2021 Volume 46 Issue 4 Pages 382-392
2021 Volume 46 Issue 4 Pages 382-392
Plants synthesize and accumulate a wide variety of compounds called secondary metabolites. Secondary metabolites serve as chemical barriers to protect plants from pathogens and herbivores. Antimicrobial secondary metabolites are accumulated to prevent pathogen infection. These metabolites are classified into phytoalexins (induced in response to pathogen attack) and phytoanticipins (present prior to pathogen infection). The antimicrobial compounds in the grass family (Poaceae) were studied from the viewpoint of evolution. The studies were performed at three hierarchies, families, genera, and species and include the following: 1) the distribution of benzoxazinoids (Bxs) in the grass family, 2) evolutionary replacement of phytoanticipins from Bxs to hydroxycinnamic acid amide dimers in the genus Hordeum, and 3) chemodiversity of flavonoid and diterpenoid phytoalexins in rice. These studies demonstrated dynamic changes in secondary metabolism during evolution, indicating the adaptation of plants to their environment by repeating scrap-and-build cycles.
Plants accumulate numerous compounds with diverse structures, which are referred to as secondary metabolites. The number of secondary metabolites has been roughly estimated to be one million.1) The principal role of secondary metabolites is to help plants adapt to their environment. Regarding interactions between plants and pathogenes, the role of secondary metabolites is to prevent plants from pathogen infection by their antimicrobial activities. Among the antimicrobial secondary metabolites, those accumulated in response to pathogen attacks are referred to as phytoalexins,2) and those accumulated prior to pathogen attacks are phytoanticipins.3)
The presence of phytoalexins was suggested by Müllar and Börger.4) They showed that the inoculation of an incompatible pathogen lacking the ability to transmit the disease to potato (Solanum tuberosum) tuber discs resulted in resistance to the second inoculation of a compatible pathogen. The acquired resistance was considered to be due to the accumulation of antimicrobial compounds, which were named phytoalexins. Subsequently, inducible compounds with antimicrobial activity were isolated from various plants attacked by pathogens, which proved that the hypothesis proposed by Müllar and Börger was correct. In the early 1980s, the following concept of phytoalexins gained consensus: “phytoalexins are low-molecular weight, antimicrobial compounds that are both synthesized by and accumulated in plants after exposure to microorganisms.”2,5) Oppositely, phytoanticipins were defined as “low molecular weight, antimicrobial compounds that are present in plants before challenge by microorganisms or are produced after infection solely from preexisting constituent.”3) The numerous compounds that fit these definitions have been identified in various plant species to date.
Are these antimicrobial compounds useful for the protection of plants from pathogenic infection? Many studies have addressed this issue. In Arabidopsis thaliana, the mutant strain pad3, which did not accumulate its phytoalexin, camalexin, was generated and its response to pathogen infection was compared with that of wild-type plants.6) The inoculation of Alternaria barassicicola (the causal agent of dark leaf spot disease of Brassica species) to the leaves of the pad3 mutant, resulted in the formation of larger lesions, compared to those in the wild type. This indicates the importance of camalexin in the reduction of damage caused by the infection. In addition, the gene encoding biosynthetic enzyme for resveratrol, a phytoalexin of the grapevine (Vitis vinifera), was introduced to the leaves of the tobacco (Nicotiana tabacum) plant by genetic modification.7) The transformed tobacco leaves exhibited smaller lesions than the leaves of wild-type plants in response to an inoculation with conidia of Botrytis cinerea (causal agent of gray mold in various plants). Furthermore, the correlation of the resistance of plants to pathogen infection with the amount and velocity of phytoalexin accumulation have been demonstrated in various plants. Similar experiments were performed to examine the functions of phytoanticipins. For example, the mutant strain of the diploid oat (Avena strigoza), which does not accumulate its phytoanticipins (avenacins), is more susceptible to the take-all disease caused by Gaeumannomyces graminis var. tritici, in comparison with the wild-type strain.8) These examples indicate that the antimicrobial secondary metabolites are effective to defend the plants against pathogenic infection, at least in some combinations of the plants and their pathogens.
