.
In the course of the above studies, when the substituent R5 on the pyrazole ring was converted to 1-alkoxyethyl ethyl carbonate such as 22, a large improvement in the activity was observed as compared with the S-ethyl thiocarbonate derivative 10. Therefore, further optimization of substituents on the pyrazole ring was carried out as shown in Table 2.
2. Introduction of substituents on the pyrazole ringAmong the current commercial pyrazole-based 4-HPPD inhibitors, N-methylpyrazole moiety is employed as a key substructure. In order to examine the effect of substituents R3, we prepared several derivatives and compared their activities. Ethyl (23) and isopropyl (24) derivatives showed almost the same level of herbicidal activities as methyl derivative 22, but n-propyl derivative 25 tended to reduce the activity, especially on SETVI, suggesting that the size of steric tolerance is not very large. The same was true for the substituent R4, and the herbicidal activity tended to decrease as the bulkiness increased. Non-substituted pyrazole derivative 22 showed the highest activity, whereas placing any substituents such as methyl (26) or cyclopropyl (27) decreased the activity.
Since the optimal substitution of R3 was found to be Me, Et or isopropyl without any substitution at the 3-position (R4) of the pyrazole ring, the effect of R5 on herbicidal activity was evaluated again. Although benzyl (29) and acetyl (30) derivatives showed good activity, further in-depth evaluation revealed that there was a problem with crop safety. Small alkyl groups such as methyl (28) resulted in a significant decline in herbicidal activity. As a conclusion, 1-alkoxyethyl alkyl carbonate derivatives were found to be suitable for excellent herbicidal activity and corn selectivity.
Based on the above results, several promising compounds were selected and direct comparison tests were conducted. Compound 1a (tolpyralate), which was excellent in activity and crop selectivity under various field conditions, was finally chosen as a development compound.
A quantitative structure–activity relationship (QSAR) study on R2, R3, R4, and R5 is also described in Supplemental calculation method S1.
3. Field evaluationThe herbicidal efficacy of tolpyralate under field conditions is summarized in Table 3. Tolpyralate showed moderate herbicidal efficacy against IPOHE, but excellent efficacy against annual grass and broadleaf weeds at the use range without causing significant phytotoxicity. It provided better control of SETFA, ELEIN, and POROL than tembotrione or mesotrione. These findings indicate that tolpyralate has excellent herbicidal performance against a wide variety of annual weed species.
| Compounds | Dose (g a.i./ha) | Herbicidal efficacy (%) | |||||
|---|---|---|---|---|---|---|---|
| DIGSA | ECHCG | SETFA | ELEIN | SOLNI | CHEAL | ||
| Tolpyralate | 50 | 86 | 91 | 96 | 87 | 94 | 100 |
| 40 | 84 | 89 | 93 | 84 | 95 | 99 | |
| 30 | 81 | 83 | 96 | 83 | 96 | 97 | |
| Tembotrione | 92 | 94 | 93 | 70 | 88 | 96 | 99 |
| Mesotrione | 144–150 | 82 | 83 | 28 | 74 | 100 | 100 |
| Compounds | Dose (g a.i./ha) | Herbicidal efficacy (%) | |||||
| POLLN | AMBEL | AMARE | POROL | GASCI | IPOHE | ||
| Tolpyralate | 50 | 99 | 99 | 98 | 97 | 100 | 52 |
| 40 | 93 | 95 | 98 | 96 | 95 | 63 | |
| 30 | 88 | 96 | 98 | 96 | 94 | 52 | |
| Tembotrione | 92 | 98 | 98 | 98 | 67 | 95 | 73 |
| Mesotrione | 144–150 | 93 | 95 | 100 | 76 | 97 | 99 |
Approximate leaf-stages of applied plants are followings: grass weeds: 2–7 L and broad-leaved weeds: 2–10 L. Tolpyralate 400SC, Tembotrione 420 g/L and Mesotrione 480 g/L formulations were used in this study. Visual assessments were conducted at 28–36 days after application.