The grass family (Poaceae) includes staple crops that are indispensable for human survival, such as rice (Oryza sativa), wheat (Triticum aestivum), and maize (Zea mays). Naturally, the grass family contains approximately 768 genera and 11,506 species,9,10) and these species are estimated to inhabit approximately 40% of the land area, except Greenland and Antarctica.11) Thus, Poaceae is regarded as one of the most prosperous plant groups on Earth. The species in Poaceae expanded their habitat to diverse environments ranging from lakes and wetlands to dry savannas and steppes and adapted to land inhabited by humans. Many weeds in farmlands and cities belong to the grass family. The first appearance of this family was estimated to be in the Middle Cretaceous period.12) Accordingly, herbivorous dinosaurs would have fed on grasses. Vascular bundles of grasses were discovered in the fossils of dinosaur feces. Phylogenetically, the grasses, except for a few primitive subfamilies, are divided into two clades: the BOP clade consisting of the subfamilies Bambusoideae, Oryzoideae, and Pooideae and the PACMAD clade containing the subfamilies Panicoideae, Aristidoideae, Chloridoideae, Micrairoideae, Arundinoideae, and Danthonioideae.9,10,13) The subfamilies Oryzoideae and Pooideae in the BOP clade contain rice and wheat, respectively, while the Panicoideae in the PACMAD clade contains maize and sorghum (Sorghum bicolor).
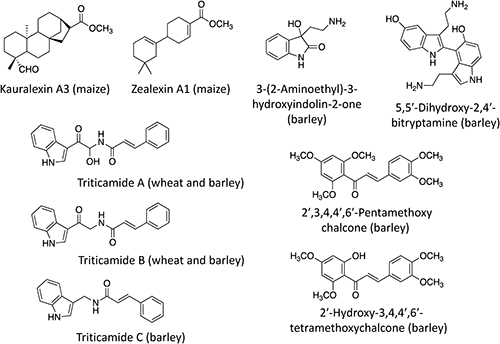
Regarding the accumulation of antimicrobial secondary metabolites, the Poaceae family is not an exception. The presence of phytoalexins was elucidated in early phytoalexin studies in rice,14) oats (Avena sativa),15,16) and sorghum.17) Recently, phytoalexins have been discovered in the grass family that had long been thought to lack phytoalexins. These phytoalexins include indole amine derivatives18) and methoxychalcones19) in barley, triticamides in wheat and barley (Hordeum vulgare),20,21) and kauralexins22) and zealexins23) in maize (Fig. 1). The presence of various phytoanticipins has also been reported in the Poaceae family. For example, cyanogenic glycosides occur in many grass species,24) as exemplified by the presence of sutherlandin and epidermin in barley,25) taxiphyllin in bamboo,26) and dhurrin in sorghum.27)
Compared to central metabolism, secondary metabolism is deduced to be evolvable28) for the following reasons. 1) Considering that central metabolism is directly related to the survival and reproduction of organisms, in many cases, its mutation has pleiotropic effects and is thus occasionally lethal. Conversely, a mutation in the gene involved in secondary metabolism typically has limited effects. This is also related to the fact that many of the central metabolic pathways form a circuit, while the secondary metabolic pathways form a cascade. 2) Subtle changes in the amino acid sequences of biosynthetic enzymes for secondary metabolism may significantly alter the profile of products, as shown for the enzymes involved in terpenoid biosynthesis.29) In central metabolism, the alteration of the product results in the loss of function of the pathway, while the changes in the structure of final products in secondary metabolism may not affect their ecological roles. 3) The duplication of genes for secondary metabolites provides an opportunity to create a new pathway without affecting the original pathway. 4) Secondary metabolism is occasionally expressed at a specific time in a specific organ. Mutations in the genes involved in secondary metabolism would have limited effects and hinder adverse effects on the producing plants.