The corn selectivity of tolpyralate under various field conditions is summarized in Table 4. Temporal changes caused by phytotoxicity such as chlorosis and bleaching were slightly observed after application. However, they were transient and did not affect the subsequent growth of corn. These findings indicate that tolpyralate has excellent selectivity not only for field corn but also for sweet corn.19,20)
| Chlorosis symptoms | |||||||
|---|---|---|---|---|---|---|---|
| Compounds | Dose (g a.i./ha) | Number of Field Corn out of 41 varieties | Number of Sweet Corn out of 16 varieties | ||||
| Little or none chlorosis | Accetable chlorosis | Unaccetable chlorosis | Little or none chlorosis | Accetable chlorosis | Unaccetable chlorosis | ||
| Tolpyralate | 100 | 38 | 3 | 0 | 16 | 0 | 0 |
| 50 | 41 | 0 | 0 | 16 | 0 | 0 | |
Chlorosis symptoms was observed at 4–5 days after application with rating Little or none: 0–1.0, Acceptable: 1.1–1.5, Unacceptable: more than 1.6.
| Culm length (comparison with untreated plot: %) | |||||||
|---|---|---|---|---|---|---|---|
| Compounds | Dose (g a.i./ha) | Number of Field Corn out of 41 varieties | Number of Sweet Corn out of 16 varieties | ||||
| Little or none influence | Acceptable level | Unacceptable level | Little or none influence | Acceptable level | Unacceptable level | ||
| Tolpyralate | 100 | 41 | 0 | 0 | 15 | 1 | 0 |
| 50 | 41 | 0 | 0 | 16 | 0 | 0 | |
Culm length was observed at 47–59 days after application. Comparison with untreated plot was calculated following formula: (application plot/untreated plot) ×100. Little or none influence: more than 95%, Acceptable level: 94–90%, Unacceptable level: less than 90%
As shown in Table 5, tolpyralate exhibited equivalent or better control of susceptible weeds at 30 g a.i./ha than those of comparative compounds. Furthermore, it showed the same level of herbicidal activity against glyphosate-resistant weeds as susceptible species.21) Therefore, tolpyralate provides an important tool in the management of resistant weeds.
| Compounds | Dose (g a.i./ha) | Herbicidal efficacy (%) | |||||
|---|---|---|---|---|---|---|---|
| AMATU | AMBTR | ERICA | |||||
| Sensitive | Resistant | Sensitive | Resistant | Sensitive | Resistant | ||
| Tolpyralate | 30 | 91 | 99 | 99 | 96 | 100 | 98 |
| Tembotrione | 92 | 64 | 80 | 99 | 99 | 100 | 99 |
| Mesotrione | 88 | 71 | 65 | 99 | 99 | 77 | 85 |
| Topramezone | 17 | 76 | 55 | 98 | 88 | 65 | 71 |
| Glyphosate | 800 | 94 | 40 | 78 | 9 | 84 | 0 |
Approximate leaf-stages of applied plants are followings: AMATU: 4–6 L, AMBTR: 4–6 L and ERICA: 5-6 cm. Tolpyralate 400SC, Tembotrione 420 g/L, Mesotrione 480 g/L and Topramezone 336 g/L formulations were used in this study. Visual assessments were conducted at 29–30 days after application.
We conducted a research to discover a new 4-HPPD inhibiting herbicide based on the pyrazolone structure and led to the discovery of tolpyralate. The 1-alkoxyethyl methyl carbonate group on the N-ethylpyrazole moiety was distinctive and important for high herbicidal activity and good crop safety. Further, the methoxyethoxy group at the 3-position on the benzene ring also played an important role in improving the activity. Tolpyralate exhibited excellent herbicidal activity against annual grass and broadleaf weeds at a rate of 30–50 g a.i./ha in the field tests. Furthermore, since tolpyralate shows high herbicidal activity against glyphosate-resistant weeds, it also provides an important tool from the perspective of managing resistant weeds, which has become a major issue worldwide. We hope that tolpyralate will improve agricultural production worldwide and ensure the safety of food production.
The authors are grateful to the all members of the research and development division of Ishihara Sangyo Kaisha, Ltd. who provided great advice and support for this research. We would also like to take this opportunity to thank our former colleagues, Mr. Nobuyuki Sakashita and Dr. Hiroshi Shimoharada for insightful comments and valuable discussion.
The online version of this article contains supplementary materials (Supplemental calculation method S1, Supplemental Tables S1 and S2), which are available at https://www.jstage.jst.go.jp/browse/jpestics/.