I have been investigating the secondary metabolism involved in defense reactions mainly in the grass family. Through this investigation, many new antimicrobial compounds were identified, and the metabolic pathways leading to the compounds were characterized by biochemical and molecular biological approaches. Herein, I present the advances of this study and discuss new findings on plant defense mechanisms involving secondary metabolism from the viewpoint of evolution and its potential for future utilization in agriculture.
Bxs are characteristic secondary metabolites in grass family plants, such as wheat, rye (Secale cereale), and maize.30–32) The major Bxs are 2,4-dihydroxy-1,4-benzoxazin-3-one (DIBOA) and its 7-methoxy derivative (DIMBOA) (Fig. 2A). The concentration of Bxs is high in the seedlings immediately after germination. Bxs are stored in the vacuole as glycosides and release aglycons via the action of β-glucosidase present in plastids when the compartmentation of cells is broken by pathogenic infection or by herbivoral attack. The aglycones and their degradation products, benzoxazolinones, exert antimicrobial and antifeeding activities; therefore, Bxs are classified as phytoanticipins.

The biosynthetic genes for Bxs were first identified in maize33,34) and subsequently in wheat35,36) and rye.37) The comparison of the identified biosynthetic genes showed that the wheat genes were orthologous to the maize genes, indicating that Bxs were biosynthesized by common enzymes in maize in the PACMAD clade and wheat in the BOP clades. Thus, the common ancestor of these species already had the Bx biosynthetic pathway. Therefore, the grass family plants acquired the Bx biosynthetic pathway before splitting into the BOP and PACMAD clades.
How were the biosynthetic abilities of Bxs passed down in the evolutionary history of grasses? If the Bx biosynthetic pathway was invented by the common ancestor of grasses, it was assumed that Bxs may be present in more grasses than previously known. To address this issue, 69 species were collected from the subfamilies Pooideae, Bambusoideae, and Oryzoideae in the BOP clade and the subfamilies Panicoideae, Arundinoideae, and Chloridoideae in the PACMAD clade, and the presence of DIBOA-Glc and DIMBOA-Glc was analyzed in the species (Fig. 2B).38) Bxs were extracted from the shoots and roots immediately after germination or from new buds formed from the rhizome for the vegetatively propagating species because these organs contain Bxs in high concentrations. Bxs were detected from the species in Poodieae in the BOP clade and Panicoideae in the PACMAD clade. However, they were not detected in species from other subfamilies, suggesting that the biosynthetic pathway of Bxs was lost in subfamilies other than Pooideae and Panicoideae at the early stages of divergence in these subfamilies.
Here, we identified new Bx-accumulating species. In Pooideae, Bxs had been found only in the Triticeae tribe; however, we observed that Bxs were accumulated in Dactylis glomerata, Briza maxima, and Phalaris arundinacea in the Poae tribe, indicating that Poae was another center of distribution for Bxs. Similarly, Bxs have been reported only in Andropogoneae in Panicoideae; however, Panicum maximum, Oplismenus undulatifolius, var. undulatifolius, Echinochloa esculenta, and Echinochloa crus-galli var. caudata in the Paniceae tribe were observed to accumulate Bxs. Thus, the distribution of Bxs in the subfamilies Pooideae and Panicoideae was considerably wider than previously thought.
In contrast, we observed that many species in Pooideae and Panicoideae do not accumulate Bxs. In addition, species even in the same genus showed a difference in the presence or absence of Bxs. For instance, Panicum maximum is a Bx-accumulating species, while P. miliaceum is not. Similarly, Hordeum brachyantherum accumulates Bxs, while H. vulgare does not. Considering that the Bx-accumulating species are limited to Pooideae and Panicoideae and that multiple species in these subfamilies do not accumulate Bxs, the loss of biosynthesis of Bxs was deduced to have occurred at various times in the evolutionary history of Poaceae.
Studies on Bx biosynthetic genes by Nomura et al. demonstrated the losing process of Bx biosynthesis in barley36) and diploid wheat T. boeticum39) during evolution. The aglycones of Bxs are biosynthesized from indole 3-glycerol phosphate, an intermediate of the tryptophan pathway, by BX1–BX5. In maize, the genes encoding these enzymes were shown to be located on the short arm of chromosome 4.33,34) In hexaploid wheat, the orthologs of these genes are dispersed on groups-4 and -5 chromosomes. In barley that lacks Bxs, none of the Bx genes were detected by Southern hybridization.36) This implied that the Bx genes formed a cluster in the Bx-accumulating ancestor of barley and that the cluster was eliminated during the evolution of the barley. This issue can be addressed by analyzing the chromosomal location of Bx genes in Bx-accumulating Hordeum species. On the other hand, in the diploid wheat T. boeoticum, there were Bx-accumulating and nonBx-accumulating accessions.39) The sequencing of Bx1 gene in T. boeoticum accessions indicated that the nonBx-accumulating accessions had a single nucleotide substitution from G to C at the 3′-end of the first intron. This substitution probably interferes with the correct splicing of the intron, leading to a lack of enzyme activity of BX1. As exemplified in these cases, various genetic events may have caused the loss of Bx accumulation in the evolution of Poaceae.
For the species that have lost the ability to accumulate Bxs, are phytoanticipins no longer necessary for their protection? In this regard, the study performed for the genus Hordeum provided an insight.40) Hordeum is a group of 31 species, whose phylogenetic relationships have been well studied. The genus is classified into four clades: H, Xu, Xa, and I, among which H and Xu comprise a monophyletic group, and I and Xa comprise another.41–43) The cultivated barley, H. vulgare, belongs to the H clade and produces the unique phytoanticipins, hordatines. Hordatines are hydroxycinnamic acid amide (HCAA) dimers (lignanamides) that are biosynthesized by a pathway different from the Bx pathway. Hordatine A is a dimer of p-coumaroylagmatine units, while hordatine B is a heterodimer of p-coumaroylagmatine and feruloylagmatine units (Fig. 3A).44–46) Regarding the recently proposed nomenclature of lignanamides,47) hordatines A and B are expressed as CouAgm-4-O-7′/3-8′-DCouAgm and FerAgm-4-O-7′/3-8′-DCouAgm, respectively. We analyzed the secondary metabolites in the young shoots of 13 accessions of 10 species from the four clades of Hordeum.40) The analysis indicated that all the species belonging to the I and Xa clades accumulated DIBOA-Glc, while the species in the H clade accumulated hordatines. However, neither Bxs nor hordatines were detected in H. murinum ssp. glaucum, which is a member of the Xu clade. This prompted us to analyze the antifungal secondary metabolites in this species.

The HPLC analysis of the extract of the shoots of H. murinum ssp. glaucum showed two peaks, which appeared at retention times different from those of the Bxs and hordatines. We purified the compounds corresponding to the peaks and found that these compounds inhibited the germination of conidia and elongation of germ tubes of the phytopathogenic fungi Bipolaris sorokiniana and Fusarium asiaticum. This indicates that they function as phytoanticipins. Spectroscopic analyses demonstrated that these compounds were dimers of feruloylagmatine, as shown in Fig. 3. Since these compounds have not been identified to date, they were named as murinamides A (FerAgm-2-7′/8-8′-FerAgm) and B (FerAgm-8-8′/9-N-7′-DFerAgm). Murinamides are also present in H. murinum ssp. leporinum in the Xu clade and H. bulbosum in the H clade. As shown in Fig. 3A, hordatines and murinamides are considered dimers of HCAAs, although the positions of the linkages between the two HCAA units are different. Thus, in the genus Hordeum, the species belonging to the I and Xa clades inherited the Bx biosynthetic pathway; however, the ancestors of the H and Xu clades created a new pathway leading to the formation of HCAA dimers (Fig. 3B). The first created compounds were probably murinamides because these compounds are present in H. murinum and H. bulbosum in the Xu and H clades, respectively. Thereafter, a minor change in the biosynthetic pathway occurred in the lineage to the cultivated barley in the H clade, resulting in the biosynthesis of hordatines. For the H and Xu clades in the genus Hordeum, the defensive secondary metabolites (Bxs) were completely replaced by HCAA dimers.
Hordatines and murinamides were deduced to be biosynthesized by a radical coupling reaction between two HCAA units. The precursors of hordatines and murinamides, p-coumaroylagmatine and ferulogylagmatine, did not exhibit antimicrobial activity.40,46) Dimerized phenolic compounds have been found in other Poaceae species. The dimers of avenanthramide phytoalexins in oats48–50) and a dimer of serotonin in barley18) are probably biosynthesized by similar radical coupling reactions. The dimerization reaction by radical coupling can create a new skeleton that is completely different from the precursor; therefore, it greatly contributes to the diversification of plant secondary metabolites.
The replacement of phytoanticipins in Hordeum raises the question on the molecular basis for the major evolution of secondary metabolism, such as the creation of pathways leading to HCAA dimers. To answer this question, the biosynthetic genes involved in the synthesis of HCAA dimers in barley are currently been identified.
Studies on phytoalexins were typically conducted on a specific cultivar of a plant species. This is reasonable because when a large number of cultivars are studied, additional factors must be considered, complicating the interpretation of the results. However, a study with a single cultivar does not fully elucidate the chemical defense response of the species. In addition, studying intraspecific (natural) variations in various cultivars or strains may lead to a better understanding of the function and evolution of phytoalexins.
Rice is one of the most cultivated crops worldwide. The presence of countless elite cultivars and landraces reflects their wide distribution and long history of cultivation. Rice has been reported to accumulate more than 20 phytoalexins consisting of diterpenoids,51) hydroxycinnamic acid amides,52–55) and a flavonoid.56) However, the difference in the composition of accumulated phytoalexins in various cultivars has not been addressed. The agricultural bioresources genebank of the National Institute of Agrobiological Sciences (NIAS) provides the “World Rice Core Collection (WRC)” (https://www.gene.affrc.go.jp/databases-core_collections_wr.php). This collection comprises 69 cultivars that cover 90% of restriction fragment length polymorphism (RFLP) alleles detected in approximately 300 accessions that were selected based on passport data from the whole rice collection (approximately 30,000 accessions) maintained at the NIAS genebank.57,58) This collection was used to study the diversity of phytoalexin production in rice.
The accumulation of the flavonoid phytoalexin in rice, sakuranetin, is effectively induced by a treatment with jasmonic acid.59) Here, third leaves from rice cultivars in the WRC were treated with 1 mM jasmonic acid, and the accumulation of sakuranetin was examined.60) The results indicated that certain cultivars accumulated high concentrations of sakuranetin, while others accumulated naringenin that is the immediate precursor for sakuranetin (Fig. 4). In addition, certain cultivars accumulated both compounds at concentrations below the detection limits. The phenotype related to the composition of accumulated secondary metabolites is called the chemotype. Multiple chemotypes were observed concerning the accumulation of flavonoid phytoalexins (sakuranetin and naringenin). Rice is classified into two subspecies, japonica and indica; however, the differences in the chemotypes did not correspond to the differences in the subspecies.

The molecular basis of the differential accumulation of flavonoid phytoalexins in rice was addressed. Fortunately, the model rice cultivars Nipponbare and Kasalath showed different chemotypes: Nipponbare accumulated high concentrations of sakuranetin, while Kasalath accumulated a higher concentration of naringenin. Therefore, the chromosomal location of the causal gene for the differential accumulation of flavonoid phytoalexins was determined by a quantitative trait locus (QTL) analysis using backcross inbred lines (BILs) and by a mapping using chromosomal segment substitution lines (CSSLs) derived from these cultivars. These analyses indicated that a causal gene was located in a region of chromosome 12. A database search of the genes in this region revealed that the presence of a gene encoding naringenin-7-O-methyltransferase (NOMT), which catalyzes the conversion of naringenin to sakuranetin.61) Possibly, the difference in the NOMT sequences between Nipponbare and Kasalath was responsible for the differential accumulation of flavonoid phytoalexins. The expression of the NOMT gene greatly reduced in the jasmonic acid-treated leaves of Kasalath, compared to that of Nipponbare. In addition, it was observed that the substitution of Thr-130 in amino acid sequences of Nipponbare NOMT by Pro in the Kasalath NOMT impaired the enzymatic function with a 4.5-fold increase in the Km values for S-adenosylmethionin, the donor of the methyl group in the enzymatic reaction.
The expression of NOMT was analyzed in various cultivars. The transcript amounts of NOMT were low in the cultivars with low sakuranetin chemotypes, indicating that the suppressed expression of NOMT is the other cause of the chemotype in these cultivars. The comparison of nucleotide sequences of NOMT in the rice cultivars and accessions of the wild relative of rice, O. rufipogon, demonstrated that the NOMT sequences of cultivars and strains with low-sakuranetin chemotype formed two clusters. One of the clusters includes NOMT sequences from Kasalath, and the NOMT in this cluster commonly contains the amino acid substitution from Thr-130 to Pro. The presence of two clusters of NOMT sequences indicated that the loss of function in NOMT occurred at least twice in the evolution of rice (Fig. 4).
Certain cultivars with low-sakuranetin chemotype accumulated high concentrations of naringenin, in response to the jasmonic-acid treatment. Considering that naringenin accumulation might be relevant to the interaction with microorganisms, the antimicrobial activity of naringenin was compared with that of sakuranetin. Sakuranetin inhibited the germination of the conidia of fungal pathogens, including Pyricularia oryzae, the causal agent of rice blast, as reported.56,62) However, it did not suppress the growth of bacterial pathogens, such as Xanthomonas oryzae and Burkholderia glumae, the causal agents of bacterial blight and bacterial panicle blight, respectively. Naringenin showed the opposite trend in antimicrobial activity; it suppressed the growth of bacterial pathogens at a lower concentration than that of sakuranetin and exhibited weaker activity in the inhibitory assay for conidial germination of fungal pathogens. Thus, naringenin can be regarded as a phytoalexin for bacterial pathogens. These characteristics of antimicrobial activity in naringenin imply that the naringenin-accumulating cultivars may have been selected in a region where bacterial diseases were very difficult to treat, thereby decreasing the yield of rice. Noteworthily, rice blast is a serious problem worldwide, while bacterial leaf blight is the most severe problem in tropical and subtropical Asia.63)
In addition to flavonoid phytoalexins (sakuranetin and naringenin), the natural variation in diterpenoid phytoalexins, including oryzalexin A,64) momilactones, and phytocassanes were investigated.65) The treatment with jasmonic acid did not induce the accumulation of diterpenoid phytoalexins66); therefore, the leaves of rice seedlings were irradiated with ultraviolet (UV) light. The analysis indicated that the concentration of oryzalexin A was lower than the detection limit in majority of the cultivars in the WRC, regardless of the difference in the subspecies (Fig. 5A). Oryzalexin A is thought to be a representative phytoalexin in rice because it was discovered early in rice phytoalexin studies67,68); however, it is a minor compound detected in only a limited number of cultivars. Similar to sakuranetin, the model cultivar Nipponbare accumulated oryzalexin A, while Kasalath did not. The causal genes were explored using BILs produced from Nipponbare and Kasalath. The analysis suggested that the causal gene was present in a region in chromosome 12, where KSL10, the key biosynthetic gene for oryzalexins, is located. The qRT-PCR analysis showed the suppressed expression level of KSL10 in Kasalath, although the upstream and downstream biosynthetic genes, CPS2 and CYP701A8, were expressed at similar levels with those in Nipponbare. In addition, the expression levels of KSL10 were well correlated with the accumulated amount of oryzalexin A among the cultivars in the WRC; a high level of the KSL10 transcript was detected in the cultivars accumulating high concentrations of oryzalexin A and vice versa. Therefore, it was concluded that the reduced expression level of KSL10 caused the nonaccumulation of oryzalexin A in majority of the rice cultivars.

Large differences in accumulated amounts among cultivars were observed for momilactones A and B and phytocassanes A and D.65) However, the differences were not as extreme as those observed for oryzalexin A, and most of the cultivars accumulated momilactones and phytocassanes to a great or less extent. Therefore, momilactones and phytocassanes can be regarded as basic phytoalexins in rice. The natural variation in the accumulated amounts of these phytoalexins exists among the accessions of O. rufipogon, indicating that the origin of the natural variation in phytoalexin accumulation is older than the domestication of rice.
During this study, we discovered new phytoalexins (oryzalactone and phytocassane G) (Fig. 5B).65) Oryzalactone was accumulated in only three cultivars in the WRC, whereas phytocassane G was accumulated in virtually all the cultivars. It was speculated that oryzalactone is a recently created phytoalexin in the evolutionary history of rice because of its limited distribution in rice cultivars. Accordingly, it is anticipated that the analysis of the biosynthetic genes for oryzalactone will solve the mystery of the invention of a novel pathway for secondary metabolism.
Recently, a large natural variation was shown to be present for the accumulation of the diterpenoid casbene-type phytoalexin, 5,10-diketo-casbene (ent-10-oxodepressin).69) A genome-wide association study identified a cluster of biosynthetic genes for this phytoalexin. Considered with this study, natural variation seems to be a general characteristic in phytoalexin accumulation.
The diversity in secondary metabolism can be divided into three categories: α-, β-, and γ-chemodiversity.70) α-chemodiversity is the diversity of secondary metabolites in the same individual, including temporal and spatial differences such as changes in metabolites prior to and after pathogenic infection and variation among organs. β-chemodiversity is the variation of secondary metabolites in a population of the same species. γ-chemodiversity refers to the diversity of secondary metabolites within a plant community consisting of multiple plant species. It is difficult to apply this concept directly to crops because a crop population or plant community cultivated in an agroecosystem cannot be defined. However, considering that it is potentially possible to artificially cross any two individuals from an entire crop species, the discussion on β-chemodiversity can be applied to the intraspecific diversity of secondary metabolism in crops.
The existence of β-chemodiversity is frequently explained in terms of gene-environment interactions (G×E) for a natural population.70) Gene-environment interaction refers to a phenomenon in which individuals with distinct genotypes respond differently to environmental differences. Individuals with a particular genotype could be advantaged or disadvantaged in their survival under different environmental conditions. This situation may result in the simultaneous presence of multiple genotypes in a population. Therefore, the accumulation of different phytoalexins is probably advantageous or disadvantageous under different environmental conditions. As mentioned above, the chemodiversity in flavonoid phytoalexin accumulation in rice can be explained from the viewpoint of G×E.
A domesticated plant species is cultivated in a wider area than the natural habitat of its wild relative. During an expansion period, crops acquire adaptations de novo or by selecting standing traits as they colonize new regions with different environmental conditions.71) Exposure to a variety of biotic stresses caused by different environments can contribute to the maintenance of phytoalexin diversity in rice. Furthermore, the history of rice after domestication may have influenced the diversity of phytoalexins. According to Huang et al., O. sativa ssp. japonica was first domesticated from group III of O. rufipogon in South China, and O. sativa ssp. indica was established through a crossing with a local wild rice O. rufipogon group I during the expansion of rice cultivation.72) Accordingly, the introgression of biosynthetic genes for phytoalexins from the local populations of O. rufipogon may have been attributed to the large chemodiversity of phytoalexins in rice. In addition, the gene flow from the wild to cultivated rice may have occurred after the establishment of the subspecies, because the habitat of O. rufipogon was widely overlapped with the rice cultivation area. The gene flow from Z. mays ssp. mexicana, the wild teosinte native to the highland environment, into cultivated maize domesticated from Balsas teosinte (Zea mays ssp. parviglumis) has been suggested to be responsible for conveying adaptive phenotypes for cold and high altitudes, including a substantially high density of macrohairs and increased pigmentation.73)
Chemodiversity may be useful in agroecosystems. As in the case of sakuranetin and naringenin in rice, each antimicrobial compound has a unique antimicrobial spectrum. Therefore, it may be possible to breed a strain with an effective composition of antimicrobial substances against a specific pathogen using genes that cause chemodiversity of antimicrobial compounds. Consequently, it is important to prepare a large β-chemodiversity in advance and to analyze the mechanisms leading to different chemotypes. Additionally, it is possible to discover the secondary metabolites that were lost in elite cultivars by exploring landraces and closely related wild species. These secondary metabolites can be introduced into elite cultivars by crossbreeding.
On the other hand, it may be possible to introduce a chemodiversity of antimicrobial compounds into a single cultivar and create a set of strains (multiline) containing different antimicrobial compounds. A multiline variety using rice blast resistant isolines bearing different resistant genes was already developed as Koshihikari BL.74) Cultivating a mixture of cultivars with different compounds may also be possible. In these mixed plantings, if a pathogen overcomes the chemical defense of an individual, there will still be individuals accumulating different antimicrobial compounds in their neighborhood, which will hinder the spread of the pathogen. Indeed, the practice of planting mixtures has been widely adopted in traditional subsistence agriculture.73) In the Andean region of South America, various varieties of potatoes are simultaneously cultivated in a single field.75,76) It is presumed that a reason for the mixed cultivation is risk hedging against pests and diseases. Traditional bean varieties including the Andean and mesoamerican domesticates of Phaseolus vulgaris, are grown by households in Uganda. These varieties exhibit different sensitivities against anthracnose and angular leaf spot caused by Colletotrichum lindemuthianum and Pseudocercospora girseola, respectively.77)
Secondary metabolism is defined as metabolism that is not directly involved in the survival or reproduction of an organism. It plays a highly important specialized role in the relationship between plants and their biotic or abiotic environments. Consequently, secondary metabolism has been increasingly referred to as specialized metabolism.78,79) Herein, I presented recent findings on antimicrobial secondary metabolites, phytoalexins and phytoanticipins, in three hierarchies, families, genera, and species in the grass family from an evolutionary perspective. Our study on Bxs, which are retained in certain species in the Pooideae and Panicoideae subfamilies in the Poaceae, revealed a repeated loss in the Bx pathway during evolution from the emergence of Poaceae. The study on phytoanticipins in the genus Hordeum demonstrated the generation of new metabolic pathways. Future studies will elucidate the mechanisms underlying the generation of metabolic pathways. In addition, studies on the natural variation in phytoalexin accumulation in rice have provided insights into the significance of chemodiversity in crops regarding interactions between plants and pathogens. Summarily, these studies highlight the plasticity of secondary metabolism. It is considered that such studies are meaningful for understanding agroecosystems created by humanity and making them robust against variable biological stresses in an ever-changing environment.
This work was supported by many individuals in the Laboratory of Biofunction Chemistry, Graduate School of Agriculture, Kyoto University and the Laboratory of Natural Product Chemistry, Faculty of Agriculture, Tottori University. This work was performed in collaboration with many researchers. I would like to express my sincere gratitude to all those involved. In particular, I would like to express my heartfelt gratitude to Dr. Naoki Ube, Faculty of Agriculture, Tottori University (currently Toyama Prefectural University), who played a central role throughout the entire study. I would like to express my sincere gratitude to Prof. Naoki Mori and Prof. Yutaka Okumoto (presently at Setsunan University) at Kyoto University for helping me take up the challenge of a new field of research. Furthermore, I cannot sufficiently express my gratitude to Professor Emeritus Hajime Iwamura and Professor Hisashi Miyagawa at Kyoto University for inviting me to this research field. Finally, I believe that I received this award as a representative of all the students who supported this work at Kyoto and Tottori Universities. I thank them from the bottom of my heart.